Abstract
IP3-dependent Ca2+ signaling controls a myriad of cellular processes in higher eukaryotes and similar signaling pathways are evolutionarily conserved in Plasmodium, the intracellular parasite that causes malaria. We have reported that isolated, permeabilized Plasmodium chabaudi, releases Ca2+ upon addition of exogenous IP3. In the present study, we investigated whether the IP3 signaling pathway operates in intact Plasmodium falciparum, the major disease-causing human malaria parasite. P. falciparum-infected red blood cells (RBCs) in the trophozoite stage were simultaneously loaded with the Ca2+ indicator Fluo-4/AM and caged-IP3. Photolytic release of IP3 elicited a transient Ca2+ increase in the cytosol of the intact parasite within the RBC. The intracellular Ca2+ pools of the parasite were selectively discharged, using thapsigargin to deplete endoplasmic reticulum (ER) Ca2+ and the antimalarial chloroquine to deplete Ca2+ from acidocalcisomes. These data show that the ER is the major IP3-sensitive Ca2+ store. Previous work has shown that the human host hormone melatonin regulates P. falciparum cell cycle via a Ca2+-dependent pathway. In the present study, we demonstrate that melatonin increases inositol-polyphosphate production in intact intraerythrocytic parasite. Moreover, the Ca2+ responses to melatonin and uncaging of IP3 were mutually exclusive in infected RBCs. Taken together these data provide evidence that melatonin activates PLC to generate IP3 and open ER-localized IP3-sensitive Ca2+ channels in P. falciparum. This receptor signaling pathway is likely to be involved in the regulation and synchronization of parasite cell cycle progression.
Keywords: Calcium Imaging, Calcium Intracellular Release, Inositol Phosphates, Parasitology, Phospholipase C, Malaria, Melatonin, Plasmodium
Introduction
Malaria, caused by the obligate Plasmodium parasite, infects over 300 million people annually and resistance to current antimalarial drugs is an increasing problem (1–5). The intraerythrocytic phase of Plasmodium falciparum, the most lethal human malaria parasite, is the primary cause of malaria morbidity and mortality. Therefore, arrest of the red blood cell (RBC)4 stage of Plasmodium life cycle is a clear pharmaceutical target. The RBC cycle of P. falciparum occurs over a period of 48 h (the life cycles of other Plasmodium species are also multiples of 24 h) and consists of three stages of parasite development known as ring, trophozoite, and schizont. Proliferation occurs by lysis of the RBC to release merozoites, which are the product of the end of shizogony. This is followed by rapid reinvasion of uninfected RBCs to complete the cycle (6–9). The ability to overcome host defenses relies upon the synchrony of merozoite release into the blood stream, usually at a specific time of day (10, 11). Therefore, key to P. falciparum survival is synchronous maturation within the RBC. Clear evidence supports a role of host circadian rhythm in this process, mediated by melatonin and/or related host hormones (12–15).
Parasites like most eukaryotes, utilize second messenger signaling cascades involving Ca2+ and cAMP to coordinate cell function (6, 14, 16–20). The Ca2+ signaling toolkit in vertebrates is now well characterized (21, 22) and genetic (18, 23, 24) and pharmacological studies (14, 25) are increasing our knowledge of the signaling proteins that are evolutionarily conserved from Apicomplexa (the Plasmodium phylum). To date, key components of the classical Ca2+ release cascade have been described in Apicomplexeans; including sequences of four putative heptahelical receptors (26), G-proteins, implied by the sensitivity of gametogenesis to cholera and pertusis toxins (27) and sequences of PLCδ-like isoenzymes (23, 28). Furthermore, Ca2+ pumps such as SERCA and a plethora of Ca2+-regulated proteins have been identified (18, 29–33). A clear indication of the importance of Ca2+ homeostasis and Ca2+ regulated signaling events in these organisms. However, a canonical IP3 receptor transcript has yet to be identified in the genome of any Apicomplexean. Nevertheless, pharmacological data clearly demonstrate P. falciparum and the rodent malaria parasite P. chabaudi maintain intracellular Ca2+ stores (14, 16, 34) and IP3-dependent Ca2+ release has been demonstrated in isolated, permeabilized P. chabaudi (35). Importantly, evidence for the generation of the precursor of IP3-dependent signaling, PIP2, has also been shown in P. knowlesi and P. falciparum (36, 37). To date, in Apicomplexeans a PLC-like enzyme has been cloned only from Toxoplasma gondii and interestingly the activity of this enzyme was greater with phosphatidylinositol rather than PIP2 as a substrate (28). Nevertheless, IP3 and DAG increases have been reported during P. falciparum gametocyte exflagellation involved in the sexual cycle and transmission to the mosquito vector (38) and Elabbadi et al., (36) reported ionomycin-induced elevations in IP3 in the asexual RBC stage of the life cycle, indicating an enzyme capable of PIP2 hydrolysis is present in P. falciparum.
It is now well established that the host hormone melatonin (12), and its precursors N-acetylserotonin, tryptamine, serotonin, and N(1)-acetyl-N(2)-formyl-5-methoxykynuramine (AFMK) affect the intraerythrocytic P. falciparum cell cycle (13, 14, 39). These molecules were able to induce Ca2+ release from cultured P. falciparum and P. chabaudi and importantly these responses were blocked by PLC inhibition and melatonin receptor antagonism (14). Similarly, the ability of melatonin and other tryptophan derivatives to synchronize P. falciparum cultures were also blocked by inhibition of PLC and melatonin receptors (13, 14, 40). Whereas, in the intraerythrocytic stages of P. berghei and P. yoelii, two rodent parasites that show asynchronous development (not linked to circadian rhythm) in vivo, melatonin does not modulate their cell cycle or elicit an elevation in intracellular Ca2+ (41).
There is clear evidence that P. falciparum and other plasmodium obligate parasites contain the molecular machinery for IP3-dependent Ca2+ release (14, 35, 38). In the present study, we demonstrate unequivocally that intact P. falciparum, within their natural erythrocyte host cell, release Ca2+ in response to IP3. Furthermore, we provide clear evidence that melatonin acts in P. falciparum to activate PLC and induce concurrent elevations in IP3. This key process in P. falciparum survival depends on IP3 receptor function during the trophozoite stage of the intraerythrocytic life cycle. Considering the likely vast genetic divergence between mammalian and plasmodium IP3 receptors, this protein is a strong candidate for novel therapeutic intervention.
EXPERIMENTAL PROCEDURES
P. falciparum Culture
P. falciparum (D37) parasites were maintained in culture as described (42). Briefly, P. falciparum were cultured in RPMI media supplemented with 50 mg/liter hypoxanthine; 40 mg/liter gentamycin; 435 mg/liter NaHCO3; 5% A+ or O human red blood cells and 10% A+ or O human blood serum in an atmosphere of 5% CO2; 3% O2; 92% N2 at 37 °C. Media was changed every 24 h and RBCs replaced every 48 h. Parasitemia and the development stage of synchronized cultures were determined by Giemsa-stained smears.
Photorelease of Caged-IP3 and Ca2+ Imaging
P. falciparum infected erythrocytes were washed in HEPES-buffered saline solution (HBSS) (in mm: 25 HEPES, 121 NaCl, 5 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.0 CaCl2, 10 glucose, 0.04 probenecid, and 0.25% (w/v) fatty acid-free BSA, pH 7.4) and co-loaded, in suspension, with caged-IP3 (2 μm; siChem) and Fluo4-AM (5 μm; Invitrogen; 37 °C) for 45 min. Cells were washed with HBSS and seeded onto borosilicate glass coverslips coated with ploy-l-lysine and incubated for 15 min at room temperature to enable cell adherence. Cells were washed and mounted on the stage of an Axiovert2000 (Zeiss) spinning disc confocal microscope. Fluo4-AM fluorescence images (Argon laser excitation 488 nm, emission >510 nm) were acquired at 2 Hz with a cooled charge-coupled device (CCD) camera using the data acquisition software Piper ControlTM (Stanfordphotonics). Photo release of caged-IP3 was achieved by light pulses (1ns duration with a wavelength of 337 nm and 1.45 mJ of energy) from a nitrogen charged UV flash lamp (Photon Technology International) guided through the objective (C-Achromatx40/1.2). Data analysis was performed using ImageJ (NIH).
Measurement of [H3]Inositol Polyphosphates
The parasites were synchronized with sorbitol treatment (43) and cultured to a parasitemia of >5%. At late trophozoite stage the culture was resuspended in RPMI medium complemented with 5% human serum containing 2.5 μCi/ml [H3-myo]inositol (Perkin Elmer). Labeling continued until young trophozoite phase and >10% parasitemia. The infected erythrocyte culture was then washed in HBSS and preincubated in for 20 min with 10 mm LiCl2 to block inositol monophosphate hydrolysis prior to addition of melatonin or other test agents. The incubation was terminated by addition of ice-cold tricholoroacetic acid, the water soluble [H3]inositol containing components were then extracted by addition of tri-n-octylamine:1,1,2-trichlorofluoroethane (1:1 ratio) and [H3]inositol phosphates were separated by ion exchange chromatography (44) using Dowex resin in the formate form. Lower order inositols and glycerophospholipids were removed by elution with 40 ml 0.4 m ammonium formate/0.1 m formic acid. IP3 and higher order inositols were then eluted with 10 ml of 1.2 m ammonium formate/0.1 m formic acid. Ultima-Flo (Perkin Elmer) was added to the eluate and DPM determined using liquid scintillation counting. Data are expressed as a fold increase over non-infected red blood cell controls loaded with 2.5 μCi/ml [H3-myo]inositol in parallel.
RESULTS
Photorelease of Caged IP3 Induces Ca2+ Mobilization in Intact P. falciparum
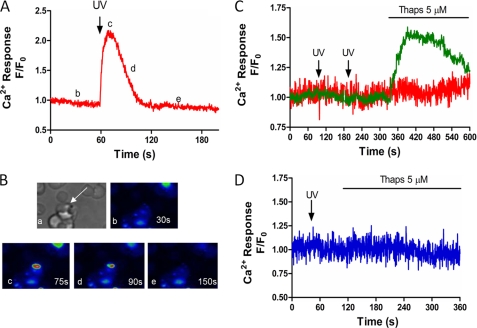
In this study, IP3-dependent Ca2+ release has been examined in intact P. falciparum within the host erythrocyte using flash photolysis of cell permeant caged-IP3. The development of this cell permeant form of caged-IP3 (45) provides a sophisticated tool to manipulate cytosolic IP3 levels under physiological conditions, and is particularly well suited to the intraerythrocytic malaria parasite because this is intractable to other methods to modify cytosolic IP3 levels. Infected erythrocytes were co-loaded with both the cell permeant caged-IP3 and Fluo4-AM for 45 min, a period sufficient to enable de-esterification of these molecules in mammalian systems (46). UV flash photolysis of caged-IP3 under these conditions elicited a rapid and transient increase in intracellular Ca2+ in RBCs infected with P. falciparum (Fig. 1A, representative trace of 81 cells from 15 independent experiments and Fig. 1B confocal images with Ca2+ changes shown in pseudocolor). A number of controls were performed to confirm that photorelease of caged IP3 is acting specifically on receptors in P. falciparum within RBCs. Firstly, we assessed the effect of IP3 photolysis on uninfected erythrocytes. Our data demonstrate RBCs are insensitive to UV laser pulses in both the absence Fig. 1C and presence Fig. 1D of caged-IP3. This result was not unexpected as mammalian RBCs lack endoplasmic reticulum. However, these results confirm that these cells are devoid of any IP3 sensitive Ca2+ store and, therefore, do not contribute to the Ca2+ response shown in Fig. 1, A and B. Importantly, we also demonstrate UV laser excitation is without effect on P. falciparum-infected RBC in the absence of caged-IP3 (Fig. 1C). These data also confirm that our protocol to photolyze the chemical cage on IP3 does not result in a Ca2+ response mediated by any cytotoxic effect of UV excitation in infected erythrocytes.
FIGURE 1.
Flash photolysis of caged-IP3 induces calcium release in P. falciparum-infected RBC. A and B, P. falciparum-infected RBCs were loaded in HBSS with Fluo4-AM (5 μm) and caged-IP3 (2 μm) for 45 min, then allowed to adhere to poly-l-lysine-coated coverslips. Changes in intracellular Ca2+ were monitored at 2 Hz using a spinning disc confocal microscope coupled to a CCD camera. Flash photolysis of caged-IP3 was achieved with a nitrogen-charged UV laser. A, representative trace of UV-induced Ca2+ increase in intact P. falciparum (UV flash indicated by arrow at 60 s). B, confocal images of the cell in Panel A to show: (a) transmitted light image depicting P. falciparum within RBC (arrow); (b–e) changes in Ca2+ are shown in pseudocolor (blue lowest and red highest [Ca2+]) at (b) baseline (t = 30 s) (c) peak Ca2+ transient (t = 75 s), (d) half-peak height (t = 90 s), and (e) return to baseline (t = 150 s). Data are representative of 81 cells from 15 experiments. C, representative traces of infected (green) and uninfected (red) RBC loaded with Fluo4-AM in the absence of caged-IP3 (UV flashes at 75 and 180 s). D, representative trace of uninfected RBC in the presence of caged-IP3 (UV flash at 40 s). Thapsigargin (5 μm, Thaps) was added as indicated.
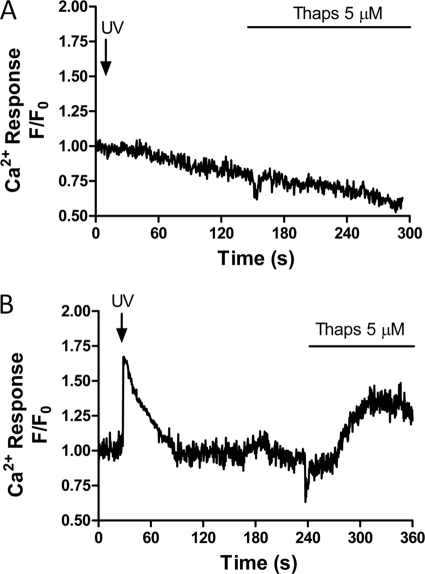
All experiments were performed in the presence of 40 μm probenecid, a nonspecific inhibitor of organic anion transport (47, 48) to block cellular loss and compartmentalization of the Ca2+ indicator. Previous studies have shown that Ca2+ indicator dyes accumulate in the parasite acidic food vacuole (49, 50), complicating measurements of cytosolic Ca2+ in the intraerythrocytic parasite. Consistent with this, in the absence of probenecid we observed no Ca2+ response to photolysis of caged-IP3 and little or no response to SERCA blockade with thapsigargin in P. falciparum-infected RBCs (Fig. 2A) compared with responses in the presence of probenecide (Fig. 2B). Thus in P. falciparum in the absence of probenecid, anion transporters appear to allow Fluo-4AM accumulation in intracellular compartments.
FIGURE 2.
Anion transport inhibition is required to detect changes in parasite cytosolic Ca2+. P. falciparum-infected RBCs were loaded with Fluo4-AM and caged-IP3 in the presence or absence of the anion transport inhibitor probenecid (40 μm) prior to activation with caged-IP3 and thapsigargin (5 μm). Shown are representative traces of changes in intracellular Ca2+ in the absence (A; representative of 36 cells from 11 independent experiments) and presence of probenecid (B; 17 cells from three experiments), respectively.
Investigation of IP3-sensitive Stores in P. falciparum
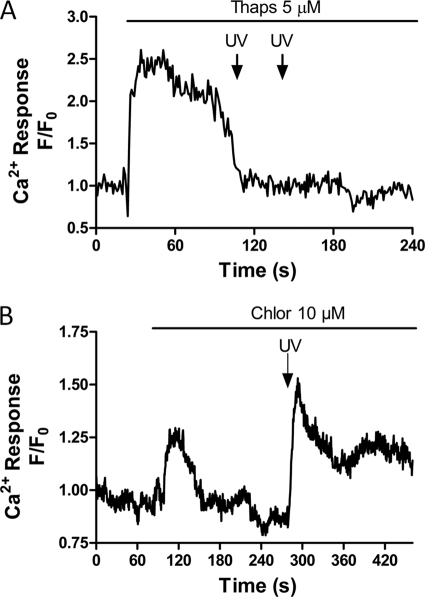
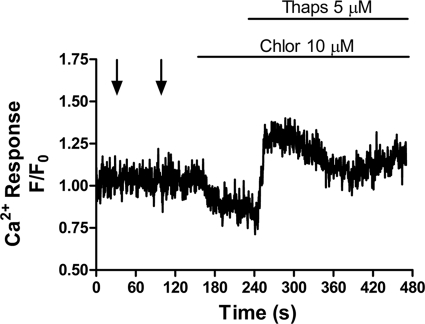
Previous studies in isolated permeabilized malaria parasites have revealed the presence of both ER and acidic vacuole Ca2+ stores within P. chabaudi and P. falciparum (16, 35). To establish the sensitivity of these organelles to IP3 in intact P. falciparum, the ability of caged-IP3 to elicit Ca2+ release after depletion of each compartment was characterized in intraerythrocytic parasites. Depletion of the acidic pool with chloroquine (10 μm) did not affect the ability of the P. falciparum to respond to photolysis of IP3 (Fig. 3B, representative trace of 11 cells from three independent experiments). However, depletion of ER Ca2+ with thapsigargin (5 μm) abolished IP3-mediated Ca2+ release (Fig. 3A, representative trace of 12 cells from three experiments). These data suggest that in P. falciparum the ER is the major IP3-sensitive Ca2+ store.
FIGURE 3.
IP3-dependent Ca2+ release arises from the thapsigargin-sensitive store. Endoplasmic reticulum and acidic compartment Ca2+ stores were discharged independently with thapsigargin (5 μm) or chloroquine (10 μm, Chlor) before photolysis of caged-IP3. A, representative trace (12 cells from three experiments) to show thapsigargin depletes all IP3-sensitive Ca2+ stores in P. falciparum and B, representative trace (11 cells from three experiments) to show chloroquine releases Ca2+ but does not deplete the IP3-sensitive Ca2+ store.
Melatonin Activates PLC to Increase Inositol Polyphosphates in Intact P. falciparum
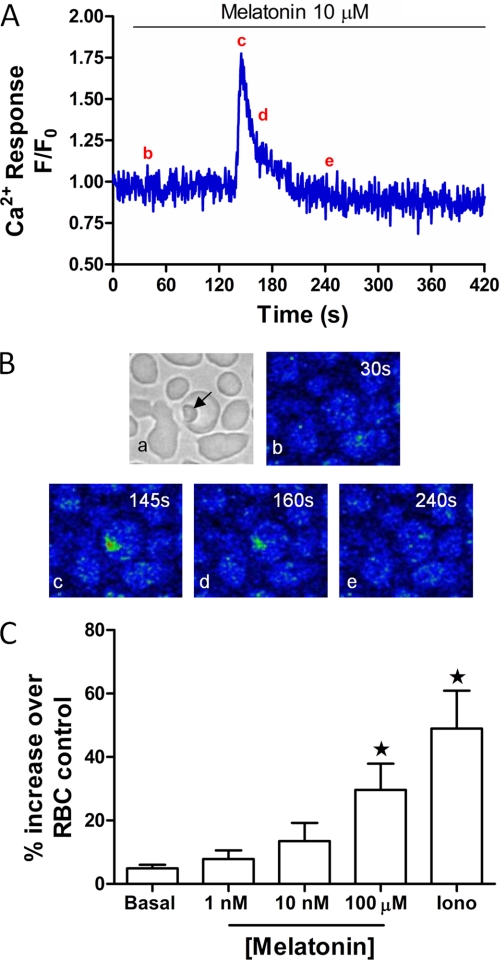
We have previously demonstrated that the host hormone melatonin, and its metabolites, elicit Ca2+ increases in intact P. chabaudi (12)- and P. falciparum (13)-infected RBCs. Fig. 4, A and B show the melatonin-induced Ca2+ signals with high temporal and spatial resolution. To test the hypothesis that hormone-induced Ca2+ release proceeds via a canonical PLC/IP3 receptor pathway in Plasmodia, we examined the effect of melatonin on polyphosphoinositide levels. Sorbitol treatment was used to synchronize the cultures so that all of the parasites were at the same stage (43), and a parasitemia of >5% infected RBCs was obtained prior to [3H-myo]inositol labeling. It has been reported that de novo synthesis of polyphosphoinositides (the lipid precursors for IP3) is greatest during mature parasite development (trophozoite and schizont) and high during invasion and early ring stages of the RBC lifecycle (36, 51). Therefore, in our experiments [3H-myo]inositol loading commenced at the late trophozoite stage and the parasites were allowed to go through one RBC invasion cycle to ensure maximum incorporation into the lipid pool. We have found that the Ca2+ responses to melatonin occur predominantly at the trophozoite stage. Therefore, incubations with melatonin were performed after 36 h incubation with [3H-myo]inositol at the early trophozoite phase, which was confirmed with Giemsa-stained smears. At this point, the parasitemia was typically about 10%. Mature erythrocytes, turnover membrane lipids slowly and are devoid of the machinery for de novo inositol lipid synthesis, and thus incorporation of [3H-myo]inositol into the RBC lipids is minimal in comparison to P. falciparum (37). Consistent with the work of Elabbadi et al., (36), ionomycin (2 μm) was capable of eliciting a robust increase in inositol polyphosphates of 49 ± 20% compared with a basal level of 5 ± 2% in untreated infected RBCs (Fig. 4C) (data from three independent experiments performed in triplicate and expressed as percentage increase over non-infected RBC control). In the same series of experiments, melatonin (100 μm) elicited an increase of 30 ± 14% in inositol polyphosphate generation (Fig. 4C). Increases of inositol polyphosphate formation at lower levels of melatonin followed the same trend, but did not rise to significance when compared with the control cells without added melatonin (basal).
FIGURE 4.
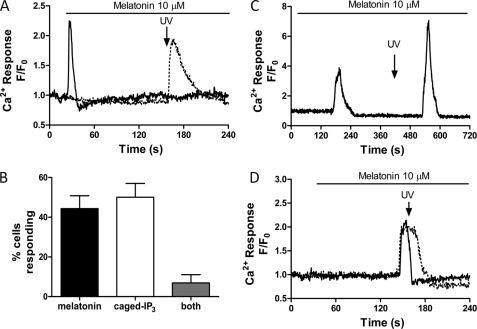
Melatonin-induced inositol polyphosphate production in P. falciparum cultures. A, representative trace of melatonin (10 μm) -induced Ca2+ increase in infected RBC. B, confocal images of Ca2+ changes in the cell shown in panel A (representative of 12 cells from three independent experiments); B shows confocal images to show: (a) transmitted light image depicting P. falciparum within RBC (arrow); (b–e) changes in Ca2+ shown in pseudocolor (blue lowest and red highest [Ca2+]) at (b) baseline (t = 30 s) (c) peak Ca2+ transient (t = 145 s), (d) half-peak height (t = 160 s) and (e) return to baseline (t = 240 s). C, melatonin-induced increases in inositol polyphosphate formation were measured in infected RBC cultures as described under “Experimental Procedures.” Prior to melatonin stimulation (1 nm, 10 nm, 100 μm; 20 min) cells were washed three times with HBSS then incubated for 20 min with LiCl2 (10 μm). Data are presented as mean ± S.E. from three independent experiments performed in triplicate (*, p < 0.05) compared with background [H3]inositol levels in non-infected RBC loaded in parallel.
Melatonin Pretreatment Prevents IP3-induced Ca2+ Release in Intact P. falciparum
To establish whether melatonin and caged-IP3 release Ca2+ from the same intracellular store, we assessed the ability of P. falciparum to respond to photolysis of caged-IP3 after challenging the cells with a maximal dose of melatonin (10 μm). In any given microscope field, a melatonin-induced Ca2+ increase was observed in approximately half of the infected erythrocytes (44 ± 14% in five independent experiments, 54 total cells examined), and almost all of those cells that did not respond to melatonin released Ca2+ upon photolysis of IP3 (50 ± 15%). Fig. 5A, shows representative traces of melatonin and IP3-sensitive P. falciparum from the same coverslip. Interestingly, very few cells were capably of eliciting sequential Ca2+ transients to both melatonin (10 μm) and photolysis of caged-IP3. Only 2 out of the 54 cells (7 ± 4%) generated Ca2+ transients to both stimuli (Fig. 4B). These data showing apparent overlap of the melatonin- and IP3-sensitive Ca2+ intracellular stores are summarized in Fig. 5C. Furthermore, photorelease of caged-IP3 during a melatonin-dependent rise in intracellular Ca2+ did not potentiate the Ca2+ response (Fig. 5D; representative of 4 cells from three independent experiments). Thus, these data provide clear evidence that melatonin releases Ca2+ from the ER IP3-sensitive Ca2+ store in P. falciparum.
FIGURE 5.
Ins1,4,5P3-induced Ca2+ increases are abolished after melatonin stimulation. Infected RBCs co-loaded with Fluo4-AM and caged-IP3 were challenged with melatonin (10 μm) prior to flash photolysis of caged-IP3. A, representative trace of cells from the same coverslip responding to melatonin (solid line) or IP3 uncaging (dotted line). Similar results were obtained in 5 separate experiments, with 54 total cells analyzed. B, representative trace of cells (2 out of 54) which released Ca2+ to both stimuli. C, percentage of cells responding to melatonin (10 μm), photolysis of caged-IP3 or both (data are the mean ± S.E. from five experiments). D, representative traces of cells in which flash photolysis of caged-IP3 was performed during the melatonin-induced Ca2+ transient, showing no further Ca2+ release with the uncaging of IP3 (representative of 4 cells from three independent experiments).
P. falciparum Are Insensitive to IP3 at the Schizont Stage of the Intraerythrocytic Cell Cycle
The above data all describe responses in P. falciparum at the trophozoite stage of the erythrocyte life cycle. Since we postulate that the IP3-dependent signaling cascade plays a vital role in the cell cycle progression, UV photolysis of caged-IP3 was investigated at the schizont stage. Interestingly the ability of IP3 to mobilize Ca2+ in RBC infected by P. falciparum was not observed during the schizont stage (Fig. 5A, representative trace of 12 cells from 4 experiments). Importantly, Ca2+ release was observed upon the addition of thapsigargin confirming the integrity of the intracellular Ca2+ store under these conditions. Moreover, we have shown previously that Ca2+ in the parasitophorous vacuole is necessary for the maintenance of Ca2+ stores in the intraerythrocytic parasite (48) (and see “Discussion”), so these data suggest that mature schizonts rather than merozoites were being stimulated. Similarly, melatonin was not able to induce elevations in cytosolic Ca2+ levels when applied to parasites in the schizont phase (data not shown) or ring stage (13). These findings provide evidence for differential sensitivity to IP3 and melatonin as P. falciparum parasites pass through the different intraerythrocytic developmental stages.
DISCUSSION
Recent studies have begun to identify components of intracellular signaling cascades in Plasmodium. However it remains unclear when and how these signaling molecules act to trigger Plasmodium maturation, division, differentiation, and reinvasion during the asexual stage that takes places within red blood cells (30). As discussed, in the Introduction, there is substantial evidence that Plasmodia and in particular, P. falciparum possess the molecular machinery for IP3-dependent signaling (12, 14, 16, 18, 48). Indeed, this pathway is integral to the maturation and survival within the host of this obligate parasite. In the present study, we utilized cell permeant caged-IP3 to demonstrate unequivocally that IP3-induces Ca2+ release from intracellular stores within intact P. falciparum. Moreover, our experiments were carried out with parasites developing inside the host red blood cell, demonstrating that Ca2+ mobilization in response to IP3 occurs in the normal physiological environment. It should also be noted that native (uninfected) red blood cells do not have intracellular Ca2+ stores, and consistent with this uncaging of IP3 did not elicit any change in cytosolic Ca2+ in these host cells.
One potential question is how does the malaria parasite maintain intracellular Ca2+ stores for signaling while it is sequestered within the RBC cytoplasm? The parasitophorous vacuole is formed by invagination of the RBC plasma membrane during parasite invasion, and is believed to include a number of ion pumps that would serve to transport ions, including Ca2+, from the host erythrocyte cytoplasm into the lumen of the vacuole. The vacuole may also communicate directly with the extracellular medium surrounding the RBC through a parasitophorous duct that is permeable to small molecules and ions (52). Thus, the parasitophorous vacuole plays a key role in providing a relatively Ca2+-rich environment to the intraerythrocytic parasite for use in Ca2+ signaling. In a previous study (48), we have measured the Ca2+ concentration in the vacuole using Ca2+ indicator dyes sequestered into this compartment during merozoite invasion of the RBC. The measured free Ca2+ concentration in the vacuole was ∼40 μm, which is low relative to plasma free Ca2+, but is apparently sufficient to sustain the filling of intracellular Ca2+ stores within the parasite and hence maintain cytosolic Ca2+ signaling in the intraerythrocytic Plasmodia (48). Experiments with isolated parasites have shown a transient cytosolic Ca2+ response in the absence of external Ca2+ that has a second phase of increase following Ca2+ readdition, suggesting a potential role of capacitive calcium entry (14).
Pharmacological effectors of the known intracellular Ca2+ stores in malaria parasites were used to investigate the source of Ca2+ mobilized by IP3. Thapsigargin was used to inhibit SERCA and release Ca2+ from the ER, and chloroquine was used to collapse the pH gradient and release Ca2+ from the acidic pool. These experiments demonstrate that the ER is the major IP3-sensitive Ca2+ store in P. falciparum, since we show IP3-dependent Ca2+ release was abolished after SERCA inhibition, but was unaffected by chloroquine. We have previously reported that thapsigargin did not fully block the Ca2+ release by exogenous IP3 addition (5 μm) to permeabilized P. chabaudi parasites (35). This residual increment of IP3-induced Ca2+ release in the permeabilized parasites was apparently derived from the chloroquine-sensitive Ca2+ pool. This discrepancy between the present and previous studies may reflect a difference between P. chabaudi (rodent malaria) and P. falciparum (human malaria) in terms of IP3 receptor location and/or density. However, it should be noted that this present study assesses the sensitivity of the acid compartments to IP3 under much more physiological conditions, because the cell-permeant caged-IP3 does not require isolation and permeabilization of the parasites prior to IP3 addition as used in previous investigations. Moreover, flash photolysis releases only a fraction of the 2 μm caged-IP3 included in the loading buffer, as indicated by the ability of cells to respond to more than one round of IP3 uncaging and the need to use multiple pulses to reach the threshold for Ca2+ release in some experiments. These data also provide evidence that the IP3 levels generated by a single photolysis pulse were not saturating for the P. falciparum IP3-receptor Ca2+ channel. Therefore, the fact that Ca2+ release elicited by IP3 uncaging occurred as an all-or-nothing response (amplitude and kinetics), suggests there may be positive feedback on the Ca2+ release channel(s) as observed in mammalian IP3-receptors (21, 22). When our intact red blood cell parasite cultures were incubated with higher concentrations of caged-IP3 (3–5 μm) UV flash photolysis often resulted in irreversible elevations in Ca2+ and consequently cell death (data not shown). This observation suggests the Plasmodia IP3 receptors, unlike their mammalian analogues (21), may not be sensitive to Ca2+-dependent inhibition.
The present study provides the first direct evidence that the host hormone melatonin elicits a rise in intracellular IP3 levels in the malaria parasite. Previous studies in which P. falciparum was labeled with [3H-myo]inositol have shown that the Ca2+ ionophore, ionomycin is capable of increasing inositol phosphate levels (presumably by Ca2+-dependent activation of PLC) (36). Our data clearly demonstrate a receptor coupled event leading to increased inositol polyphosphate levels and strongly support the role of a G-protein and PLC dependent signaling cascade in this organism. In mammalian systems it has been possible to separate individual inositol phosphate isomers (53), however because the number of infected RBCs is low (∼10%), and the parasites occupy only a small fraction of the RBC volume, there was not sufficient signal to measure individual inositol phosphate isomers in our experiments. Instead, the anion exchange column method was used to elute total IP3 and IP4 isomers together (IP3+IP4) in the presence of LiCl to inhibit inositol phosphate breakdown (36, 53, 54). However, as we assume IP4 is derived from the generation of IP3 this is the first report of hormone-induced IP3 generation in P. falciparum.
As mentioned in the “Results,” increases in inositol polyphosphates by melatonin concentrations below 100 μm were not significant. Melatonin in the lower range is capable of exerting effects on life cycle progression when included in RBC malaria parasite cultures (13, 40). However, these cell cycle progression effects of melatonin occur on a much slower timescale than the 20-min incubations in the present experiments, and the associated Ca2+ increases are also slower and of lower amplitude (40). Melatonin is relatively hydrophobic (Log p = 1.6) and is expected to cross the erythrocyte and parasitophorous membranes. However, we cannot be sure of the actual concentration of melatonin perceived by the parasite after only 20 min of exposure. Thus higher concentrations may be necessary to elicit maximal amplitude and immediate responses at the level of inositol phosphate generation and Ca2+ mobilization.
Previous studies from our laboratory have shown that the effects of melatonin on parasite Ca2+release and synchronized progression through the cell cycle are blocked by the PLC inhibitor U73122 (13, 14, 40). Importantly, the activation of PLC by melatonin in P. falciparum is corroborated in this study without the use of pharmacological inhibitors and the potential nonspecific effects of these compounds. In humans, melatonin receptors MT1 and MT2 couple predominately to Gαi (55) and thus mediate their cellular effects via inhibition of adenylate cyclase and PKA. However, melatonin receptors can also couple to G-proteins that lead to PLC activation and IP3 generation (56–58), including in Xenopus melanocytes (59) and unicellular eukaryotic dinoflagellates (60). In common with the IP3 receptor, the lack of an identified melatonin receptor in the Plasmodium genome database suggests that the molecular identity of the P. falciparum melatonin receptor protein is far removed from the human host. Indeed, we have demonstrated that cAMP levels and PKA activity are actually increased by melatonin in P. falciparum, and this plays an important role in parasite synchronization (17). This melatonin-induced increase in cAMP is a secondary consequence of the activation of PLC and associated Ca2+ mobilization. Further evidence for the presence of a P. falciparum melatonin receptor comes from the finding that the antagonist luzindole (61) inhibits melatonin-induced Ca2+ release and the synchronization of cell cycle progression in the parasite (12, 13). Interestingly, this antagonist shows more than 10-fold greater selectivity for MT2 over MT1 melatonin receptors, and it is also an effective antagonist of the Xenopus melatonin receptor (62).
In contrast to our observations of Ca2+ mobilization by IP3 uncaging and melatonin addition at the trophozoite stage of P. falciparum, we did not observe any Ca2+ response to either agent in intraerythrocytic schizonts. This raises the interesting possibility that PLC-dependent signaling is regulated by stage specific expression of components of the intracellular Ca2+ signaling pathway. This is of particular relevance when considering the multitude of Ca2+-dependent kinases and binding proteins operative at different stages of both sexual and asexual development (30–32, 63, 64). It was recently reported that parasite egress from erythrocytes depends on the calcium-dependent protein kinase PfCDPK5 (33) a process that occurs during late schizgony. Moreover, another calcium-dependent kinase, PfPKB, is believed to be involved in the reinvasion of erythrocytes by the released merozoites (31, 32). It has also been reported that cytosolic Ca2+ increases in free merozoites in response to the change in K+ ion concentration when they are released from the red blood cell (9). This Ca2+ increase and the PfPKB activation are both blocked by the PLC inhibitor U73122 (9, 31, 32), implying that PLC/IP3-dependent Ca2+ signaling may also be active during the late schizont and merozoite stage of the P. falciparum lifecycle, albeit activated by different extracellular signals. In our experiments we were not able to measure Ca2+ signals in intraerythrocytic segmented (late phase) schizonts (Fig. 6) and free merozoites were not observed.
FIGURE 6.
IP3 receptor function is lost at schizony in P. falciparum. P. falciparum cells in the schizont phase were insensitive to photolysis of caged-IP3. A representative trace of a schizont stimulated with two sequential pulses of UV (arrows), followed by chloroquine (10 μm), and thapsigargin (5 μm) (trace representative of 12 cells from four independent experiments). Thapsigargin-dependent release confirms that the ER Ca2+ store is intact but insensitive to changes in IP3 and chloroquine.
In the present study, we provide clear and direct evidence that a classical PLC-dependent intracellular Ca2+ release pathway exists in P. falciparum. This Ca2+ signaling pathway is activated by melatonin, which provides a mechanism for coordination of parasite development and release by the human host hormone associated with circadian rhythm. Periodic fever due to synchronized parasite release is characteristic of human malaria, and may provide a mechanism for the parasite to overwhelm the immune system during release and reinvasion of new erythrocytes. Once inside the RBC, the parasite is protected from immunological recognition. Thus, blockade of the Plasmodium melatonin signaling pathway has pharmaceutical potential in preventing the synchronization of the parasite within the host.
Acknowledgments
We thank Dr. Fiddok and C. Brownback (Columbia University, Departments of Microbiology and Medicine, Hammer Health Sciences Center, New York) and Dr. Mohandas (Lindsey F. Kimball Research Institute, NY Blood Center) for kindly providing us with cultured P. falciparum.
This work was supported, in whole or in part, by the Thomas P. Infusino Endowment (to A. P. T.), National Institutes of Health Grant DK082954, Fapesp (07/52924-0), Malaria Pronex, and INCT-INBqMed.
- RBC
- red blood cell
- IP3
- Ins(1,4,5)P3
- ER
- endoplasmic reticulum
- SERCA
- sarco/endoplasmic reticulum Ca2+ ATPase
- Chlor
- chloroquine
- Thaps
- thapsigargin.
REFERENCES
- 1. Breman J. G. (2009) Sci. Prog. 92, 1–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skinner-Adams T. S., Stack C. M., Trenholme K. R., Brown C. L., Grembecka J., Lowther J., Mucha A., Drag M., Kafarski P., McGowan S., Whisstock J. C., Gardiner D. L., Dalton J. P. (2010) Trends Biochem. Sci. 35, 53–61 [DOI] [PubMed] [Google Scholar]
- 3. Ekland E. H., Fidock D. A. (2007) Curr. Opin. Microbiol. 10, 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kappe S. H., Vaughan A. M., Boddey J. A., Cowman A. F. (2010) Science 328, 862–866 [DOI] [PubMed] [Google Scholar]
- 5. Kooij T. W., Janse C. J., Waters A. P. (2006) Nat. Rev. Microbiology 4, 344–357 [DOI] [PubMed] [Google Scholar]
- 6. Doerig C., Baker D., Billker O., Blackman M. J., Chitnis C., Dhar Kumar S., Heussler V., Holder A. A., Kocken C., Krishna S., Langsley G., Lasonder E., Menard R., Meissner M., Pradel G., Ranford-Cartwright L., Sharma A., Sharma P., Tardieux T., Tatu U., Alano P. (2009) Parasite 16, 169–182 [DOI] [PubMed] [Google Scholar]
- 7. Haldar K., Mohandas N. (2009) Hematology Am. Soc. Hematol. Educ. Program 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia C. R., de Azevedo M. F., Wunderlich G., Budu A., Young J. A., Bannister L. (2008) Int. Rev. Cell Mol. Biol. 266, 85–156 [DOI] [PubMed] [Google Scholar]
- 9. Singh S., Alam M. M., Pal-Bhowmick I., Brzostowski J. A., Chitnis C. E. (2010) PLoS. Pathog. 6, e1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia C. R., Markus R. P., Madeira L. (2001) J Biol. Rhythms. 16, 436–443 [DOI] [PubMed] [Google Scholar]
- 11. Bannister L., Mitchell G. (2003) Trends Parasitol. 19, 209–213 [DOI] [PubMed] [Google Scholar]
- 12. Hotta C. T., Gazarini M. L., Beraldo F. H., Varotti F. P., Lopes C., Markus R. P., Pozzan T., Garcia C. R. (2000) Nat. Cell Biol. 2, 466–468 [DOI] [PubMed] [Google Scholar]
- 13. Beraldo F. H., Garcia C. R. (2005) J. Pineal Res. 39, 224–230 [DOI] [PubMed] [Google Scholar]
- 14. Beraldo F. H., Mikoshiba K., Garcia C. R. (2007) J. Pineal Res. 43, 360–364 [DOI] [PubMed] [Google Scholar]
- 15. Srinivasan V., Spence D. W., Moscovitch A., Pandi-Perumal S. R., Trakht I., Brown G. M., Cardinali D. P. (2010) J. Pineal Res. 48, 1–8 [DOI] [PubMed] [Google Scholar]
- 16. Varotti F. P., Beraldo F. H., Gazarini M. L., Garcia C. R. (2003) Cell Calcium 33, 137–144 [DOI] [PubMed] [Google Scholar]
- 17. Beraldo F. H., Almeida F. M., da Silva A. M., Garcia C. R. (2005) J. Cell Biol. 170, 551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagamune K., Sibley L. D. (2006) Mol. Biol. Evol. 23, 1613–1627 [DOI] [PubMed] [Google Scholar]
- 19. Krishna S., Squire-Pollard L. (1990) Parasitol. Today 6, 196–198 [DOI] [PubMed] [Google Scholar]
- 20. Docampo R., de Souza W., Miranda K., Rohloff P., Moreno S. N. J. (2005) Nat. Rev. Microbiology 3, 251–261 [DOI] [PubMed] [Google Scholar]
- 21. Berridge M. J. (2002) Cell Calcium 32, 235–249 [DOI] [PubMed] [Google Scholar]
- 22. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 23. Gardner M. J., Hall N., Fung E., White O., Berriman M., Hyman R. W., Carlton J. M., Pain A., Nelson K. E., Bowman S., Paulsen I. T., James K., Eisen J. A., Rutherford K., Salzberg S. L., Craig A., Kyes S., Chan M.-S., Nene V., Shallom S. J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M. W., Vaidya A. B., Martin D. M. A., Fairlamb A. H., Fraunholz M. J., Roos D. S., Ralph S. A., McFadden G. I., Cummings L. M., Subramanian G. M., Mungall C., Venter J. C., Carucci D. J., Hoffman S. L., Newbold C., Davis R. W., Fraser C. M., Barrell B. (2002) Nature 419, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagamune K., Beatty W. L., Sibley L. D. (2007) Eukaryot. Cell 6, 2147–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lovett J. L., Sibley L. D. (2003) J. Cell Sci. 116, 3009–3016 [DOI] [PubMed] [Google Scholar]
- 26. Madeira L., Galante P. A., Budu A., Azevedo M. F., Malnic B., Garcia C. R. (2008) PLoS. ONE 3, e1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dyer M., Day K. (2000) Mol. Biochem. Parasitol. 110, 437–448 [DOI] [PubMed] [Google Scholar]
- 28. Fang J., Marchesini N., Moreno S. N. (2006) Biochem. J. 394, 417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Billker O., Lourido S., Sibley L. D. (2009) Cell Host. Microbe. 5, 612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koyama F. C., Chakrabarti D., Garcia C. R. (2009) Mol. Biochem. Parasitol. 165, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vaid A., Sharma P. (2006) J. Biol. Chem. 281, 27126–27133 [DOI] [PubMed] [Google Scholar]
- 32. Vaid A., Thomas D. C., Sharma P. (2008) J. Biol. Chem. 283, 5589–5597 [DOI] [PubMed] [Google Scholar]
- 33. Dvorin J. D., Martyn D. C., Patel S. D., Grimley J. S., Collins C. R., Hopp C. S., Bright A. T., Westenberger S., Winzeler E., Blackman M. J., Baker D. A., Wandless T. J., Duraisingh M. T. (2010) Science 328, 910–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gazarini M. L., Garcia C. R. (2004) Biochem. Biophys. Res. Commun. 321, 138–144 [DOI] [PubMed] [Google Scholar]
- 35. Passos A. P., Garcia C. R. (1998) Biochem. Biophys. Res. Commun. 245, 155–160 [DOI] [PubMed] [Google Scholar]
- 36. Elabbadi N., Ancelin M. L., Vial H. J. (1994) Mol. Biochem. Parasitol. 63, 179–192 [DOI] [PubMed] [Google Scholar]
- 37. Wengelnik K., Vial H. J. (2007) Res. Microbiol. 158, 51–59 [DOI] [PubMed] [Google Scholar]
- 38. Martin S. K., Jett M., Schneider I. (1994) J. Parasitol. 80, 371–378 [PubMed] [Google Scholar]
- 39. Budu A., Peres R., Bueno V. B., Catalani L. H., Garcia C. R. (2007) J. Pineal Res. 42, 261–266 [DOI] [PubMed] [Google Scholar]
- 40. Hotta C. T., Markus R. P., Garcia C. R. (2003) Braz. J Med. Biol. Res. 36, 1583–1587 [DOI] [PubMed] [Google Scholar]
- 41. Bagnaresi P., Alves E., da Silva H. B., Epiphanio S., Mota M. M., Garcia C. R. (2009) Int. J. Gen. Med. 2, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trager W., Jensen J. B. (1976) Science 193, 673–675 [DOI] [PubMed] [Google Scholar]
- 43. Lambros C., Vanderberg J. P. (1979) J. Parasitol. 65, 418–420 [PubMed] [Google Scholar]
- 44. Berridge M. J., Downes C. P., Hanley M. R. (1982) Biochem. J. 206, 587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dakin K., Li W. H. (2007) Cell Calcium 42, 291–301 [DOI] [PubMed] [Google Scholar]
- 46. Smith I. F., Wiltgen S. M., Parker I. (2009) Cell Calcium 45, 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Di Virgilio F., Steinberg T. H., Silverstein S. C. (1990) Cell Calcium 11, 57–62 [DOI] [PubMed] [Google Scholar]
- 48. Gazarini M. L., Thomas A. P., Pozzan T., Garcia C. R. (2003) J. Cell Biol. 161, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Biagini G. A., Bray P. G., Spiller D. G., White M. R., Ward S. A. (2003) J. Biol. Chem. 278, 27910–27915 [DOI] [PubMed] [Google Scholar]
- 50. Rohrbach P., Friedrich O., Hentschel J., Plattner H., Fink R. H., Lanzer M. (2005) J. Biol. Chem. 280, 27960–27969 [DOI] [PubMed] [Google Scholar]
- 51. Olszewski K. L., Morrisey J. M., Wilinski D., Burns J. M., Vaidya A. B., Rabinowitz J. D., Llinás M. (2009) Cell Host Microbe 5, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pouvelle B., Spiegel R., Hsiao L., Howard R. J., Morris R. L., Thomas A. P., Taraschi T. F. (1991) Nature 353, 73–75 [DOI] [PubMed] [Google Scholar]
- 53. Thomas A. P., Alexander J., Williamson J. R. (1984) J. Biol. Chem. 259, 5574–5584 [PubMed] [Google Scholar]
- 54. Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. (1985) Biochem. J. 229, 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jockers R., Maurice P., Boutin J. A., Delagrange P. (2008) Br. J. Pharmacol. 154, 1182–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brydon L., Roka F., Petit L., de Coppet P., Tissot M., Barrett P., Morgan P. J., Nanoff C., Strosberg A. D., Jockers R. (1999) Mol. Endocrinol. 13, 2025–2038 [DOI] [PubMed] [Google Scholar]
- 57. Lai F. P., Mody S. M., Yung L. Y., Kam J. Y., Pang C. S., Pang S. F., Wong Y. H. (2002) J. Neurochem. 80, 736–745 [DOI] [PubMed] [Google Scholar]
- 58. Steffens F., Zhou X. B., Sausbier U., Sailer C., Motejlek K., Ruth P., Olcese J., Korth M., Wieland T. (2003) Mol. Endocrinol. 17, 2103–2115 [DOI] [PubMed] [Google Scholar]
- 59. Mullins U. L., Fernandes P. B., Eison A. S. (1997) Cell Signal. 9, 169–173 [DOI] [PubMed] [Google Scholar]
- 60. Tsim S. T., Wong J. T., Wong Y. H. (1997) J. Cell Sci. 110, 1387–1393 [DOI] [PubMed] [Google Scholar]
- 61. Dubocovich M. L. (1988) J. Pharmacol. Exp. Ther. 246, 902–910 [PubMed] [Google Scholar]
- 62. Sugden D. (1992) Eur. J. Pharmacol. 213, 405–408 [DOI] [PubMed] [Google Scholar]
- 63. Billker O., Dechamps S., Tewari R., Wenig G., Franke-Fayard B., Brinkmann V. (2004) Cell 117, 503–514 [DOI] [PubMed] [Google Scholar]
- 64. Doerig C., Billker O., Haystead T., Sharma P., Tobin A. B., Waters N. C. (2008) Trends Parasitol. 24, 570–577 [DOI] [PubMed] [Google Scholar]