An article in this issue of the Biophysical Journal by Li et al. (1) reports what is believed to be the first detailed atomic model of tropomyosin bound to the actin filament (F-actin), addressing a question that has puzzled scientists for years.
Tropomyosin is a coiled-coil protein that associates end-to-end to form long helical strands along the two long-pitch helices of F-actin (Fig. 1 a). In striated muscle, Ca2+ controls the position of tropomyosin on F-actin via the troponin complex. The troponin complex consists of three subunits: tropomyosin-binding (TnT), inhibitory (TnI), and Ca2+ sensor (TnC). At low Ca2+, TnI competes with tropomyosin for a common binding site on F-actin, forcing tropomyosin into a position where it blocks the binding of myosin heads and hence muscle contraction. This constrained position of tropomyosin is known as the blocked (B)-state. On activation, Ca2+ binds to the N-lobe of TnC, which then recruits TnI, enabling tropomyosin to translocate azimuthally ∼25° on the surface of F-actin. This new position of tropomyosin is approximately equivalent to its equilibrium position in the absence of troponin, and is referred to as the closed (C)-state because tropomyosin still partially covers the myosin-binding site. Yet, tropomyosin is bound loosely in the C-state, such that myosin can bind to the filament, leading to an additional ∼10° azimuthal translocation of tropomyosin on F-actin to a position known as the open (M)-state. In this way, tropomyosin's azimuthal plasticity on the surface of F-actin serves as the basis for its gatekeeper function (i.e., controlling the access of myosin to the actin filament in striated muscle). Similarly, in nonmuscle cells lacking troponin, a large number of tropomyosin isoforms control the access and activities of various actin-binding proteins (2), as well as the specific organization of actin networks and cell adhesions (3).
Figure 1.
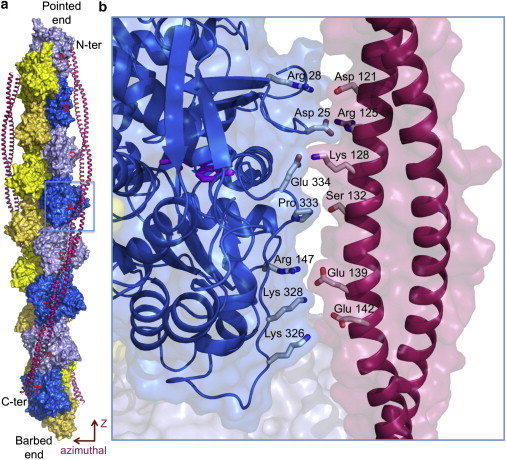
Model of tropomyosin on the actin filament as proposed by Li et al. (1). (a) Tropomyosin winds symmetrically along the two long-pitch helices of the actin filament, making similar contacts with each actin subunit (numbered) along its 385-Å-long path. (b) Close-up view of tropomyosin pseudo-repeat-4 (contoured in panel a, but slightly rotated), which together with pseudo-repeat-5 makes the strongest contacts with the filament (4). Positively charged amino acids of actin subdomain 4 (Arg-147, Lys-326, and Lys-328) face negatively charged amino acids in each pseudo-repeat of tropomyosin. Pro-333 in a protruding loop of actin, running from subdomain 3 to 1, faces a shallow area of tropomyosin. Additional contacts may involve Glu-334, Arg-28, and Asp-25 in actin subdomain 1, which face oppositely charged amino acids in tropomyosin. Similar contacts are observed along all seven pseudo-repeats of tropomyosin.
How tropomyosin achieves such remarkable conformational plasticity on F-actin has been the subject of much research (4,5). Although definitive answers are not yet available, various features of the tropomyosin structure undoubtedly play a role. Chief among these is shape complementarity with F-actin, a concept that Holmes and Lehman have termed gestalt-binding (6). Gestalt-binding reflects the notion that the intrinsic conformation of the tropomyosin coiled coil displays a right-handed supercoil twist that is designed to match precisely to the shape of F-actin along a 385-Å-long path encompassing seven actin subunits of the long pitch helix. Other coiled-coil proteins lack this twist, which in tropomyosin is made possible by the presence of periodic clusters of alanine residues (5). Tropomyosin's coiled coil is also quite rigid, with a persistence length of 423 nm, and thus local azimuthal movements are propagated over a relatively long distance. Another feature is the low binding affinity of individual tropomyosin molecules for F-actin. Because only end-to-end associated tropomyosin polymers have any significant affinity for the filament, azimuthal movements of tropomyosin can be achieved at a low energy cost. Finally, a distinctive attribute of tropomyosin is the presence of pseudo-repeats of ∼40 amino acids (seven repeats in striated muscle, and four to six repeats in truncated nonmuscle isoforms), with each repeat bridging the distance across one actin subunit of the long pitch helix. Every repeat presents a cluster of acidic residues that are thought to interact with basic residues on the actin surface (5). The presence of these acidic clusters, along with the salt dependence and low affinity of the interaction, have led to the assumption that tropomyosin binds to F-actin mainly through electrostatic contacts, as opposed to hydrophobic or stereospecific contacts.
Although the azimuthal positions of tropomyosin on F-actin have been well characterized by electron microscopy (EM) and fiber diffraction, its longitudinal (z)-position along the filament (Fig. 1 a) and thus the exact nature of tropomyosin-actin contacts have remained elusive. Li et al. (1) used a combination of EM and computational chemistry to find the optimal z-position of tropomyosin on F-actin in the equilibrium state. Their computational search focused exclusively on finding the most favorable electrostatic interactions between tropomyosin and actin by exploring ∼6000 combinations of different pseudo-rotations and azimuthal and longitudinal positions of tropomyosin. The search converged to a model in which amino acids of opposite charges face each other along the tropomyosin-actin interface (Fig. 1 b). Li et al. (1) then turned to their EM reconstruction, paying particular attention to a well-known but previously unexplored feature of F-actin-tropomyosin reconstructions: the tropomyosin density broadens and tapers periodically, corresponding to the wide and narrow faces of a coiled-coil viewed face-on. This led to a unique fit of the atomic model of tropomyosin derived from crystal structures of overlapping segments into the EM volume. Remarkably, the models that were obtained independently by computational chemistry and fitting into the EM volume are nearly identical and reveal electrostatic contacts similar to those proposed by Brown and Cohen (5) based on analysis of crystal structures of tropomyosin. On the actin side of the interface, most of the charges are positive and belong to subdomains 1 and 3, whereas the tropomyosin pseudo-repeats contribute mainly negative charges. Pro-333, located in a protruding loop that runs from subdomain 3 to 1 of actin, faces a slight depression on the tropomyosin surface. The electrostatic interactions observed at the interface can easily be satisfied intramolecularly or through solvation, which combined with the lack of stereospecific contacts may explain tropomyosin's significant degree of azimuthal freedom on F-actin.
Many questions remain unresolved by this study, including the position and interactions of the end-to-end overlap region, the interactions between actin and other tropomyosin isoforms, and the effects of troponin and other actin-binding proteins on the position of tropomyosin on F-actin. At the pointed end of the filament in muscle cells, the end-to-end interaction of tropomyosin is substituted by interactions with tropomodulin, a pointed end-capping protein, and Lmod, an actin filament nucleator. Our limited understanding of these important interactions, and the newly found relevance of tropomyosin for controlling actin dynamics in nonmuscle cells ensure that the field of tropomyosin research is likely to remain very active.
Acknowledgments
This work was supported by National Institutes of Health grant R01 GM073791.
References
- 1.Li X.E., Tobacman L.S., Lehman W. Tropomyosin position on F-actin revealed by EM reconstruction and computational chemistry. Biophys. J. 2011;100:1005–1013. doi: 10.1016/j.bpj.2010.12.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunning P., O'Neill G., Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol. Rev. 2008;88:1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- 3.Bach C.T., Creed S., O'Neill G.M. Tropomyosin isoform expression regulates the transition of adhesions to determine cell speed and direction. Mol. Cell. Biol. 2009;29:1506–1514. doi: 10.1128/MCB.00857-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hitchcock-DeGregori S.E., Singh A. What makes tropomyosin an actin binding protein? A perspective. J. Struct. Biol. 2010;170:319–324. doi: 10.1016/j.jsb.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J.H., Cohen C. Regulation of muscle contraction by tropomyosin and troponin: how structure illuminates function. Adv. Protein Chem. 2005;71:121–159. doi: 10.1016/S0065-3233(04)71004-9. [DOI] [PubMed] [Google Scholar]
- 6.Holmes K.C., Lehman W. Gestalt-binding of tropomyosin to actin filaments. J. Muscle Res. Cell Motil. 2008;29:213–219. doi: 10.1007/s10974-008-9157-6. [DOI] [PubMed] [Google Scholar]


