Abstract
Calcium levulinate (4-ketopentanoate) is used as an oral and parenteral source of calcium. We hypothesized that levulinate is converted in the liver to 4-hydroxypentanoate, a new drug of abuse, and that this conversion is accelerated by ethanol oxidation. We confirmed these hypotheses in live rats, perfused rat livers, and liver subcellular preparations. Levulinate is reduced to (R)-4-hydroxypentanoate by a cytosolic and a mitochondrial dehydrogenase, which are NADPH- and NADH-dependent, respectively. A mitochondrial dehydrogenase or racemase system also forms (S)-4-hydroxypentanoate. In livers perfused with [13C5]levulinate, there was substantial CoA trapping in levulinyl-CoA, 4-hydroxypentanoyl-CoA, and 4-phosphopentanoyl-CoA. This CoA trapping was increased by ethanol, with a 6-fold increase in the concentration of 4-phosphopentanoyl-CoA. Levulinate is catabolized by 3 parallel pathways to propionyl-CoA, acetyl-CoA, and lactate. Most intermediates of the 3 pathways were identified by mass isotopomer analysis and metabolomics. The production of 4-hydroxypentanoate from levulinate and its stimulation by ethanol is a potential public health concern.
Keywords: Alcohol, Dehydrogenase, Drug Metabolism, Fatty Acid Oxidation, Isotopic Tracers, 4-Hydroxyacids, γ-Hydroxyvalerate, Acyl-CoA, α-Oxidation, Mass Isotopomer Analysis
Introduction
Levulinate (4-ketopentanoate) is a food additive allowed by the Food and Drug Administration. As a calcium salt, it has been used for many years as an oral and intravenous form of calcium administration (1, 2). It is listed in the pharmacopeias of a number of countries (USA, Europe, India, China, Japan, etc). It is also listed in quality control documents of the World Health Organization. The dry salt is sold on the internet as a non-prescription dietary supplement. To the best of our review of the literature, the catabolism of levulinate has not been investigated in mammalian cells. We found one report on the identification of the reduced form of levulinate, 4-hydroxypentanoate, in the urine of children with β-ketothiolase deficiency treated with intravenous calcium levulinate (3). We also found an unexplained report of induction of lactic acidosis in premature babies treated with oral calcium levulinate for hypocalcemia (4).
Our interest in levulinate arose from our recent study of the metabolism of 4-hydroxyacids, which are products of lipid peroxidation (C9, C6) or drugs of abuse (C4, C5) (5, 6). We showed that 4-hydroxyacids with 5 or more carbons are metabolized by two new pathways. The first pathway involves the isomerization of 4-hydroxyacyl-CoAs to 3-hydroxyacyl-CoAs via 4-phosphoacyl-CoAs, a new class of acyl-CoA esters. After isomerization, the 3-hydroxyacyl-CoAs are catabolized via classical β-oxidation to acetyl-CoA and, in the case of odd-chain 4-hydroxyacids, to propionyl-CoA. The second pathway involves a sequence of β-, α-, and β-oxidation steps leading to acetyl-CoA, formate and, in the case of even chain 4-hydroxyacids, to propionyl-CoA.
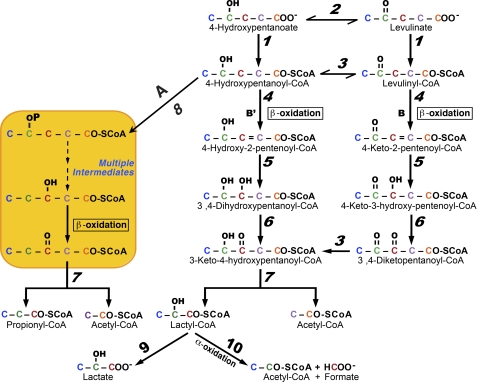
The five-carbon 4-hydroxypentanoate, as a racemic mixture, is a new drug of abuse used in lieu of 4-hydroxybutyrate, which is now a controlled substance in the United States (7–11). Based on our previous work, we hypothesized that calcium levulinate could be used as a precursor of 4-hydroxypentanoate, i.e. as a pro-drug of abuse. The reduction of levulinate to 4-hydroxypentanoate would occur in the liver and other organs, possibly catalyzed by NADH- and/or NADPH- dependent dehydrogenase(s) (Fig. 1, Reaction 2). We further hypothesized that the reduction of levulinate to 4-hydroxypentanoate could be stimulated by ethanol ingestion. The oxidation of ethanol in liver increases the [NADH]/[NAD+] ratios in cytosol and mitochondria (12), and would stimulate the reduction of levulinate by a hepatic NADH-dehydrogenase. We also considered that the metabolism of levulinate in cells could generate both enantiomers of 4-hydroxypentanoate. Last, we hypothesized that the metabolism of levulinate and 4-hydroxypentanoate would follow the reactions of the scheme outlined in Fig. 1. This scheme has features demonstrated previously, i.e. the isomerization of 4-hydroxypentanoyl-CoA to 3-hydroxypentanoyl-CoA via 4-phosphopentanoyl-CoA followed by β-oxidation to propionyl-CoA + acetyl-CoA (Pathway A). In addition, we hypothesized that 4-hydroxypentanoyl-CoA and levulinyl-CoA are degraded by two parallel β-oxidation processes: pathways B (previously described for 4-hydroxynonanoate (5)) and B′ (hypothetical) that converge at 3-keto-4-hydroxypentanoyl-CoA (Fig. 1). The latter would undergo thiolytic cleavage to acetyl-CoA + the putative lactyl-CoA, which has not been identified in mammalian cells. Lactyl-CoA could be hydrolyzed to lactate, and/or α-oxidized to acetyl-CoA + formate.
FIGURE 1.
Proposed scheme for the metabolism of levulinate and 4-hydroxypentanoate. The hypothetical enzyme activities, designated by numbers in italics are: 1, acid-CoA ligase; 2, hydroxyacid dehydrogenase; 3, 4-hydroxyacyl-CoA dehydrogenase; 4, acyl-CoA dehydrogenase; 5, enoyl-CoA hydratase; 6, 3-hydroxyacyl-CoA dehydrogenase; 7, 3-ketoacyl-CoA thiolase; 8, 4-hydroxyacyl-CoA kinase; 9, acyl-CoA hydrolase; 10, α-oxidation enzymes. The “multiple reactions” mentioned between 4-phosphopentanoyl-CoA and 3-hydroxypentanoyl-CoA result in the isomerization of 4-hydroxypentanoyl-CoA to 3-hydroxypentanoyl-CoA (5).
To test these hypotheses, we tried to design a [13Cn]levulinate substrate that would yield (i) different mass isotopomers2 of the two acetyl-CoAs presumably derived from C-1 + 2 and C-4 + 5 of levulinate, and (ii) allow tracing the production of formate from C-3 of levulinate. In our previous study, we had designed 4-hydroxy-[3,4-13C2]nonanoate, which yielded M2 and M1 acetyl-CoA via Pathways A and B, respectively. However, we could not design a single [13Cn]levulinate that would allow tracing of all the processes outlined in Fig. 1. We settled on M5 [13C5]levulinate for most of the planned experiments. We also synthesized [3-13C]levulinate and [1,2,4,5-13C4]levulinate to test for the production of formate from C-3 of levulinate.
We previously showed that, among livers perfused with one of the C4 to C11 4-hydroxyacids, the highest concentrations of 4-phosphoacyl-CoA and related acyl-CoAs were found in livers perfused with 4-hydroxypentanoate (see Fig. 2 and supplemental Fig. 3S of Ref. 5). This raised the question of whether the metabolism of levulinate ± ethanol in the liver would lead to substantial CoA trapping, a process linked to a number of perturbations of intermediary metabolism (13). We tested the above hypotheses in live rats and in perfused rat livers using a combination of metabolomics (14, 15) and mass isotopomer analysis (16). Our data confirm that levulinate is converted to 4-hydroxypentanoate, and that it is metabolized by the three pathways outlined in Fig. 1.
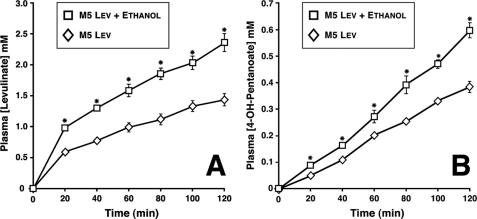
FIGURE 2.
Profiles of plasma concentrations of levulinate (A) and 4-hydroxypentanoate (B) in rats infused intravenously with sodium levulinate ± ethanol. Data are presented as mean ± S.E. (n = 6).
EXPERIMENTAL PROCEDURES
Materials
Sigma and Isotec supplied most chemicals and the following isotopically labeled compounds: [2H7]butyric acid, [13C3]propionic acid, [2H9]pentanoic acid, sodium [13C2]acetate, and sodium [13C]formate. [3,3,5,5,5-2H5]Levulinate was prepared by isotopic exchange between unlabeled levulinate, 2H2O, and NaO2H (17). 4-Hydroxy-[3,3,4,5,5,5-2H6]pentanoate was prepared by reducing [3,3,5,5,5-2H5]levulinate with NaB2H4. The same procedure was used to prepare (R,S)-3-hydroxy-[2H5]pentanoate from 3-ketopentanoate ethyl ester. Variously 13C-labeled levulinates ([13C5], [3-13C], and [1,2,4,5-13C4]) were prepared as described in Ref. 18. The purity of synthesized compounds was verified by gas chromatography-mass spectrometry (GC-MS) and NMR. The lactones of all 4-hydroxypentanoates were hydrolyzed with 10% excess NaOH at 60 °C for 1 h. [2H5]Propionyl-CoA and [2H9]pentanoyl-CoA (internal standards for acyl-CoA profiles) were prepared from the acids as described in Ref. 19.
In Vivo Experiments
Overnight fasted male Sprague-Dawley rats were anesthetized with 2% isoflurane and fitted with carotid and jugular catheters. After a 20-min equilibration, Na-levulinate was infused intravenously, as a 150 mm solution, at 2, 4, 6, 8, 10, or 12 μmol min−1 kg−1 for 2 h. In half of the rats, a bolus of 10% ethanol (1.7 m) in saline was injected intraperitoneally at −15 min in an amount calculated to achieve 10 mm in total body water. This was followed by a constant intravenous ethanol infusion at 40 μmol min−1 kg−1. Arterial blood was sampled every 20 min for 2 h.
Perfused Liver Experiments
Four groups of livers from male rats (200–250 g) were perfused (20) with recirculating bicarbonate buffer containing 4% dialyzed, fatty acid-free, bovine serum albumin, 4 mm glucose, ± 2 mm M5 levulinate ± 20 mm ethanol (or 20 mm ethanol without levulinate). Perfusate was sampled every 20 min. Livers were quick frozen at 2 h.
Analytical Procedures
The concentrations and mass isotopomer distributions of the various acids, keto acids, and hydroxyacids were assayed by GC-MS of trimethylsilyl or pentafluorobenzyl derivatives, using analog unlabeled or labeled compounds as internal standards. The concentration and labeling of acetate and formate were assayed using negative chemical ionization of the pentafluorobenzyl derivatives (38). The concentrations and mass isotopomer distributions of acyl-CoA esters were assayed as in Ref. 5. The chromatographic conditions optimize the separation of short- and medium-chain acyl-CoAs. Because the peaks of levulinyl-CoA and 4-hydroxypentanoyl-CoA partially overlapped (retention times: 14.3 and 14.7 min, respectively), peak integrations were conducted on the front 50% of the levulinyl-CoA peak and the rear 50% of the 4-hydroxypentanoyl-CoA peak. For the assay of the concentrations of new acyl-CoA esters for which unlabeled and labeled standards are not available, we used a calibration curve of acetyl-CoA concentration with an internal standard of [2H9]pentanoyl-CoA prepared from the acid (19).
To assay the distribution of chiral enantiomers of 4-hydroxypentanoate and lactate, we prepared the (R)-2-butyl-O-acetyl derivatives by a modification of the procedure of Struys et al. (21). This procedure involves reacting the samples with 2(R)-butanol + HCl, extracting the 2(R)-butyl hydroxyester, and acetylating the latter with acetic anhydride. We achieved the double derivatization in one step by reacting the samples with 2(R)-butanol + acetyl chloride. The derivatives were assayed by electron ionization GC-MS. The concentration of ethanol in rat plasma was assayed by head space GC-MS with an internal standard of 1-propanol (22).
Pilot Enzymatic Assays
Rat liver mitochondria and cytosolic extracts (100,000 × g supernatants) were prepared as described in Ref. 23. Mitochondria (2.5 or 6 mg) were incubated in 1.2 ml of buffer (100 mm KCl, 50 mm MOPS, 5 mm K-phosphate, 1 mm EGTA, pH 7.4) containing 4.2 mm glutamate, and 6 μm rotenone at 30 °C in Mitocell MT200 incubation chambers. The kinetics were started by adding 5 mm acetoacetate (controls) or levulinate. Incubations were conducted for 30 (acetoacetate) or 120 min (levulinate) with multiple sampling. Cytosolic extracts (2.4 mg of protein) were incubated for up to 2 h with 0.3 mm NADPH and 2.8 mm levulinate in a total volume of 3.5 ml of 50 mm potassium phosphate buffer, pH 7.4, at 30 °C. The production of 4-hydroxypentanoate was assayed as the trimethylsilyl derivative and as the chiral 2(R)-butyl-O-acetyl derivative.
Calculations
Correction of measured mass isotopomer distributions for natural enrichment was performed using the CORMAT software (24). The data points shown in the figures represent means of duplicate GC-MS or LC-MS/MS injections, which differed by <2%. The statistical differences between some profiles were tested using an unpaired t test (Graph Pad Prism Software, version 3).
RESULTS
Reduction of Levulinate to 4-Hydroxypentanoate in Vivo and Isolated Livers; Stimulation by Ethanol
In orientation in vivo experiments, we infused anesthetized rats with Na-levulinate at rates ranging from 2 to 12 μmol min−1 kg−1. At infusion rates of levulinate from 2 to 6 μmol min−1 kg−1, plasma levulinate concentrations plateaued at up to 0.8 mm (not shown). At higher rates of levulinate infusions (6 to 12 μmol min−1 kg−1), the levulinate concentration kept increasing almost linearly. In all cases, we observed the accumulation of 4-hydroxypentanoate, which increased linearly without plateauing at all rates of levulinate infusion, reaching 0.47 mm after 2 h of levulinate infusion at 12 μmol min−1 kg−1 (not shown). Because this plasma 4-hydroxypentanoate concentration is in the range measured in humans addicted to 4-hydroxypentanoate (7, 11), we settled on a levulinate infusion rate of 12 μmol min−1 kg−1 for our rat in vivo experiments. We infused 12 overnight-fasted anesthetized rats with 12 μmol min−1 kg−1 levulinate for 2 h. At −15 min, 6 rats received an intraperitoneal injection of 10% ethanol in saline. The dose was calculated to induce ethanol concentrations of about 10 mm in total body water. The same 6 rats were infused intravenously with 10% ethanol in saline at 40 μmol min−1 kg−1 from 0 to 120 min to compensate for ethanol metabolism. This rate of infusion corresponds to the reported activity of alcohol dehydrogenase in the whole liver (12). The plasma ethanol concentration at 120 min was 3.7 ± 1 mm (n = 6). Because the half-maximal ethanol uptake by perfused rat livers was observed at a perfusate concentration of 0.25 mm (25), ethanol oxidation was operating at maximal capacity throughout the experiment. The other 6 rats, used as controls (infused with levulinate but no ethanol), were injected and infused with saline.
Fig. 2 shows the profiles of plasma levulinate and 4-hydroxypentanoate concentrations in the two groups of rats. Compared with the levulinate controls, ethanol administration resulted in higher plasma levulinate and 4-hydroxypentanoate concentrations, and significantly increased [lactate]/[pyruvate] ratios (not shown). These experiments demonstrated that (i) levulinate is reduced to 4-hydroxypentanoate in vivo, (ii) ethanol stimulates the reduction of levulinate to 4-hydroxypentanoate, and (iii) ethanol decreases total levulinate metabolism in vivo. However, because we do not know the volumes of distribution of levulinate and 4-hydroxypentanoate in rats, we could not calculate metabolic rates from the data of Fig. 2. This is why we turned to perfused rat liver experiments.
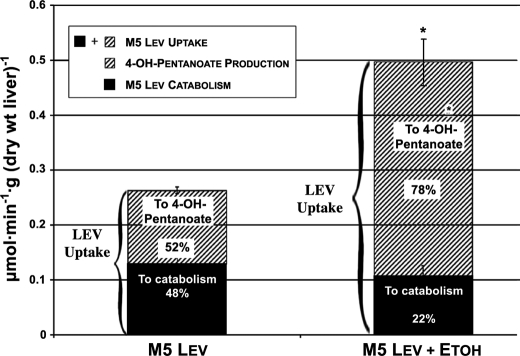
We perfused 4 groups of isolated rat livers for 2 h with recirculating buffer containing initially 4 mm glucose and (i) nothing (controls), (ii) 2 mm M5 levulinate, (iii) 2 mm M5 levulinate + 20 mm ethanol, or (iv) 20 mm ethanol. Fig. 3 shows for groups ii and iii, which contained M5 levulinate, the total levulinate uptake and 4-hydroxypentanoate production over 2 h. Contrary to what we observed in vivo, ethanol almost doubled the uptake of levulinate by the liver. Also, ethanol tripled the production of 4-hydroxypentanoate from levulinate by the isolated liver. Ethanol shifted the distribution of levulinate metabolism between 4-hydroxypentanoate production and catabolism from 1/1 to 4/1. Chiral assay of 4-hydroxypentanoate in the final perfusates of groups ii (levulinate) and iii (levulinate + ethanol) revealed that the proportions of the (R)-enantiomer were 84 ± 0.3 and 79 ± 1.4%, respectively (p = 0.007).
FIGURE 3.
Effect of ethanol on levulinate uptake and metabolism in perfused rat livers. The lower section of each bar, labeled “To catabolism” was calculated as the difference between the uptake of levulinate and the release of 4-hydroxypentanoate. It corresponds to the disposal of levulinate via Pathways A, B, and B′. Data are presented as mean ± S.E. (n = 6).
Evidence That Levulinate and 4-Hydroxypentanoate Are Catabolized as Outlined in Fig. 1
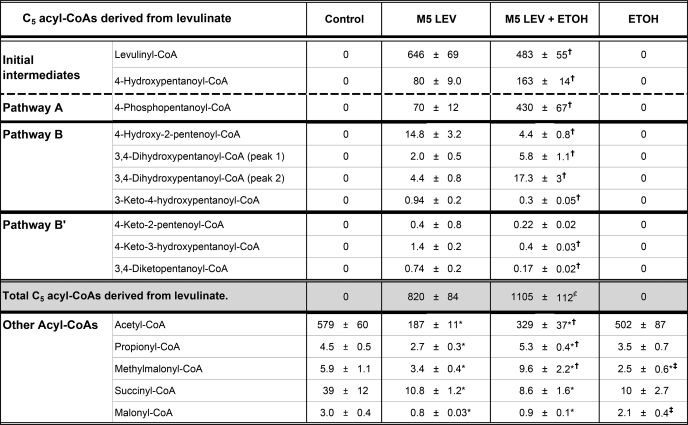
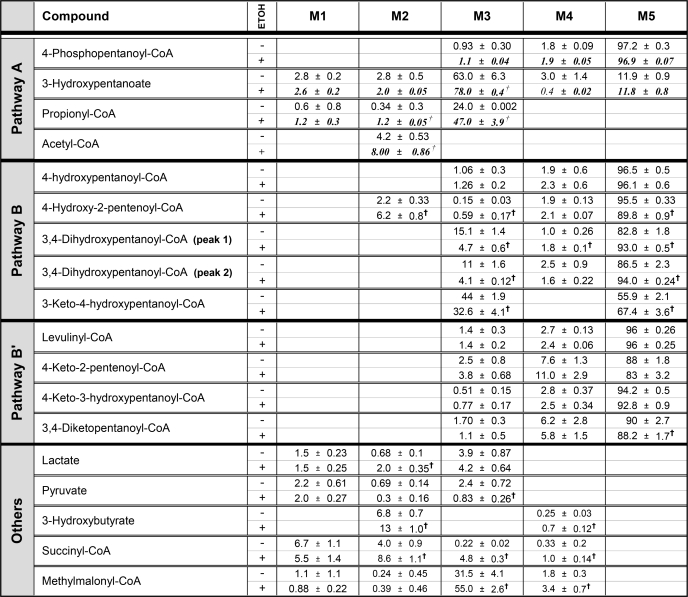
The putative pathways of levulinate metabolism were confirmed by the identification of a number of acyl-CoAs, as well as by the assays of their concentrations and mass isotopomer distributions (Tables 1 and 2). Further confirmation was obtained from the mass isotopomer distribution of some carboxylic acids (Table 2). In livers perfused with an initial 2 mm M5 levulinate ± 20 mm ethanol, the three C5 acyl-CoAs initially derived from the substrate (levulinyl-CoA, 4-hydroxypentanoyl-CoA and 4-phosphopentanoyl-CoA, Fig. 1) accumulated in large amounts (Table 1). These three acyl-CoAs, which are the initial intermediates of Pathways B′, B, and A, respectively (Fig. 1), were only M5 labeled as expected. Consistent with Pathway A, propionyl-CoA was 24 and 47% M3-labeled in livers perfused with M5 levulinate or M5 levulinate + ethanol, respectively (Table 2). We did not identify the products of 4-hydroxypentanoyl-CoA isomerization, i.e. 3-hydroxypentanoyl-CoA and the latter's oxidized form, i.e. 3-ketopentanoyl-CoA in liver tissue. However, we identified the corresponding 3-hydroxypentanoate in the perfusate. Unexpectedly, the mass isotopomer distribution of the latter was not only M5, but was mostly M3 with a small component of M5 (Table 2). The only possible explanation of the isotopomer distribution of 3-hydroxypentanoate is the combined reversibility of the reactions catalyzed by 3-hydroxyacyl-CoA dehydrogenase and 3-ketoacyl-CoA thiolase (Pathway A) in intact liver cells. This combined reversibility results in the exchanges of (i) the C-1 + 2 moiety of 3-hydroxypentanoyl-CoA with free acetyl-CoA, and (ii) the C-3 + 4+5 moiety of 3-hydroxypentanoyl-CoA with propionyl-CoA. However, the propionyl moiety exchanges much more slowly than the acetyl moiety (26). This explains the much lower M2 than M3 enrichment of 3-hydroxypentanoate (Table 2). The very different rates of exchange by thiolase of the moieties of 3-ketoacyl-CoAs had been originally reported by Hüth et al. (27) for acetoacetyl-CoA.
TABLE 1.
Concentrations of acyl-CoAs in perfused rat livers
Livers were perfused for 2 h with buffer containing 4 mm glucose and either nothing (control), 2 mm [13C5]levulinate (M5 LEV), 2 mm [13C5]levulinate + 20 mm ethanol (M5 LEV + ETOH) or 20 mm ethanol (ETOH). Concentrations of acyl-CoAs are expressed in nanomole/g dry weight (mean ± S.E.; n = 6). Statistics: *, significantly different from controls; †, significantly different from M5 LEV; ‡, significantly different from M5 LEV + ETOH (p < 0.05);  , p = 0.06 compared to M5 LEV.
, p = 0.06 compared to M5 LEV.
TABLE 2.
Mass isotopomer distribution of products of [13C5]levulinate in perfused rat livers
Livers were perfused for 2 h with buffer containing 4 mm glucose and either 2 mm [13C5]levulinate or 2 mm [13C5]levulinate + 20 mm ethanol. Data are presented as percent distributions of mass isotopomers (mean ± S.E.; n = 6). The absence or presence of ethanol in the pefusate is indicated by a − or + sign in the third column. Data from perfusions with [13C5]levulinate + ethanol are shown in italics. Statistics: †, significantly different from M5 LEV (p < 0.05).
Consistent with Pathways B and B′, we identified the six C5 acyl-CoAs that we had hypothesized to derive from 4-hydroxypentanoyl-CoA and levulinyl-CoA (Fig. 1). The identifications were based on LC-MS/MS with specific mother/daughter ion relationships and on the mass isotopomer distributions of the compounds. The mass isotopomer distribution was instrumental at differentiating the isomeric compounds 4-keto-3-hydroxypentanoyl-CoA and 3-keto-4-hydroxypentanoyl-CoA (Fig. 1), which have the same mother/daughter ion pairs. We had anticipated that 3-keto-4-hydroxypentanoyl-CoA, a presumed substrate of a reversible thiolase, would have a component of M3 labeling as a result of isotopic exchange with acetyl-CoA. This was the case: 56% M5 and 44% M3. In contrast, we anticipated that 4-keto-3-hydroxypentanoyl-CoA, which would not be a substrate for a thiolase, would be mostly M5 labeled. This was the case: 94% M5.
3,4-Dihydroxypentanoyl-CoA (Fig. 1, Pathway B) yielded two peaks, in relative abundance of about 40:60, because it is a mixture of diastereomers. The OH on C-3 is (S)-, because of the specificity of enoyl-CoA hydratase. The OH on C-4 is either (R)- or (S)-, because 4-hydroxypentanoate derived from levulinate is 85% (R)- and 15% (S)- (more on this under “Discussion”). The two diastereomers of 3,4-dihydroxypentanoyl-CoA had similar mass isotopomer distributions: 83% M5 and 15% M3, versus 87% M5 and 11% M3 (Table 2). The presence of M3 isotopomers of the two diastereomers of 3,4-dihydroxypentanoyl-CoA shows that the acetyl moiety of each diastereomer exchanges with acetyl-CoA via 3-hydroxyacyl-CoA dehydrogenase and 3-ketoacyl-CoA thiolase.
We did not identify lactyl-CoA, which would be formed after the convergence of Pathways B and B′, via thiolytic cleavage of 3-keto-4-hydroxypentanoyl-CoA. However, we found that perfusate lactate was mostly M3 labeled, and was more M3 labeled than pyruvate (Table 2). This showed a precursor to product relationship of lactate to pyruvate supporting the formation of M3 lactate by hydrolysis of the putative lactyl-CoA.
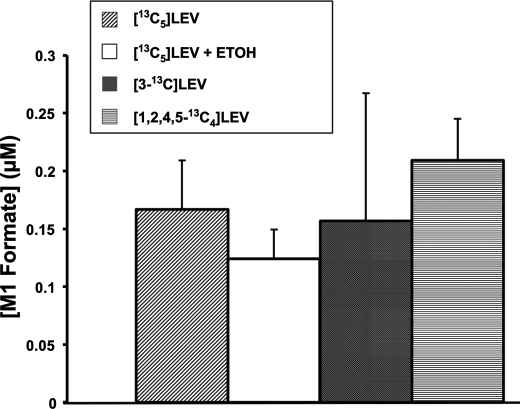
Our hypothetical scheme of levulinate catabolism involved the α-oxidation of lactyl-CoA, which would yield formate. As expected, M1 formate accumulated in perfusions with M5 levulinate (Fig. 4). To check whether the production of M1 formate was derived indeed from C-3 of levulinate, we perfused 2 livers with [3-13C]levulinate and 2 livers with [1,2,4,5-13C4]levulinate. As expected M1 formate was generated from [3-13C]levulinate. To our surprise, M1 formate was also generated from [1,2,4,5-13C4]levulinate. Fig. 4 shows the concentrations of M1 formate at 120 min in the perfusates of the experiments with [13C5]levulinate (± ethanol), [3-13C]levulinate, and [1,2,4,5-13C4]levulinate. The data are presented as M1 formate concentrations because of the ubiquitous contamination of reagents with traces of unlabeled formate. Formyl-CoA, a very unstable intermediate of fatty acid α-oxidation (28), was not detected in any of the livers. We suspected that M1 formate was formed, not from C-3 of levulinate via α-oxidation, but by an indirect route: levulinate →→ propionyl-CoA (via Pathway A) → anaplerosis → citric acid cycle intermediates → PEP →→ 3-phosphoglycerate →→ serine → glycine → formate. Consistent with this long process, we found in the final perfusates of experiments with [13C5]levulinate, [3-13C]levulinate, or [1,2,4,5-13C4]levulinate that tissue glycine was M1 labeled in the range of 0.6 to 2.1% without a significant difference between the labels. Thus, the production of M1 formate from [13Cn]levulinate does not reflect an α-oxidation process generating formate from C-3 of levulinate. However, we cannot exclude that a very small amount of formate is formed by α-oxidation of lactyl-CoA.
FIGURE 4.
Release of [13C]formate by rat livers perfused with [13Cn]levulinate. Shown are concentrations of [13C]formate at the end of 120-min perfusions with [13C5]levulinate (n = 6), [13C5]levulinate + 20 mm ethanol (n = 6), [3-13C]levulinate (n = 2), or [1,2,4,5-13C5]levulinate (n = 2).
Last, in perfusions with [13C5]levulinate ± ethanol, the M2 enrichment of acetyl-CoA was 4.2 and 8.0%, respectively. Based on the scheme of Fig. 1, the catabolism of 1 molecule of levulinate yields 1 acetyl-CoA via Pathway A or 1 to 2 acetyl-CoA via Pathways B + B′. Thiolytic cleavage of 3-keto-4-hydroxypentanoyl-CoA yields 1 acetyl-CoA. Decarboxylation of pyruvate derived from lactate can generate up to 1 more acetyl-CoA. However, there is very little pyruvate decarboxylation in livers from overnight-fasted rats, as demonstrated by Koeppe et al. (29) from the labeling pattern of liver glutamate after injection of [2-14C]pyruvate. The decarboxylation of pyruvate is further decreased by ethanol oxidation, which inhibits citric acid cycle activity (12). Therefore, only 1 acetyl-CoA is formed per molecule of levulinate. Thus, the M2 enrichment of acetyl-CoA represents the contribution of levulinate to acetyl-CoA production.
Pilot Enzymatic Assays
To test for the existence of a mitochondrial dehydrogenase that would reduce levulinate, we incubated intact liver mitochondria with glutamate + rotenone (NADH-generating system) + 5 mm levulinate at 30 °C. We observed the production of 4-hydroxypentanoate that was assayed by GC-MS of the trimethylsilyl derivative. The production of 4-hydroxypentanoate was linear with time for 120 min, and with protein concentration (for 0.25 to 0.75 mg of protein/incubation). The apparent activity at 5 mm levulinate and 30 °C was 0.48 ± 0.03 nmol min−1 (mg of protein)−1 or 12.0 ± 0.8 nmol min−1 (g of liver)−1 (S.E., n = 7) based on 25 mg of mitochondrial protein/g of liver. In parallel control incubations of mitochondria with 5 mm acetoacetate instead of levulinate, the production of 3-hydroxybutyrate was 32 ± 1.3 nmol min−1 (mg of protein)−1 or 789 ± 32 nmol min−1 (g of liver)−1 (n = 7). The rates of acetoacetate reduction are similar to published values. Chiral GC-MS assays showed that 85 ± 0.5% of the 4-hydroxypentanoate formed from levulinate was the (R)-enantiomer (n = 7 different livers). In the same chiral assay, a pure standard of (R)-4-hydroxypentanoate (Sigma) yielded an apparent 97% of the (R)-enantiomer.
To test for a cytosolic dehydrogenase that would reduce levulinate, we incubated a 100,000 × g supernatant of liver homogenate with levulinate + NADPH. We observed a linear production of 4-hydroxypentanoate. The apparent activity at 30 °C was 0.043 ± 0.003 nmol min−1 (mg of protein)−1 or 4.6 ± 0.36 nmol min−1 (g liver)−1 (S.E., n = 6). When the assay was conducted in the presence of NADH, the activity was less than 20% of the activity measured with NADPH. Chiral GC-MS assay of 4-hydroxypentanoate formed in one cytosolic incubation showed 94% of the (R)-enantiomer. Because of the low sensitivity of the chiral assay, this assay was conducted on the pooled cytosolic extracts of 6 livers.
The total apparent activity (mitochondrial + cytosolic) of enzymes that reduce levulinate to 4-hydroxypentanoate in vitro is about 16.5 ± 0.95 nmol min−1 (g liver wet weight)−1 at 30 °C. This rate is equivalent to 82.5 nmol min−1 (g liver dry weight)−1 at 37 °C, assuming a (37 °C)/(30 °C) activity ratio of 1.5 and a (dry weight)/(wet weight) of 0.31 for in vivo liver (16.5 × 1.5 ÷ 0.31 = 82.5). This rate, measured in subcellular fractions of non-perfused livers, accounts for 62 and 21% of the rates of production of 4-hydroxypentanoate measured in rat livers perfused with 2 mm levulinate ± ethanol (134 and 390 nmol min−1 (g liver dry weight)−1, respectively, at 37 °C (see Fig. 2, upper sections of bars)). The above pilot enzymatic assays that identify activities that convert levulinate to 4-hydroxypentanoate are the basis of future studies that will characterize the kinetics of the uncovered activities.
DISCUSSION
Interconversion of Levulinate and 4-Hydroxypentanoate
In live rats and perfused rat livers, we demonstrated the reduction of levulinate to 4-hydroxypentanoate (Figs. 2 and 3). Also, in livers perfused with (R,S)- or (R)-4-hydroxypentanoate we observed the accumulation of levulinate (not shown). Thus, there is at least one dehydrogenase system that interconverts levulinate and 4-hydroxypentanoate in liver. We could not find any report on an enzyme system that interconverts 4-keto acids and 4-hydroxyacids longer than 4 carbons in mammalian cells. Kaufman's group (30–32) reported on two enzymes that interconvert succinic semialdehyde and 4-hydroxybutyrate: a cytosolic NADP+-dehydrogenase and a mitochondrial transhydrogenase catalyzing the reaction 4-hydroxybutyrate + α-ketoglutarate ↔ succinate semialdehyde + α-hydroxyglutarate. The transhydrogenase has an absolute requirement for α-ketoglutarate.
In our pilot enzymatic studies, the slow cytosolic NADPH-dependent reduction of levulinate we detected may be catalyzed by the cytosolic 4-hydroxybutyrate dehydrogenase described by Kaufman (30). The more rapid reduction of levulinate in mitochondria incubated with glutamate and rotenone must be catalyzed by one or more enzymes different from the transhydrogenase of Kaufman (30). The reduction of levulinate by the 4-hydroxybutyrate transhydrogenase would be presumably inhibited by α-ketoglutarate derived from glutamate. Because the addition of glutamate to mitochondria forms a NADH generating system, the reduction of levulinate to 4-hydroxypentanoate is most likely catalyzed by at least one NADH-dehydrogenase. This conclusion is supported by the stimulation of levulinate reduction by ethanol in perfused livers (Fig. 3). The oxidation of ethanol leads to marked increases in the cytosolic and mitochondrial [NADH]/[NAD+] ratios (12). Both enantiomers of 4-hydroxypentanoate were generated in mitochondria incubated with levulinate (85% (R)) and in livers perfused with levulinate alone (84% (R)), or with levulinate + ethanol (79% (R)).
The data suggest that the reduction of levulinate in liver mitochondria is catalyzed by: (i) one (R)- and one (S)-NADH-dehydrogenase; (ii) one (R)-NADH-dehydrogenase and a racemase; or (iii) one (R)-NADH-dehydrogenase and a racemase system. The (R)-dehydrogenase could be (R)-3-hydroxybutyrate dehydrogenase, the enzyme that interconverts the physiological ketone bodies, acetoacetate and (R)-3-hydroxybutyrate (33). In our mitochondria incubations, the rate of levulinate reduction to 4-hydroxypentanoate was 1.6% of the rate of acetoacetate reduction to 3-hydroxybutyrate. Thus, the reduction of levulinate may be a side reaction of (R)-3-hydroxybutyrate dehydrogenase, which also interconverts (R)-3-hydroxypentanoate and 3-ketopentanoate (26, 34).
A possible racemase system would follow the sequence: levulinate → levulinyl-CoA → (S)-4-hydroxypentanoyl-CoA → (S)-4-hydroxypentanoate (Fig. 1, reactions 1, 3, and reversal of reaction 1). Although there is no information on the enzyme that interconverts levulinyl-CoA and 4-hydroxypentanoyl-CoA, it may be similar (or identical) to the (S)-3-hydroxyacyl-CoA dehydrogenase involved in fatty acid β-oxidation. A fraction of the (S)-4-hydroxypentanoyl-CoA would be hydrolyzed to (S)-4-hydroxypentanoate.
If the above interpretation is correct, (S)-4-hydroxypentanoyl-CoA is formed from levulinyl-CoA via (S)-3-hydroxyacyl-CoA dehydrogenase or a similar enzyme. (R)-4-Hydroxypentanoyl-CoA is formed via the activation of (R)-4-hydroxypentanoate derived from the reduction of levulinate by (R)-3-hydroxybutyrate dehydrogenase. The 15% (S)-component of 4-hydroxypentanoate derived from levulinate represents a minimal (S)-component of 4-hydroxypentanoyl-CoA.
Ethanol decreases slightly but significantly the fraction of (R)-4-hydroxypentanoate derived from levulinate in perfused livers (from 84 to 79%). This effect results probably from the inhibition of the three oxidation reactions catalyzed by 3-hydroxyacyl-CoA dehydrogenase (Fig. 1, reactions 6) by the increase in the [NADH]/[NAD+] ratio resulting from ethanol oxidation. The decrease in the catabolism of (S)-4-hydroxypentanoyl-CoA probably increases its hydrolysis.
Pathways of Levulinate Catabolism
In our previous study (5), we showed that 4-hydroxyacids with at least 5 carbons are metabolized by two pathways listed in Fig. 1 as A and B. Pathway A involves the isomerization of 4-hydroxyacyl-CoAs to 3-hydroxyacyl-CoAs via 4-phosphoacyl-CoAs. Pathway B involves a sequence of β-oxidation and α-oxidation steps. In the present study, we demonstrated that an additional β-oxidation process (pathway B′) proceeds from levulinyl-CoA in parallel to pathway B, linking with the latter at 3-keto-4-hydroxypentanoyl-CoA. Pathways B and B′ differ by the presence of a hydroxy or a keto group on carbon 4 of the five-carbon intermediates. The identification of acyl-CoAs, which are intermediates in pathways B and B′ (Fig. 1) demonstrates that the presence of a keto or a hydroxy group on C-4 of a fatty acid does not prevent any of the reactions of β-oxidation to proceed. In perfusions with (R,S)-4-hydroxypentanoate, the two enantiomers are taken up at the same rate (not shown). One can thus conclude that the configuration of the hydroxyl on C-4 of some acyl-CoA metabolites of levulinate does not affect their flux rates through Pathways A and B (in Pathway B′, C-4 is a carbonyl). Further information on the enantiomeric and diastereomeric distribution of levulinate metabolites will require the development of mass spectrometry techniques to measure the chiral composition of hydroxy- and dihydroxyacyl-CoAs.
In the presence of ethanol, we observed marked increases in the uptake of levulinate and in its conversion to 4-hydroxypentanoate by perfused livers. The increase in the [NADH]/[NAD+] induced by ethanol oxidation (12) explains the increase in the reduction of levulinate to 4-hydroxypentanoate. The subsequent decrease in intracellular levulinate concentration stimulates levulinate uptake. The small but significant decrease in the catabolism of levulinate (Fig. 3, lower bars) results from the inhibition of the three reactions catalyzed by 3-hydroxyacyl-CoA dehydrogenase in Pathways A, B, and B′ (Fig. 1, reactions 6). In an apparent contradiction, the inhibition of [13C5]levulinate catabolism by ethanol was accompanied by marked increases in the M2 enrichment of acetyl-CoA (from 4.2 to 8%, Table 2) and in the M3 enrichment of propionyl-CoA (from 24 to 47%). The most likely explanation of these shifts in metabolite enrichments is that the production of unlabeled acetyl-CoA and propionyl-CoA from endogenous long-chain fatty acids and amino acids was more inhibited by ethanol oxidation than the production of labeled acetyl-CoA and propionyl-CoA from [13C5]levulinate. When ethanol is oxidized, the total acetyl-CoA production by the liver is decreased because the respiratory chain is fueled mostly by reducing equivalents formed in the oxidation of ethanol to acetate (12).
In livers perfused with [13C5]levulinate ± ethanol, the mass isotopomer distribution of lactate and pyruvate (Table 2) is compatible with labeled lactate being formed directly from levulinate rather than from pyruvate. In most cases, pyruvate is the precursor of lactate, except when lactate is generated from the oxidation of 1,2-propanediol (35). In the presence of [13C5]levulinate, the higher M3 enrichment of lactate compared with pyruvate demonstrates a precursor to product relationship where lactate is the precursor of pyruvate. This occurred despite the production of unlabeled pyruvate from glucose and amino acids. The enrichment ratio (M3 lactate)/(M3 pyruvate) increased from 1.6 to 5.1 in the presence of ethanol. This reflects the decreased isotopic equilibration between lactate and pyruvate as a result of the increase in the [NADH]/[NAD+] ratio.
Although the M3 isotopomer of lactate is formed from [13C5]levulinate, M2 and M1 isotopomers of lactate were also detected. This reflects the redistribution and loss of label in the pyruvate → oxaloacetate → PEP → pyruvate cycle with continuous partial isotopic equilibration between lactate and pyruvate. Although some (S)-lactate should be formed from (S)-4-hydroxypentanoyl-CoA via Pathway B (Fig. 1), no (S)-lactate was detected in the perfusate. This results from the abundant formation of (R)-lactate (L-lactate) via glycolysis and other reactions of intermediary metabolism.
Ethanol increased levulinate uptake in isolated livers, but it decreased the disposal of levulinate in vivo (compare Figs. 2 and 3). Because peripheral tissues do not oxidize ethanol, one cannot consider an inhibition of levulinate catabolism by an increase in the [NADH]/[NAD+] ratio. A more likely explanation involves the peripheral oxidation of acetate derived from ethanol oxidation in liver. The group of Hellerstein (36) has documented a major decrease (73%) in whole body lipid oxidation in humans after ethanol ingestion. Because levulinate catabolism yields acetyl-CoA, its catabolism is inhibited by another precursor of acetyl-CoA, i.e. acetate. The latter is used rapidly by peripheral tissues.
Scope and Public Health Relevance
Although calcium levulinate has been administered to humans for more than a century, it is remarkable that the metabolism of levulinate in mammalian cells has not been investigated. One could then reasonably assume that, at the doses used for dietary calcium supplementation or for the occasional intravenous treatment of hypocalcemia, calcium levulinate is probably a safe compound (2). However, some tuberculosis patients treated orally with calcium levulinate (1 to 4 g/day) experienced loss of appetite and nausea (2). Also, in premature babies treated with oral calcium levulinate for hypocalcemia, serious lactic acidosis developed (4). This unexplained metabolic perturbation vanished when calcium levulinate was replaced by calcium gluconate. In other children treated with calcium levulinate, the presence of 4-hydroxypentanoate and levulinate in urine was reported as an incidental analytical finding without firm biochemical explanation (3). Although we recognize that there are differences in the metabolism of xenobiotics between humans and rats, we strongly feel that our data are relevant to human health.
The recent use of 4-hydroxypentanoate as a drug of abuse (more toxic than 4-hydroxybutyrate (11)) led us to hypothesize that levulinate could be used as a pro-drug. This hypothesis was prompted by our finding that 4-hydroxynonanoate (a metabolite of the lipid peroxidation product 4-hydroxynonenal) reversibly interconverts with 4-ketononanoate in perfused rat livers (5). The reduction of levulinate to 4-hydroxypentanoate in liver might be stimulated by ethanol oxidation as a result of the increase in cytosolic and mitochondrial [NADH]/[NAD+] ratios (12). Two other public health concerns resulted from the very high accumulation, in livers perfused with 4-hydroxypentanoate, of 4-phosphopentanoyl-CoA and a number of related acyl-CoAs (5). First, in mice with 4-hydroxybutyrate aciduria resulting from a deficiency in succinic semialdehyde dehydrogenase (37), the concentration of 4-phosphobutyryl-CoA in liver and brain was 40 times greater than in wild type mice (5). The accumulation of 4-phosphobutyryl-CoA may be related to the severe epileptic seizures suffered by these mice, and to the inability to reason in persons intoxicated with 4-hydroxybutyrate. Second, substantial CoA trapping by metabolites of levulinate might interfere with a number of CoA-dependent reactions of intermediary metabolism (13), and might explain the lactic acidosis observed in premature babies treated with calcium levulinate (4).
Our concerns about CoA trapping and the accumulation of 4-phosphoacyl-CoA are supported by the concentration profiles of acyl-CoAs derived from levulinate in livers perfused with 2 mm levulinate ± 20 mm ethanol (Table 1). The first three acyl-CoAs derived from levulinate, i.e. levulinyl-CoA, 4-hydroxypentanoyl-CoA, and 4-phosphopentanoyl-CoA accumulated to high levels. This resulted in large decreases in the concentrations of acetyl-CoA, propionyl-CoA, methylmalonyl-CoA, succinyl-CoA, and malonyl-CoA, compared with controls. The large decrease in acetyl-CoA concentration induced by levulinate is somewhat blunted by the addition of ethanol, presumably because ethanol oxidation generates acetate that is activated to acetyl-CoA. Of particular concern is the very high concentration of 4-phosphopentanoyl-CoA in livers perfused with levulinate + ethanol. After ingestion of calcium levulinate, one can expect a high concentration of levulinate in the portal vein. The stimulation by ethanol of levulinate uptake and conversion to 4-hydroxypentanoate (Fig. 3) may result in the release of high concentrations of 4-hydroxypentanoate into peripheral blood (probably in higher concentrations than after the intravenous administration of levulinate (Fig. 2)). High concentrations of 4-hydroxypentanoate reaching the brain may induce high concentrations of 4-phosphopentanoyl-CoA, which could perturb some brain functions by acting as a neuromodulator. The drug effects of 4-hydroxypentanoate are less potent than those of 4-hydroxybutyrate (11). Addicts may ingest large doses of 4-hydroxypentanoate (or levulinate + ethanol) to produce the desired effects. This is a potentially serious public health concern that calls for an investigation of the effects of the oral ingestion of calcium levulinate + ethanol on brain metabolism and on behavior in live rodents.
Acknowledgments
We thank Dr. Janos Kerner for advice on mitochondrial incubations. We also thank the Case Mouse Metabolic and Phenotyping Center for help with in vivo experiments.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grants R33DK070291 (Roadmap grant) and R01ES013925 (to H. B.) and Grant RO1HL053315 (to G. P. T.).
Mass isotopomers are designated as M, M1, M2, Mn, where n is the number of heavy atoms in the molecule. The isotopic enrichment of each mass isotopomer is expressed as mol %.
REFERENCES
- 1. Tischer R. G., Fellers C. R., Doyle B. J. (1942) J. Am. Pharmacol. Assoc. 31, 217–220 [Google Scholar]
- 2. Gordon B., Kough O. S., Proskouriakoff A. (1933) J. Lab. Clin. Med. 18, 507–511 [Google Scholar]
- 3. Kolvraa S., Gregersen N., Christensen E., Gron I. (1977) Clin. Chim. Acta 77, 197–201 [DOI] [PubMed] [Google Scholar]
- 4. Williams M., Huijmans J. G., Duran M., de Klerk J. B., van Maldegem B. T., Poll-The B. T. (2007) Ned. Tijdschr. Geneeskd. 151, 1191–1196 [PubMed] [Google Scholar]
- 5. Zhang G. F., Kombu R. S., Kasumov T., Han Y., Sadhukhan S., Zhang J., Sayre L. M., Ray D., Gibson K. M., Anderson V. A., Tochtrop G. P., Brunengraber H. (2009) J. Biol. Chem. 284, 33521–33534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadhukhan S., Han Y., Zhang G. F., Brunengraber H., Tochtrop G. P. (2010) J. Am. Chem. Soc. 132, 6309–6311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marinetti L. J. (2003) The Pharmacology of γ-Valerolactone (GVL) as Compared to γ-Hydroxybutyrate (GHB), γ-Butyrolactone (GBL), 1,4-Butanediol (1,4-BD), Ethanol (EtOH), and Baclofen (BAC) in the Rat, Ph.D. thesis, Wayne State University [Google Scholar]
- 8. Marinetti L. J., Isenschmid D. S., Hepler B. R., Kanluen S. (2005) J. Anal. Toxicol. 29, 41–47 [DOI] [PubMed] [Google Scholar]
- 9. Mercer J., Shakleya D., Bell S. (2006) J. Anal. Toxicol. 30, 539–544 [DOI] [PubMed] [Google Scholar]
- 10. Anderson I. B., Kim S. Y., Dyer J. E., Burkhardt C. B., Iknoian J. C., Walsh M. J., Blanc P. D. (2006) Ann. Emerg. Med. 42, 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carter L. P., Chen W., Wu H., Mehta A. K., Hernandez R. J., Ticku M. K., Coop A., Koek W., France C. P. (2005) Drug Alcohol Depend. 78, 91–99 [DOI] [PubMed] [Google Scholar]
- 12. Veech R. L., Felver M. E., Lakshmanan M. R., Huang M. T., Wolf S. (1981) Curr. Top. Cell Regul. 18, 151–179 [DOI] [PubMed] [Google Scholar]
- 13. Mitchell G. A., Gauthier N., Lesimple A., Wang S. P., Mamer O., Qureshi I. (2008) Mol. Genet. Metab. 94, 4–15 [DOI] [PubMed] [Google Scholar]
- 14. Fiehn O., Weckwerth W. (2003) Eur. J. Biochem. 270, 579–588 [DOI] [PubMed] [Google Scholar]
- 15. Nicholson J. K., Connelly J., Lindon J. C., Holmes E. (2002) Nat. Rev. Drug Discov. 1, 153–161 [DOI] [PubMed] [Google Scholar]
- 16. Zhang G. F., Sadukhan S., Tochtrop G., Brunengraber H. (2011) J. Biol. Chem. 286, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Des Rosiers C., Montgomery J. A., Desrochers S., Garneau M., David F., Mamer O. A., Brunengraber H. (1988) Anal. Biochem. 173, 96–105 [DOI] [PubMed] [Google Scholar]
- 18. Joubert C., Beney C., Marsura A., Luu-Duc C. (1995) J. Labelled Compounds Radiopharm. 36, 745–754 [Google Scholar]
- 19. Kasumov T., Martini W. Z., Reszko A. E., Bian F., Pierce B. A., David F., Roe C. R., Brunengraber H. (2002) Anal. Biochem. 305, 90–96 [DOI] [PubMed] [Google Scholar]
- 20. Brunengraber H., Boutry M., Lowenstein J. M. (1973) J. Biol. Chem. 248, 2656–2669 [PubMed] [Google Scholar]
- 21. Struys E. A., Verhoeven N. M., Brunengraber H., Jakobs C. (2004) FEBS Lett. 557, 115–120 [DOI] [PubMed] [Google Scholar]
- 22. Takayasu T., Ohshima T., Tanaka N., Maeda H., Kondo T., Nishigami J., Nagano T. (1995) Forensic Sci. Int. 76, 129–140 [DOI] [PubMed] [Google Scholar]
- 23. Hoppel C., DiMarco J. P., Tandler B. (1979) J. Biol. Chem. 254, 4164–4170 [PubMed] [Google Scholar]
- 24. Fernandez C. A., Des Rosiers C., Previs S. F., David F., Brunengraber H. (1996) J. Mass Spectrom. 31, 255–262 [DOI] [PubMed] [Google Scholar]
- 25. Kashiwagi T., Ji S., Lemasters J. J., Thurman R. G. (1982) Mol. Pharmacol. 21, 438–443 [PubMed] [Google Scholar]
- 26. Deng S., Zhang G. F., Kasumov T., Roe C. R., Brunengraber H. (2009) J. Biol. Chem. 284, 27799–27807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huth W., Dierich C., von Oeynhausen V., Seubert W. (1973) Hoppe Seylers Z. Physiol. Chem. 354, 635–649 [DOI] [PubMed] [Google Scholar]
- 28. Croes K., Van Veldhoven P. P., Mannaerts G. P., Casteels M. (1997) FEBS Lett. 407, 197–200 [DOI] [PubMed] [Google Scholar]
- 29. Koeppe R. E., Mourkides G. A., Hill R. J. (1959) J. Biol. Chem. 234, 2219–2222 [PubMed] [Google Scholar]
- 30. Kaufman E. E., Nelson T. (1987) J. Neurochem. 48, 1935–1941 [DOI] [PubMed] [Google Scholar]
- 31. Kaufman E. E., Nelson T. (1991) Neurochem. Res. 16, 965–974 [DOI] [PubMed] [Google Scholar]
- 32. Kaufman E. E., Nelson T. (1981) J. Biol. Chem. 256, 6890–6894 [PubMed] [Google Scholar]
- 33. Lehninger A. L., Sudduth H. C., Wise J. B. (1960) J. Biol. Chem. 235, 2450–2455 [PubMed] [Google Scholar]
- 34. Preuveneers M. J., Peacock D., Crook E. M., Clark J. B., Brocklehurst K. (1973) Biochem. J. 133, 133–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Powers L., Ciraolo S. T., Agarwal K. C., Kumar A., Bomont C., Soloviev M. V., David F., Desrochers S., Brunengraber H. (1994) Anal. Biochem. 221, 323–328 [DOI] [PubMed] [Google Scholar]
- 36. Siler S. Q., Neese R. A., Hellerstein M. K. (1999) Am. J. Clin. Nutr. 70, 928–936 [DOI] [PubMed] [Google Scholar]
- 37. Gibson K. M., Jakobs C., Pearl P. L., Snead O. C. (2005) IUBMB Life 57, 639–644 [DOI] [PubMed] [Google Scholar]
- 38. Tomcik K., Ibarra R. A., Sadukhan S., Han Y., Tochtrop G. P., Zhang G. F. (2011) Anal. Biochem. 410, 110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]