Abstract
Human hepatocellular carcinoma (HCC) is considered difficult to cure because it is resistant to radio- and chemotherapy and has a high recurrence rate after curative liver resection. Epidermal growth factor receptor variant III (EGFRvIII) has been reported to express in HCC tissues and cell lines. This article describes the efficacy of an anti-EGFRvIII monoclonal antibody (mAb CH12) in the treatment of HCC xenografts with EGFRvIII expression and the underlying mechanism of EGFRvIII as an oncogene in HCC. The results demonstrated that CH12 bound preferentially to EGFRvIII with a dissociation constant (Kd) of 1.346 nm/liter. In addition, CH12 induces strong antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in Huh7-EGFRvIII (with exogenous expression of EGFRvIII) and SMMC-7721 (with endogenous expression of EGFRvIII) cells. Notably, CH12 significantly inhibited the growth of Huh7-EGFRvIII and SMMC-7721 xenografts in vivo with a growth inhibition ratio much higher than C225, a U. S. Food and Drug Administration-approved anti-EGFR antibody. Treatment of the two HCC xenografts with CH12 significantly suppressed tumor proliferation and angiogenesis. Mechanistically, in vivo treatment with CH12 reduced the phosphorylation of constitutively active EGFRvIII, Akt, and ERK. Down-regulation of the apoptotic protectors Bcl-xL, Bcl-2, and the cell cycle regulator cyclin D1, as well as up-regulation of the cell-cycle inhibitor p27, were also observed after in vivo CH12 treatment. Collectively, these results indicate that the monoclonal antibody CH12 is a promising therapeutic agent for HCC with EGFRvIII expression.
Keywords: Antibodies, Apoptosis, ERK, Tumor Therapy, Tyrosine Protein Kinase (Tyrosine Kinase), ADCC, Angiogenesis, Biotherapeutic Agent
Introduction
Hepatocellular carcinoma (HCC)3 is the fifth most common cancer and the third most common cause of cancer-related death in the world (1). The cancer is usually diagnosed at a stage when the disease is already advanced and incurable. Surgery is the most effective treatment for HCC. However, tumor recurrence after a curative liver resection is high (2, 3). Adjuvant chemotherapy has not significantly improved survival of HCC patients (3). Sorafenib is the only targeted therapy approved by the U. S. Food and Drug Administration to treat HCC. Although sorafenib showed good tolerance in the studied populations, most patients in clinical practice suffer from underlying liver cirrhosis with impaired metabolic function and experience dose-limiting toxicities with the need to reduce the overall dose of sorafenib subsequently (4).
The epidermal growth factor receptor (EGFR) has been successfully targeted for cancer therapy (5). EGFR expression in HCC has been reported (6). EGFR expression has been suggested to contribute to the aggressive growth characteristics of HCC tumors (7–9). EGFR overexpression has been demonstrated to be positively correlated with early tumor recurrence and a negative prognostic factor in poorly differentiated HCCs (6, 10). Hence, EGFR represent a promising target for developing innovative HCC treatment strategies. However, despite the clinical success of several EGFR-targeting therapies, including the monoclonal antibody cetuximab (C225, Erbitux) (11, 12) in the treatment of colon cancer and head and neck squamous cell carcinoma (13, 14), no obvious responses were observed in HCC patients after treatment with cetuximab (15).
The presence of EGFR gene mutations may account for the limited clinical response to EGFR-targeting therapies such as cetuximab in HCC patients. Among the EGFR mutations, EGFRvIII is the most commonly described mutation, which is a ligand-independent, constitutively active variant with in-frame deletion of exons 2–7, resulting in the deletion of amino acids 6–273 in the extracellular domain and the generation of a glycine at the fusion point (16). EGFRvIII expression has been detected in glioma, non-small cell lung carcinoma, breast cancer, head and neck squamous cell carcinoma, ovarian carcinoma, as well as hepatocellular carcinoma (17–20). Recently, we also observed its expression in liver cancer cell lines such as SMMC-7721 (21). EGFRvIII mRNA has also been observed in the serum of HCC patients (22). Expression of EGFRvIII can promote tumor cell growth in vitro and in vivo (20, 21, 23). Collectively, these results suggest that EGFRvIII is a potential therapeutic target for HCC. To explore whether EGFRvIII-targeting therapy is a new option for HCC patients, we report here the development of a monoclonal antibody directed to EGFRvIII for the treatment of HCC xenografts in vivo.
EXPERIMENTAL PROCEDURES
Cell Culture
The human hepatocellular carcinoma cell lines Huh7 (expressing low levels of endogenous WT-EGFR); Huh7-EGFR (Huh7 cells with exogenous EGFR overexpression); Huh7-EGFRvIII (Huh7 cells with exogenous EGFRvIII overexpression (21) and SMMC-7721 (Chinese Academy of Science, Shanghai, China) were maintained in DMEM supplemented with 10% fetal bovine serum in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
FACS Analysis
1 × 106 cells were collected by centrifugation and incubated with 20 μg/ml primary antibody, in phosphate-buffered saline containing 1% newborn calf serum for 45 min at 4 °C. After being washed with cold phosphate-buffered saline, cells were incubated for an additional 45 min at 4 °C with an FITC-conjugated goat anti-human antibody (Kang-Chen Bio-tech, Shanghai, China) in the dark. For each sample, at least 10,000 cells were analyzed by FACS cytometry (Beckman Coulter Epics Altra, Miami, FL) and MultiCycle AV for Windows (version 5.0; Phoenix Flow Systems, San Diego, CA).
Immunological Effector Functions
ADCC was performed as described previously (24). Hepatocellular carcinoma cells Huh7, Huh7-EGFRvIII, and SMMC-7721 were used as target cells. Effector cells (peripheral blood mononucleocytes) were freshly prepared and added to the target cells to achieve effect cells:target cells ratios of 100:1. For the CDC assay, target cells were plated at a density of ∼2000 cells/well, with varying amounts of the CH12 antibody (0.01–10 μg/ml) in microtiter plates. Human complement, prepared from the blood of normal, healthy volunteers, was added to each well and incubated at 37 °C for 4 h. The percent cytotoxicity of the antibody was calculated as follows: 1 − (number of viable cells in well treated with antibody and complement/number of viable cells in well treated with the isotype control antibody and complement) × 100%. The assay was performed in triplicate.
Radiolabeling and Binding Affinity Assay
IODO-GENTM (Sigma) was used to iodinate CH12 with 125I (Amersham Biosciences). Radiolabeling was performed on the day of injection into mice. Prior to injection, the percentage of unbound radionuclide content was determined by instant thin layer chromatography, and binding ability of the final radiolabeled product was tested using a Huh7-EGFRvIII cell binding (Lindmo) assay as detailed below. Scatchard analysis was used to determine the binding constant (Ka) and number of antibody molecules bound per cell for 125I-labeled antibody.
In Vivo Antitumor Effects
Tumor volumes were measured as described previously (21). Mice were manipulated and housed according to protocols approved by the Shanghai Medical Experimental Animal Care Commission.
Western Blot Analysis
Immunoblotting experiments were performed according to standard procedures (21). The following antibodies were used: 12H23, antiphospho-EGFR (Tyr1068) (Abcam, Cambridge, UK) and anti-GAPDH (Kang-Chen Bio-tech, Shanghai, China). Antiphospho-Akt (Thr308), anti-Akt, anti-phospho-ERK, anti-ERK1, anti-cyclin D1, anti-Bcl-2, anti-Bcl-xL, and anti-p27 were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunohistochemical Analysis
To assess angiogenesis and cell proliferation in tumors, formalin-fixed paraffin-embedded tumor tissues were immunostained using a monoclonal rat anti-mouse CD34 (Abcam, Cambridge, UK) and anti-Ki-67 (Santa Cruz Biotechnology). After deparaffinization and rehydration, the tissue sections were incubated with 3% hydrogen peroxide in methanol to quench endogenous peroxidase. The sections were blocked for 30 min with 1% BSA and incubated overnight with the primary antibody at 4 °C. The sections were then washed with PBS and incubated with an HRP-conjugated secondary antibody for 30 min. The products were then visualized using a diaminobenzidine staining kit (TIANGEN Biotech, Beijing, China) and counterstained in hematoxylin.
As a measure of proliferation, the Ki-67 labeling index was determined as the ratio of labeled nuclei: total nuclei in high power (×400) fields. Approximately 2000 nuclei were counted in each case by systematic random sampling.
Microvessel density (MVD) was determined by measuring the number of stained microvessels in each section from six mice of each group as described (25). The mean microvessel count of the six most vascular areas was taken as the MVD, which was expressed as the absolute number of microvessels per 0.74 mm2 (×200 field).
TUNEL Assay
The tumor tissues sections were firstly deparaffinized and rehydrated and were then incubated with proteinase K (20 μg/ml) for 20 min at 37 °C. After several washes with PBS, the sections were incubated with TUNEL assay buffer (Beyotime Biotechnology, Nanjing, China) for 1 h at 37 °C in the dark. Then, the slides were rinsed in PBS three times and visualized under a Zeiss LSM confocal microscope (Carl Zeiss, Jena, Germany). TUNEL-positive cells were counted at ×400 magnification. The apoptotic index was calculated as a ratio of apoptotic cell number:total cell number in each field.
Statistical Analysis
All data are presented as the means ± S.E. and were analyzed by the Student's t test. p < 0.05 was considered statistically significant.
RESULTS
Selection of Anti-EGFRvIII mAbs
The protocol for preparation of the hybridomas against EGFRvIII is shown in the supplemental data. One of the hybridoma clones named 12H23 showed preferential binding with EGFRvIII and overexpressed EGFR by FACS analysis (supplemental Fig. S1). Importantly, 12H23 can significantly inhibit the growth of Huh7-EGFRvIII xenografts in vivo with a higher growth inhibition ratio than the anti-EGFR antibody C225 (supplemental Fig. S2). At the end of the study, the inhibition ratio of the C225-treated and 12H23-treated groups were 30 and 63.4%, respectively (supplemental Fig. S2). To analyze binding epitope of 12H23, several EGFR-derived recombinant proteins were expressed in Escherichia coli (supplemental Fig. S3, A and B). The antibody binds to N12-S1 (EGFR, cysteine-rich region, 161G to 313C) and EGFRvIIIex (EGFR, 1L to 623A with a deletion of amino acids 6–273) but not to N12-S2 (EGFR cysteine-rich region, 458G to 614T) recombinant protein in ELISA assay. After comparing the amino acid composition of N12-S1 and that of EGFRvIIIex, 12H23 is suggested to bind to the same epitope as that of mAb 806 (24). To map the binding epitope, several peptides related to CC16 (287CGADSYEMEEDGVRKC302) were fused with the N12 peptide, and ELISA assay confirmed that 12H23 binds to the same epitope as that of mAb 806 (supplemental Fig. S3C). Based on the binding specificity and antitumor activity, we decided to construct antibody 12H23 as a mouse-human chimera to minimize immunogenicity for potential use in clinical trials. The resultant mouse-human chimera antibody named CH12 contains the variable regions of the heavy and light chains of 12H23 linked to human γ-1 and κ constant regions. CH12 was produced in a CHO-DG44 cell line by the procedure described in the supplemental “Experimental Procedures.”
Binding Specificity and Affinity of CH12
A FACS assay was used to test the binding specificity of CH12. C225 (cetuximab), a chimeric monoclonal IgG1 antibody was included as a control. The results indicated that C225 but not CH12 was able to bind to Huh7 cells (Fig. 1A). CH12 binds with higher ratio to Huh7-EGFRvIII cells than to Huh7-EGFR cells, whereas C225 binds to the two cell lines at a similar ratio, indicating the preferential binding of CH12 to EGFRvIII (Fig. 1, B and C). We have previously described that SMMC-7721 cells expressed endogenous EGFRvIII (21). FACS analysis showed that CH12 binds to SMMC-7721 cells (although with a lower ratio than to Huh7-EGFRvIII cells (Fig. 1D)), suggesting that the EGFRvIII may be expressed at a low density on SMMC-7721 cells. The binding affinity of CH12 to EGFRvIII was assessed by radiolabeling with 125I in the presence of unlabeled antibodies. Ka value for the radiolabeled antibody was 1.346 × 109 m−1.
FIGURE 1.
FACS analysis of parental and transfected Huh7 hepatocellular carcinoma cell lines stably expressing WT-EGFR (Huh7-EGFR) or mutant EGFR (Huh7-EGFRvIII) and SMMC-7721 cells. Cells were incubated with CH12 (black line) and C225 (gray line) followed by FITC-conjugated goat anti-human IgG (H+L) antibody. The negative control (irrelevant antibody) fluorescence is plotted on each panel (white line).
Immune Effector Function
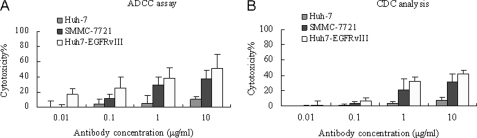
The ADCC and CDC activities of CH12 on target cells were shown in Fig. 2. High cytotoxicity was observed with CH12 against Huh7-EGFRvIII cells with 45.3% cytotoxicity at 0.1 μg/ml CH12 (Fig. 2A). A 29.4% cytotoxicity was observed targeting SMMC-7721 at 1 μg/ml CH12 (Fig. 2A). The results of the CDC analyses showed that CH12 also have high specific cytotoxicity on Huh7-EGFRvIII cells. Cytotoxicity on Huh7-EGFRvIII and SMMC-7721 cells was 32.7 and 21.1%, respectively, at 1 μg/ml CH12 (Fig. 2B). For the EGFRvIII-negative Huh7 cells, no ADCC and CDC activities were induced by CH12.
FIGURE 2.
Immune effector function of chimeric antibody CH12. A, antibody-dependent cellular cytotoxicity on Huh7, SMMC-7721, and Huh7-EGFRvIII cells at an effector:target cell ratio of 100:1 and antibody concentrations ranging from 0.01 to 10 μg/ml. B, complement-dependent cytotoxicity activity of 0.01–10 μg/ml antibody on Huh7, SMMC-7721, and Huh7-EGFRvIII cells. The mean percent cytotoxicity of triplicate determinations is presented (Bars, S.D.).
CH12 Significantly Inhibits Growth of Huh7-EGFRvIII and SMMC-7721 Xenografts
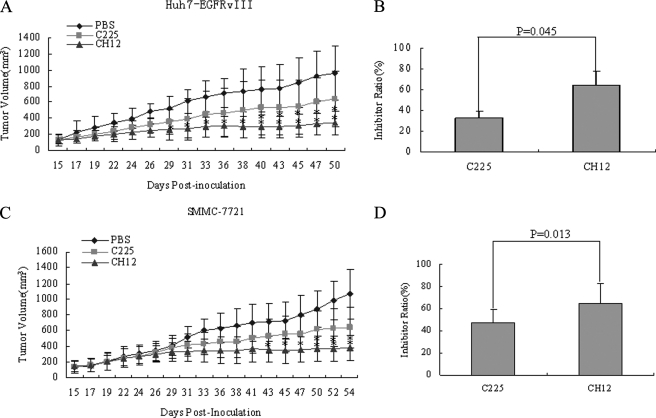
The therapeutic potential of CH12 was first examined in established Huh7-EGFRvIII HCC tumor models; and C225 was included as a control. As shown in Fig. 3 (A and B), CH12 significantly inhibited the growth of Huh7-EGFRvIII xenografts at day 29 after inoculation (CH12 versus vehicle, p < 0.05). Interestingly, when compared with the C225 control, CH12 also showed significant growth inhibition in Huh7-EGFRvIII HCC and the inhibitory ratios of C225 and CH12 at day 50 were 32.9 and 64.5%, respectively (CH12 versus C225, p < 0.05).
FIGURE 3.
Antitumor effects of CH12 and C225 on Huh7-EGFRvIII or SMMC-7721 xenografts in established models. Huh7-EGFRvIII or SMMC-7721 cells (3 × 106) were subcutaneously injected into 4-to-6-week-old nude mice when tumors had reached a mean tumor volume of 200 mm3. The mice were then randomly allocated into three groups (n = 6) and treated with (i) vehicle (sterile PBS), (ii) 25 mg/kg C225 in sterile PBS, or (iii) 25 mg/kg CH12 in sterile PBS. Injections were administered intraperitoneally three times per week for 2 weeks. *, CH12 group versus control group (p < 0.05); **, CH12 group versus C225 group (p < 0.05). In A and C, the data are expressed as mean tumor volumes. In B and D, the data are expressed as the percentage inhibition of tumor growth. (Bars, S.E.)
An established SMMC-7721 tumor model was also used to evaluate the growth inhibition rate of CH12. As shown in Fig. 3 (C and D), CH12 significantly inhibited the growth of SMMC-7721 tumor xenografts at day 43 (p < 0.05). CH12 also showed higher growth inhibition than C225 at day 50 (p < 0.05). The inhibitory ratios of C225 and CH12 54 d after inoculation were 46.9 and 65.0%, respectively.
CH12 Treatment Reduced Proliferation and Angiogenesis and Enhanced Apoptosis in Huh7-EGFRvIII Tumors
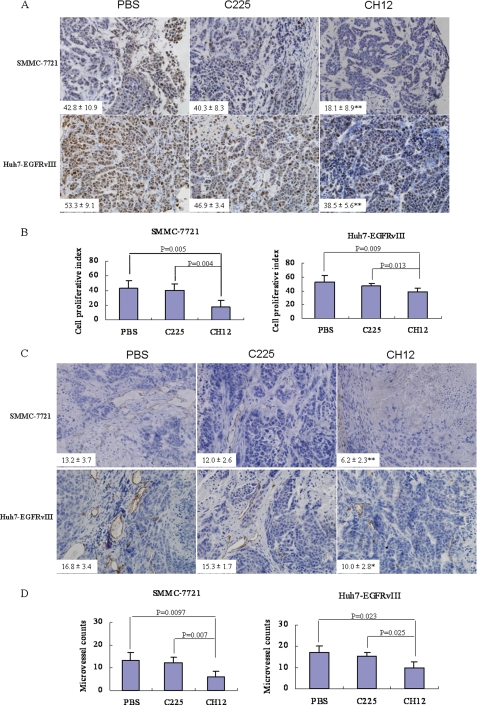
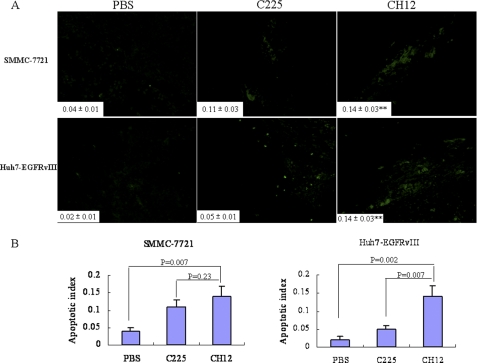
To elucidate the mechanism underlying the in vivo growth suppression caused by CH12, we determined the proliferation rate of tumors from control or treated mice. In both tumor models, the proliferative index, measured by Ki-67 staining of the CH12-treated tumors, was significantly lower than that of the vehicle control tumors (p < 0.01) or the C225-treated tumors (p < 0.05; Fig. 4, A and B). In addition, analysis of the apoptotic index through TUNEL staining demonstrated a significant increase in the number of apoptotic cells in CH12-treated tumors as compared with the vehicle control tumors (p < 0.01; Fig. 5). The number of apoptotic cells in CH12-treated Huh7-EGFRvIII tumors was also significantly higher than that in C225-treated Huh7-EGFRvIII tumors (p < 0.01; Fig. 5). In SMMC-7721 xenografts, CH12 also induced more apoptotic cells than that of C225, although the difference is not statistically significant.
FIGURE 4.
CH12 treatment leads to less growth and vasculogenesis compared with C225 in SMMC-7721 and Huh7-EGFRvIII xenograft tumors. A, tumor sections were stained for Ki-67. The cell proliferative index was assessed as the percentage of total cells that were Ki-67 positive from six randomly selected high power fields (×400) in xenografts from six mice of each group. B, the qualitative analysis of cell proliferation. C, tumor sections were immunostained with anti-CD34 antibody. MVD values were analyzed by measuring the number of stained microvessels as described under “Experimental Procedures” from six randomly selected fields (×200) in xenografts from six mice of each group. D, the qualitative analysis of tumor vascularization. Data are the mean ± S.E. All data were analyzed by Student's t test. p < 0.05 was considered statistically significant.
FIGURE 5.
CH12 treatment leads to an increase in apoptosis compared with C225 in SMMC-7721 and Huh7-EGFRvIII xenograft tumors. Apoptotic cells were detected using the TUNEL assay. The apoptotic index was assessed by the ratio of TUNEL-positive cells:total number of cells from six randomly selected high power fields (×400) in xenografts from six mice of each group. Data are the mean ± S.E. All data were analyzed by Student's t test. p < 0.05 was considered statistically significant.
The extent of tumor vascularization was analyzed by immunostaining of tumors from treated and control specimens for CD34. To quantify tumor vascularization, MVD was determined by scoring the number of stained microvessels. In Huh7-EGFRvIII tumor models, CH12-treated tumors showed 40% less MVD than that shown by vehicle control tumors and 35% than that shown by C225-treated tumors (p < 0.05; Fig. 4, C and D). In SMMC-7721 tumor models, CH12-treated tumors showed 53% less MVD than that shown by vehicle control tumors and 48% less than that shown by C225-treated tumors (p < 0.01; Fig. 4, C and D).
CH12 Treatment Reduces EGFRvIII Autophosphorylation and Down-regulates Bcl-xL and Bcl-2 Expression in Huh7-EGFRvIII and SMMC-7721 HCC Tumors
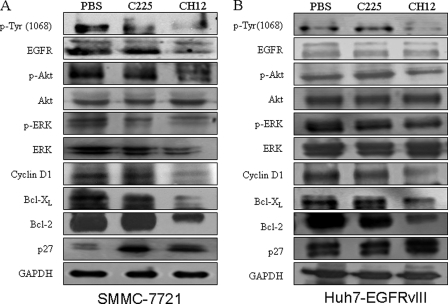
To understand the molecular events occurring in CH12-treated tumors, some key signal molecules in the EGFR pathway were examined by Western blot analysis (Fig. 6). Because the constitutively active kinase activity and autophosphorylation of the carboxyl terminus of EGFRvIII are essential for its biological functions (17, 26), we determined the EGFRvIII phosphorylation status in tumors from treated and control animals. CH12 treatment dramatically reduced EGFRvIII autophosphorylation, although receptor levels were only slightly decreased in the xenografts. Phosphorylation of Akt and ERK, the two most important downstream signal transductors of EGFRvIII was also examined. CH12 treatment reduced both Akt and ERK phosphorylation in Huh7-EGFRvIII and SMMC-7721 cell lines, although the phosphorylation level of ERK were down-regulated to a greater degree in SMMC-7721 cells than in Huh7-EGFRvIII cells (Fig. 6).
FIGURE 6.
Mechanisms of antitumor activity following treatment with CH12 and C225. Established SMMC-7721 (A) and Huh7-EGFRvIII (B) xenografts treated with PBS, CH12, or C225 as single agents were excised and prepared by homogenization in cell lysis buffer. Tumor lysates (30 μg) were then subjected to SDS-polyacrylamide gels and immunoblotted for total EGFR, phospho-EGFR (Tyr1068), total Akt, phospho-Akt, total ERK, phospho-ERK, cyclin D1, Bcl-xL, Bcl-2, and p27 as indicated.
Because CH12 significantly inhibited the proliferation of HCC, two cell cycle regulators cyclin D1 and p27 were examined. As shown in Fig. 6, cyclin D1 was significantly down-regulated by CH12 treatment. The expression of cyclin D1 in CH12 treatment group was weaker than that in C225 treatment group. CH12 treatment potently up-regulated the expression of p27 (Fig. 6).
The mechanism underlying the apoptosis promotion effect of CH12 treatment was also studied. Therefore, the expression level of Bcl-xL and Bcl-2 were examined. The results shown in Fig. 6 clearly indicate that Bcl-xL and Bcl-2 were significantly down-regulated in CH12-treated tumors. However, C225-treated tumors showed almost no reduction of Bcl-xL and Bcl-2 expression (Fig. 6).
DISCUSSION
One limitation of antibodies targeting the WT-EGFR is their significant uptake in normal tissues such as the liver and skin, which often causes side effects such as skin rash and gastrointestinal toxicity. To avoid this problem, Johns et al. (27) developed mAb 806, which binds to a specific epitope of EGFR. In a phase I clinical study, ch806 displayed excellent targeting of tumor sites in all patients with no evidence of normal tissue uptake and no significant toxicity (28). However, the highest dosage level of ch806 in the phase I clinical trial was 40 mg/m2. The authors mentioned that further dose escalation was not performed due to the limited amount of cGMP ch806 available for the trial (28), suggesting the difficulty in production of ch806. In this study, human-mouse chimeric antibody of 12H23 (CH12) displayed almost the same binding affinity to EGFRvIII as ch806 (29). Interestingly, we found that the yield of CH12 is ∼5-fold higher than that of ch806 (data not shown). Additionally, in an in vivo biodistribution assay, 24 h after antibody injection, the percentage of the injected radioactive dose per gram of tissue (% injected radioactive dose g−1) of l25I-labeled CH12 in Huh7-EGFRvIII xenografts are highest among the tested solid organs, indicating its accumulation in the tumor tissues (supplemental Fig. S4). Therefore, we decided to use CH12 to treat HCC.
Intriguingly, CH12 displayed significant ADCC and CDC effects on Huh7-EGFRvIII cells as well as SMMC-7721 cells, which bound weakly to CH12 in a FACS assay. Importantly, CH12 showed strong anti-tumor effect on Huh7-EGFRvIII and SMMC-7721 xenografts. We also tested the anti-HCC effect of the U. S. Food and Drug Administration-approved antibody C225. The results indicated that C225 displayed limited growth-inhibition effect on HCC xenografts with EGFRvIII expression. Thus, the EGFRvIII expression may be a reason for the minimal effect of C225 in the clinical trial.
Further study on the mechanism underlying the growth inhibition effect indicated that CH12 treatment can suppress angiogenesis, promote apoptosis, and decrease proliferation. Cyclin D1 is involved in regulating the G1/S transition and was observed to be amplified in HCC (30–32). p27 is a cyclin-dependent kinase (CDK) inhibitor which binds to cyclin-cyclin-dependent kinase (CDK) complexes and facilitates the inhibition of the catalytic activity of cyclin-dependent kinase (CDK), ultimately inducing G1 cell cycle arrest. It has been suggested that p27 loss occurs early in the carcinogenesis process. The more aggressive, metastasizing cancers tend to lack p27 expression (33). High p27 expression, correlated with prolonged survival, is a favorable independent prognostic parameter for HCC (33, 34). Thus, the decreased proliferation in HCC xenografts caused by CH12 treatment should be ascribed to the down-regulation of cyclin D1 expression and up-regulation of p27 expression. It has been demonstrated that the EGFRvIII can induce up-regulation of Bcl-xL (35), which has been observed to overexpress in a subset of HCC and demonstrated as a significant prognostic factor for disease progression in human HCC (36, 37). Previous study indicated that EGFR inhibitor gefitinib can suppress the expression of anti-apoptotic Bcl-2 and Bcl-xL, further rendering HCC cells prone to apoptosis (38). Therefore, the weakened expression of Bcl-2 and Bcl-xL should contribute to the apoptosis-promoting effect of CH12.
As we know, antibodies are often used with chemotherapeutic drugs in clinic. A previous report indicated that combining cetuximab with TKIs (erlotinib or AG1024) or the HMG-CoA-reductase inhibitor fluvastatin or doxorubicin resulted in synergistic antiproliferative effects on HepG2 and Huh7 hepatocellular cancer cells (39). Considering that EGFRvIII expression can decrease the sensitivity of HCC cell lines to the chemotherapeutic drug 5-fluorouracil (21), CH12 which inhibits the signal transduction of EGFRvIII, may be combined with 5-fluorouracil to enhance the antitumor effects.
CH12 can also be combined with sorafenib, which inhibits Raf-1, B-Raf, vascular endothelial growth factor receptors 1–3, and PDGFR-β (40) for HCC treatment. This combination may significantly reduce the dosage of sorafenib and thus decrease the side effects of sorafenib. Taken together, our study demonstrated that the monoclonal antibody CH12 developed here has potential to be used as therapeutics for HCC with EGFRvIII expression.
Supplementary Material
Acknowledgment
We greatly thank Dr. Zhiqiang An at the University of Texas Health Science Center (Houston, TX) for critical reading of the manuscript.
This work was supported by a supporting program of the “Eleventh Five-year Plan” for Science & Technology Research of China (Grant 2008ZX10002-024 and 2009ZX09103-701), the National Basic Research Program (Grant 2010CB529902), the National High Technology Research and Development Program of China (Grant 2007AA021203), the research fund of the State Key Laboratory of Oncogenes and Related Genes (Grant 91-09-05), the National Natural Science Foundation (Grant 30901820), and the doctoral program of the Foundation of Institutions of Higher Education of China (Grant 20090073120109).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. S1–S4.
- HCC
- hepatocellular carcinoma
- EGFR
- epidermal growth factor receptor
- ADCC
- antibody-dependent cell-mediated cytotoxicity
- CDC
- complement dependent cytotoxicity
- EGFRvIII
- epidermal growth factor receptor variant III
- MVD
- microvessel density
- ch806
- chimeric monoclonal antibody 806.
REFERENCES
- 1. Llovet J. M., Burroughs A., Bruix J. (2003) Lancet 362, 1907–1917 [DOI] [PubMed] [Google Scholar]
- 2. Carr B. I. (2004) Gastroenterology 127, S218–224 [DOI] [PubMed] [Google Scholar]
- 3. Zhu A. X. (2006) Oncologist 11, 790–800 [DOI] [PubMed] [Google Scholar]
- 4. Wörns M. A., Weinmann A., Pfingst K., Schulte-Sasse C., Messow C. M., Schulze-Bergkamen H., Teufel A., Schuchmann M., Kanzler S., Düber C., Otto G., Galle P. R. (2009) J. Clin. Gastroenterol. 43, 489–495 [DOI] [PubMed] [Google Scholar]
- 5. Gainet M., Guardiola E., Dufresne A., Pivot X. (2003) Cancer Radiother. 7, 195–199 [DOI] [PubMed] [Google Scholar]
- 6. Daveau M., Scotte M., François A., Coulouarn C., Ros G., Tallet Y., Hiron M., Hellot M. F., Salier J. P. (2003) Mol. Carcinog. 36, 130–141 [DOI] [PubMed] [Google Scholar]
- 7. DeCicco L. A., Kong J., Ringer D. P. (1997) Cancer letters 111, 149–156 [DOI] [PubMed] [Google Scholar]
- 8. Kira S., Nakanishi T., Suemori S., Kitamoto M., Watanabe Y., Kajiyama G. (1997) Liver 17, 177–182 [DOI] [PubMed] [Google Scholar]
- 9. Moser G. J., Wolf D. C., Goldsworthy T. L. (1997) Toxicol. Pathol. 25, 275–283 [DOI] [PubMed] [Google Scholar]
- 10. Zhao Y. N., Cao J., Wu F. X., Ou C., Yuan W. P., Mo Q. G., Wei W., Li Y., Su J. J., Liang A. M. (2004) Ai zheng = Aizheng = Chinese journal of cancer 23, 762–766 [PubMed] [Google Scholar]
- 11. Mendelsohn J., Baselga J. (2003) J. Clin. Oncol. 21, 2787–2799 [DOI] [PubMed] [Google Scholar]
- 12. Nygren P., Sørbye H., Osterlund P., Pfeiffer P. (2005) Acta Oncol. 44, 203–217 [DOI] [PubMed] [Google Scholar]
- 13. Harari P. M. (2004) Endocr. Relat. Cancer 11, 689–708 [DOI] [PubMed] [Google Scholar]
- 14. Huang S., Armstrong E. A., Benavente S., Chinnaiyan P., Harari P. M. (2004) Cancer Res. 64, 5355–5362 [DOI] [PubMed] [Google Scholar]
- 15. Yu X. T., Zhu S. N., Xu Z. D., Hu X. Q., Zhu T. F., Chen J. Q., Lu S. L. (2007) J. Cancer Res. Clin. Oncol. 133, 145–152 [DOI] [PubMed] [Google Scholar]
- 16. Kuan C. T., Wikstrand C. J., Bigner D. D. (2001) Endocr. Relat. Cancer 8, 83–96 [DOI] [PubMed] [Google Scholar]
- 17. Moscatello D. K., Holgado-Madruga M., Godwin A. K., Ramirez G., Gunn G., Zoltick P. W., Biegel J. A., Hayes R. L., Wong A. J. (1995) Cancer Res. 55, 5536–5539 [PubMed] [Google Scholar]
- 18. Okamoto I., Kenyon L. C., Emlet D. R., Mori T., Sasaki J., Hirosako S., Ichikawa Y., Kishi H., Godwin A. K., Yoshioka M., Suga M., Matsumoto M., Wong A. J. (2003) Cancer Sci. 94, 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ou C., Wu F. X., Luo Y., Cao J., Zhao Y. N., Yuan W. P., Li Y., Su J. J. (2005) Ai Zheng 24, 166–169 [PubMed] [Google Scholar]
- 20. Sok J. C., Coppelli F. M., Thomas S. M., Lango M. N., Xi S., Hunt J. L., Freilino M. L., Graner M. W., Wikstrand C. J., Bigner D. D., Gooding W. E., Furnari F. B., Grandis J. R. (2006) Clin. Cancer Res. 12, 5064–5073 [DOI] [PubMed] [Google Scholar]
- 21. Wang H., Jiang H., Zhou M., Xu Z., Liu S., Shi B., Yao X., Yao M., Gu J., Li Z. (2009) Cancer Lett. 279, 30–38 [DOI] [PubMed] [Google Scholar]
- 22. Zhou M., Gong B., Gu J., Li Z. (2010) Liver Int. 30, 925–927 [DOI] [PubMed] [Google Scholar]
- 23. Nishikawa R., Ji X. D., Harmon R. C., Lazar C. S., Gill G. N., Cavenee W. K., Huang H. J. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 7727–7731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang L., Jiang H., Shi B., Wang H., Li J., Wang H., Yao M., Li Z. (2010) Cancer Immunol. Immunother. 59, 1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poon R. T., Ng I. O., Lau C., Yu W. C., Yang Z. F., Fan S. T., Wong J. (2002) J. Clin. Oncol. 20, 1775–1785 [DOI] [PubMed] [Google Scholar]
- 26. Luwor R. B., Johns T. G., Murone C., Huang H. J., Cavenee W. K., Ritter G., Old L. J., Burgess A. W., Scott A. M. (2001) Cancer Res. 61, 5355–5361 [PubMed] [Google Scholar]
- 27. Johns T. G., Adams T. E., Cochran J. R., Hall N. E., Hoyne P. A., Olsen M. J., Kim Y. S., Rothacker J., Nice E. C., Walker F., Ritter G., Jungbluth A. A., Old L. J., Ward C. W., Burgess A. W., Wittrup K. D., Scott A. M. (2004) J. Biol. Chem. 279, 30375–30384 [DOI] [PubMed] [Google Scholar]
- 28. Scott A. M., Lee F. T., Tebbutt N., Herbertson R., Gill S. S., Liu Z., Skrinos E., Murone C., Saunder T. H., Chappell B., Papenfuss A. T., Poon A. M., Hopkins W., Smyth F. E., MacGregor D., Cher L. M., Jungbluth A. A., Ritter G., Brechbiel M. W., Murphy R., Burgess A. W., Hoffman E. W., Johns T. G., Old L. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4071–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panousis C., Rayzman V. M., Johns T. G., Renner C., Liu Z., Cartwright G., Lee F. T., Wang D., Gan H., Cao D., Kypridis A., Smyth F. E., Brechbiel M. W., Burgess A. W., Old L. J., Scott A. M. (2005) Br. J. Cancer 92, 1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y. J., Jiang W., Chen C. J., Lee C. S., Kahn S. M., Santella R. M., Weinstein I. B. (1993) Biochem. Biophys. Res. Commun. 196, 1010–1016 [DOI] [PubMed] [Google Scholar]
- 31. Hunter T., Pines J. (1994) Cell 79, 573–582 [DOI] [PubMed] [Google Scholar]
- 32. Nishida N., Fukuda Y., Komeda T., Kita R., Sando T., Furukawa M., Amenomori M., Shibagaki I., Nakao K., Ikenaga M., et al. (1994) Cancer Res. 54, 3107–3110 [PubMed] [Google Scholar]
- 33. Ito Y., Matsuura N., Sakon M., Miyoshi E., Noda K., Takeda T., Umeshita K., Nagano H., Nakamori S., Dono K., Tsujimoto M., Nakahara M., Nakao K., Taniguchi N., Monden M. (1999) Hepatology 30, 90–99 [DOI] [PubMed] [Google Scholar]
- 34. Fiorentino M., Altimari A., D'Errico A., Cukor B., Barozzi C., Loda M., Grigioni W. F. (2000) Clin. Cancer Res. 6, 3966–3972 [PubMed] [Google Scholar]
- 35. Nagane M., Coufal F., Lin H., Bögler O., Cavenee W. K., Huang H. J. (1996) Cancer Res. 56, 5079–5086 [PubMed] [Google Scholar]
- 36. Takehara T., Liu X., Fujimoto J., Friedman S. L., Takahashi H. (2001) Hepatology 34, 55–61 [DOI] [PubMed] [Google Scholar]
- 37. Watanabe J., Kushihata F., Honda K., Sugita A., Tateishi N., Mominoki K., Matsuda S., Kobayashi N. (2004) Surgery 135, 604–612 [DOI] [PubMed] [Google Scholar]
- 38. Höpfner M., Sutter A. P., Huether A., Schuppan D., Zeitz M., Scherübl H. (2004) J. Hepatol. 41, 1008–1016 [DOI] [PubMed] [Google Scholar]
- 39. Huether A., Höpfner M., Baradari V., Schuppan D., Scherübl H. (2005) Biochem. Pharmacol. 70, 1568–1578 [DOI] [PubMed] [Google Scholar]
- 40. Faloppi L., Scartozzi M., Maccaroni E., Di Pietro Paolo M., Berardi R., Del Prete M., Cascinu S. (2010) Cancer Treat. Rev., doi:10.1016/j.ctrv.2010.08.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.