Abstract
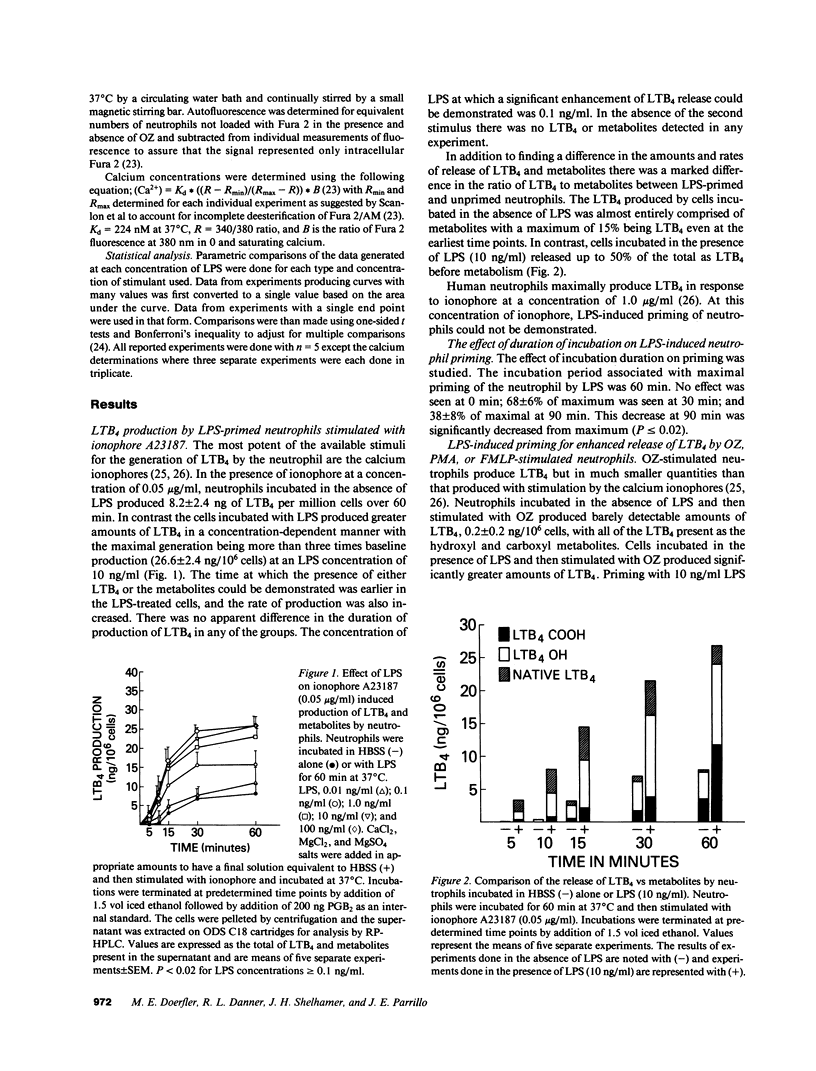
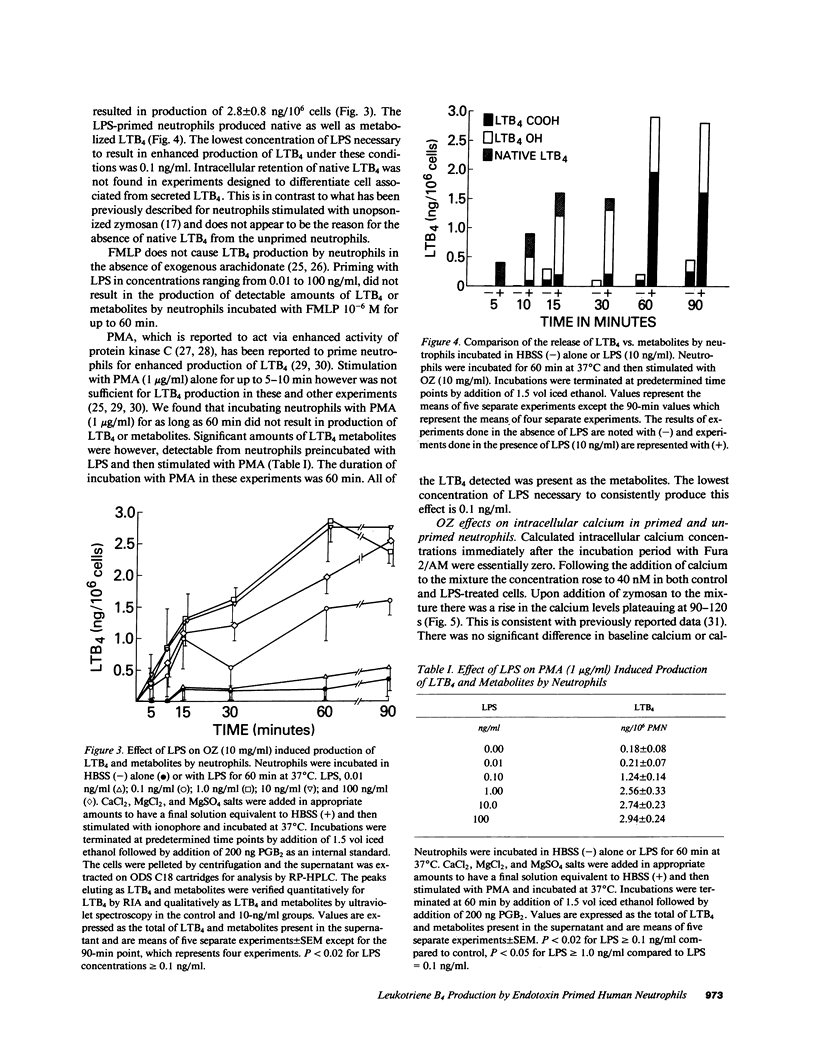
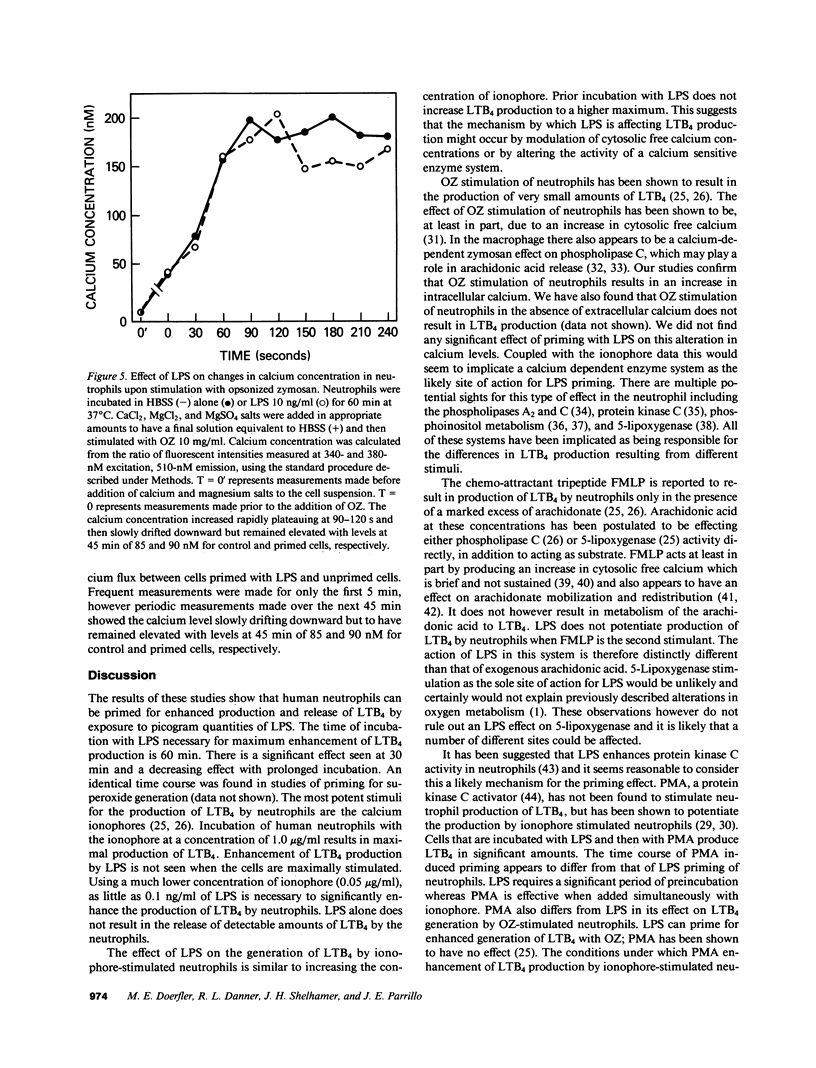
Neutrophils can be "primed" for an enhanced respiratory burst by lipopolysaccharide (LPS) in concentrations measurable in patients with septic shock. Leukotriene B4 (LTB4) is the primary eicosanoid product of neutrophils and is felt to be a mediator of host defense and inflammation. We investigated the in vitro effects of LPS on neutrophil production of LTB4 and the omega-oxidation metabolites of LTB4. Incubation of neutrophils with LPS in concentrations ranging from 0.01 to 100 ng/ml did not result in production of LTB4 or metabolites in the absence of a second stimulus. Priming neutrophils with LPS and then stimulating with opsonized zymosan, phorbol-myristate-acetate or a low concentration of the calcium ionophore A23187 resulted in enhanced production of LTB4. LPS priming of neutrophils occurred in a concentration dependent manner. LPS did not result in LTB4 production in response to the chemoattractant peptide FMLP. LPS priming of neutrophils had no effect on cytosolic calcium concentrations of resting or zymosan-stimulated cells. These results suggest that LPS might effect host defense and tissue injury by potentiating the effect of other stimulants on neutrophil production of LTB4. This LPS induced enhancement may represent an important pathogenetic pathway in patients with gram negative sepsis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINS E., HEIJN C., Jr STUDIES ON TUBERCULIN FEVER. 3. MECHANISMS INVOLVED IN THE RELEASE OF ENDOGENOUS PYROGEN IN VITRO. J Exp Med. 1965 Aug 1;122:207–235. doi: 10.1084/jem.122.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Aderem A. A., Cohen D. S., Wright S. D., Cohn Z. A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986 Jul 1;164(1):165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A. A., Keum M. M., Pure E., Cohn Z. A. Bacterial lipopolysaccharides, phorbol myristate acetate, and zymosan induce the myristoylation of specific macrophage proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5817–5821. doi: 10.1073/pnas.83.16.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Björk J., Hedqvist P., Arfors K. E. Increase in vascular permeability induced by leukotriene B4 and the role of polymorphonuclear leukocytes. Inflammation. 1982 Jun;6(2):189–200. doi: 10.1007/BF00916243. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J. Inhibition of phospholipase. Br Med Bull. 1983 Jul;39(3):260–264. doi: 10.1093/oxfordjournals.bmb.a071830. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Picard S., Vallerand P., Sirois P. Transformation of arachidonic acid in leukocytes. Isolation and structural analysis of a novel dihydroxy derivative. Prostaglandins Med. 1981 Jun;6(6):557–570. doi: 10.1016/0161-4630(81)90117-8. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Danner R. L., Joiner K. A., Parrillo J. E. Inhibition of endotoxin-induced priming of human neutrophils by lipid X and 3-Aza-lipid X. J Clin Invest. 1987 Sep;80(3):605–612. doi: 10.1172/JCI113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Emilsson A., Sundler R. Differential activation of phosphatidylinositol deacylation and a pathway via diphosphoinositide in macrophages responding to zymosan and ionophore A23187. J Biol Chem. 1984 Mar 10;259(5):3111–3116. [PubMed] [Google Scholar]
- Feinmark S. J., Lindgren J. A., Claesson H. E., Malmsten C., Samuelsson B. Stimulation of human leukocyte degranulation by leukotriene B4 and its omega-oxidized metabolites. FEBS Lett. 1981 Dec 21;136(1):141–144. doi: 10.1016/0014-5793(81)81233-1. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Noonan T. C., Jubiz W., Malik A. B. Leukotrienes and the pulmonary microcirculation. Am Rev Respir Dis. 1987 Jul;136(1):161–169. doi: 10.1164/ajrccm/136.1.161. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Pickett W. C. The human PMN leukocyte chemotactic activity of complex hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Oct;125(4):1789–1791. [PubMed] [Google Scholar]
- Gréen K., Hamberg M., Samuelsson B., Smigel M., Frölich J. C. Measurement of prostaglandins, thromboxanes, prostacyclin and their metabolites by gas liquid chromatography--mass spectrometry. Adv Prostaglandin Thromboxane Res. 1978;5:39–94. [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines K. A., Giedd K. N., Rich A. M., Korchak H. M., Weissmann G. The leukotriene B4 paradox: neutrophils can, but will not, respond to ligand-receptor interactions by forming leukotriene B4 or its omega-metabolites. Biochem J. 1987 Jan 1;241(1):55–62. doi: 10.1042/bj2410055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartiala K. T., Scott I. G., Viljanen M. K., Akerman K. E. Lack of correlation between calcium mobilization and respiratory burst activation induced by chemotactic factors in rabbit polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1987 Apr 29;144(2):794–800. doi: 10.1016/s0006-291x(87)80034-7. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Rutherford L. E., Weissmann G. Stimulus response coupling in the human neutrophil. I. Kinetic analysis of changes in calcium permeability. J Biol Chem. 1984 Apr 10;259(7):4070–4075. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Leung K. H., Ehrke M. J., Mihich E. Modulation of the development of cell-mediated immunity: possible role of the products of the cyclo-oxygenase and the lipoxygenase pathways of arachidonic acid metabolism. Int J Immunopharmacol. 1982;4(3):195–204. doi: 10.1016/0192-0561(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Lewis G. P. Immunoregulatory activity of metabolites of arachidonic acid and their role in inflammation. Br Med Bull. 1983 Jul;39(3):243–248. doi: 10.1093/oxfordjournals.bmb.a071827. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles W. C., Meier K. E., Henderson W. R. Phorbol myristate acetate and the calcium ionophore A23187 synergistically induce release of LTB4 by human neutrophils: involvement of protein kinase C activation in regulation of the 5-lipoxygenase pathway. J Immunol. 1987 May 15;138(10):3396–3402. [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Marom Z., Shelhamer J. H., Kaliner M. Human pulmonary macrophage-derived mucus secretagogue. J Exp Med. 1984 Mar 1;159(3):844–860. doi: 10.1084/jem.159.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl S. R., Hurst N. P., Cleland L. G. Modulation by phorbol myristate acetate of arachidonic acid release and leukotriene synthesis by human polymorphonuclear leukocytes stimulated with A23187. Biochem Biophys Res Commun. 1986 Dec 15;141(2):399–404. doi: 10.1016/s0006-291x(86)80186-3. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Meshulam T., Diamond R. D., Lyman C. A., Wysong D. R., Melnick D. A. Temporal association of calcium mobilization, inositol trisphosphate generation, and superoxide anion release by human neutrophils activated by serum opsonized and nonopsonized particulate stimuli. Biochem Biophys Res Commun. 1988 Jan 29;150(2):532–539. doi: 10.1016/0006-291x(88)90426-3. [DOI] [PubMed] [Google Scholar]
- Moscat J., Aracil M., Diez E., Balsinde J., Garcia Barreno P., Municio A. M. Intracellular Ca2+ requirements for zymosan-stimulated phosphoinositide hydrolysis in mouse peritoneal macrophages. Biochem Biophys Res Commun. 1986 Jan 14;134(1):367–371. doi: 10.1016/0006-291x(86)90572-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Salmon J. A. Release of leukotriene B4 from human neutrophils and its relationship to degranulation induced by N-formyl-methionyl-leucyl-phenylalanine, serum-treated zymosan and the ionophore A23187. Immunology. 1983 Sep;50(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Parker M. M., Shelhamer J. H., Natanson C., Alling D. W., Parrillo J. E. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med. 1987 Oct;15(10):923–929. doi: 10.1097/00003246-198710000-00006. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Borgeat P., Sirois P. Leukotriene B4 induces human suppressor lymphocytes. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1531–1537. doi: 10.1016/s0006-291x(82)80081-8. [DOI] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Sha'afi R. I., White J. R., Molski T. F., Shefcyk J., Volpi M., Naccache P. H., Feinstein M. B. Phorbol 12-myristate 13-acetate activates rabbit neutrophils without an apparent rise in the level of intracellular free calcium. Biochem Biophys Res Commun. 1983 Jul 29;114(2):638–645. doi: 10.1016/0006-291x(83)90828-8. [DOI] [PubMed] [Google Scholar]
- Shak S., Goldstein I. M. Omega-oxidation is the major pathway for the catabolism of leukotriene B4 in human polymorphonuclear leukocytes. J Biol Chem. 1984 Aug 25;259(16):10181–10187. [PubMed] [Google Scholar]
- Shak S. Leukotriene B4 catabolism: quantitation of leukotriene B4 and its omega-oxidation products by reversed-phase high-performance liquid chromatography. Methods Enzymol. 1987;141:355–371. doi: 10.1016/0076-6879(87)41083-5. [DOI] [PubMed] [Google Scholar]
- Taniguchi H., Taki F., Takagi K., Satake T., Sugiyama S., Ozawa T. The role of leukotriene B4 in the genesis of oxygen toxicity in the lung. Am Rev Respir Dis. 1986 May;133(5):805–808. [PubMed] [Google Scholar]
- Taylor G. W., Morris H. R. Lipoxygenase pathways. Br Med Bull. 1983 Jul;39(3):219–222. doi: 10.1093/oxfordjournals.bmb.a071822. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Waite B. M., Thomas M. J., DeChatelet L. R. Release and metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1981 Jul 25;256(14):7228–7234. [PubMed] [Google Scholar]
- Warshawski F. J., Sibbald W. J., Driedger A. A., Cheung H. Abnormal neutrophil-pulmonary interaction in the adult respiratory distress syndrome. Qualitative and quantitative assessment of pulmonary neutrophil kinetics in humans with in vivo 111indium neutrophil scintigraphy. Am Rev Respir Dis. 1986 May;133(5):797–804. [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Westcott J. Y., Stenmark K. R., Murphy R. C. Analysis of leukotriene B4 in human lung lavage by HPLC and mass spectrometry. Prostaglandins. 1986 Feb;31(2):227–237. doi: 10.1016/0090-6980(86)90049-3. [DOI] [PubMed] [Google Scholar]
- Wightman P. D., Raetz C. R. The activation of protein kinase C by biologically active lipid moieties of lipopolysaccharide. J Biol Chem. 1984 Aug 25;259(16):10048–10052. [PubMed] [Google Scholar]
- Williams J. D., Lee T. H., Lewis R. A., Austen F. Intracellular retention of the 5-lipoxygenase pathway product, leukotriene B4, by human neutrophils activated with unopsonized zymosan. J Immunol. 1985 Apr;134(4):2624–2630. [PubMed] [Google Scholar]
- Wynkoop E. M., Broekman M. J., Korchak H. M., Marcus A. J., Weissmann G. Phospholipid metabolism in human neutrophils activated by N-formyl-methionyl-leucyl-phenylalanine. Degranulation is not required for release of arachidonic acid: studies with neutrophils and neutrophil-derived cytoplasts. Biochem J. 1986 Jun 15;236(3):829–837. doi: 10.1042/bj2360829. [DOI] [PMC free article] [PubMed] [Google Scholar]


