Abstract
A previously unreported 26-membered polyene macrocyclic lactam, sceliphrolactam, was isolated from an actinomycete, Streptomyces sp., associated with the mud dauber, Sceliphron caementarium. Sceliphrolactam’s structure was determined by 1D- and 2D-NMR, MS, UV, and IR spectral analysis. Sceliphrolactam displays antifungal activity against amphotericin B-resistant Candida albicans (MIC = 4 μg/mL, 8.3 μM).
Exploring the chemistry of insect-bacterial mutualisms has recently provided new natural products with medically relevant biological activity. We have, for example, previously reported studies of a fungus-growing ant (Apterostigma dentigerum), its bacterial symbiont (Pseudonocardia sp.), and dentigerumycin, a bacterially produced depsipeptide with selective antifungal activity,(1) and the southern pine beetle (Dendroctonus frontalis), its bacterial mutualist (Streptomyces sp.), and mycangimycin, a polyene peroxide.(2) Further studies of the bacterial symbionts of the southern pine beetle system resulted in the identification of new polycyclic tetramate macrolactams, the frontalamides and their unusual biosynthetic gene cluster.(3)
Insect-bacterial mutualisms seem widespread in nature and phylogenetically diverse insects have developed symbiotic relationships with actinomycetes such as Streptomyces and Pseudonocardia, which are known to be prolific producers of bioactive secondary metabolites.(4) In related studies, the Kaltenpoth and Svatoš laboratories reported that a solitary hunting wasp (Philanthus triangulum) harbors a bacterial mutualist Streptomyces sp. in its antennae that produces small molecules that likely protect the wasps’ cocoons from fungal infestation to enhance larval survival.(5) In these three examples—a fungus-farming ant, a bark beetle, and a wasp—the bacteria were housed in specialized anatomical features that suggest a coevolutionary adaptation. In addition to these examples, other studies on bacteria-insect systems lacking anatomical evidence of adaptation have identified Streptomyces that produce small molecules.(6) Although the isolation of Actinobacteria that produce antibiotics in vitro does not prove that these bacterially produced small molecules play a role for the insect host from which the bacteria were derived, these insect-associated actinomycetes represent unexplored sources for natural product discovery. This report deals with bacteria isolated from a mud dauber’s exoskeleton and their secondary metabolites.
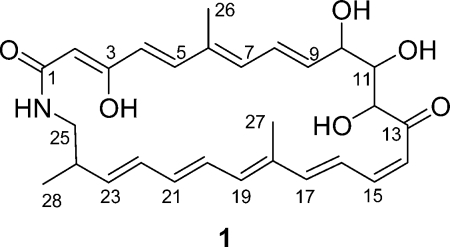
Since mud daubers, like the solitary wasps P. triangulum, construct nest burrows in soil and provide insect prey for the developing larvae, they should be good candidates for symbiotic associations with antibiotic producing bacteria. We investigated the actinomycetes associated with the black and yellow mud dauber, Sceliphron caementarium, collected in Wisconsin.(7) Chemical analysis of the secondary metabolites produced by the actinomycetes associated with S. caementarium led to a macrocyclic lactam, sceliphrolactam (1), with pronounced antifungal activity. Here we report the detailed production, isolation, structural determination, and biological activity of sceliphrolactam, a rare example of polyene macrocyclic lactams.
The crude extracts of 12 cultured Streptomyces strains, isolated from the exoskeleton of the wasp, S. caementarium, were screened by LC/MS. This preliminary survey revealed that two strains, e113 and e122, produced the molecule we eventually named sceliphrolactam (1). Compound 1 had a polyene signature in its UV (maxima at 333 and 420 nm). Its UV signature and low-resolution mass ([M + H]+ at m/z 482) spectrum, which did not match any previously reported compound, prompted us to purify and characterize it further.
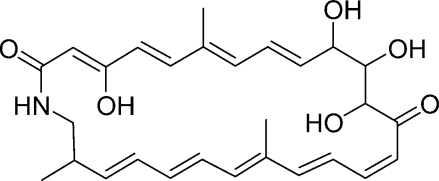
Sceliphrolactam (1)(8) was isolated as a yellow powder, with the molecular formula C28H35NO6 (11 double-bond equivalents) as derived from ESI high-resolution mass spectrometry ([M + H]+ at m/z 482.2556, calculated 482.2543) and 1H and 13C NMR spectral data. The 1H NMR spectrum displayed 15 olefinic protons from 7.38 to 4.97 ppm, along with two allylic methyl groups (δH 1.78 and 1.58), indicating that sceliphrolactam had multiple double bonds. In addition to the multiple double bond protons and allylic methyl groups, sceliphrolactam showed three carbinol protons (δH 4.24, 4.04, 3.78), three aliphatic protons (δH 3.05, 2.96, 2.22), one aliphatic methyl group (δH 0.99) and five D2O exchangeable protons (δH 13.67, 7.70, 4.95, 4.75, 4.71). The 13C NMR spectrum showed two carbonyl carbons at 201.0 and 173.3 ppm, eighteen olefinic carbons between 166.3 and 94.5 ppm, three oxygen bearing carbons at 80.3, 75.0, and 70.7 ppm, and five aliphatic carbons in the upfield (44.4 to 12.7 ppm). Together, these data indicate that sceliphrolactam (1) has 9 double bonds and 2 carbonyls, which account for 10 out of the 11 degrees of unsaturation required by the molecular formula, so sceliphrolactam must contain a single ring.
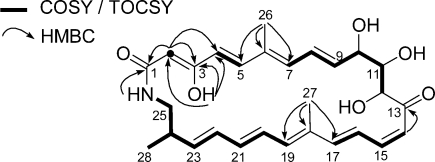
Analysis of the gHSQC NMR spectrum assigned 15 olefinic methines, 3 oxygenated methines, 1 aliphatic methine, 1 aliphatic methylene, and 3 methyl groups. Consequently, 3 olefinic carbons out of 18 must be quaternary. Interpretation of gCOSY and TOCSY NMR spectra revealed 5 distinct spin systems besides 2 methyl groups attached to quaternary olefinic carbons (C-26 and C-27). These discrete spin systems span C-2 alone, C-4 to C-5, C-7 to C-12, C-14 to C-17, and C-19 to 25-NH (Figure 1). An H-2 singlet at 4.97 ppm formed an isolated methine moiety. The COSY correlation between H-4 (δH 5.83) and H-5 (δH 6.52) revealed C-4 to C-5 connectivity. The 1H−1H three bond couplings from H-7 to H-12 observed in the COSY and the TOCSY experiments established another spin system from C-7 to C-12. The COSY and the TOCSY correlations among H-14, 15, 16, 17 showed the fourth spin system from C-14 to C-17. The last spin system, which spanned C-19 to C-25 including a branch methyl group (C-28), was identified by its 1H−1H couplings.
Figure 1.
Key COSY, TOCSY, and HMBC correlations establishing the structure of sceliphrolactam (1).
The connectivities of these discrete spin systems were secured by 13C chemical shift, gHMBC, and ROESY correlation analysis. The relatively upfield chemical shifts of H-2 and C-2 (δH 4.97; δC 94.5) indicated an α,β-unsaturated ketone system with an electron withdrawing group at the β-position. The hydrogen-bonding hydroxy proton (δH 13.67) showed HMBC correlations to C-2, C-3, and C-4, which positioned the hydroxy group at the deshielded C-3 (δC 166.3). HMBC correlations from H-2 to a carbonyl carbon (C-1; δC 173.3), C-3, and C-4 fit an α,β-unsaturated ketone with a hydroxy group at the β-position. This fragment also secured the connectivity of this moiety to the C-4 to C-5 spin system. Long-range heteronuclear couplings from methyl H3-26 (δH 1.78) to C-5, C-6, and C-7 allowed the assignment of this methyl group at C-6 and the elongation of the chain from C-5 to the C-7−C-12 spin system, which specified the tetraenone and triol moiety. HMBC correlations from the allylic methyl protons (H3-27; δH 1.58) to C-17, C-18, and C-19 assigned this methyl group at the quaternary carbon C-18 and the connectivity of the C-14 to C-17 chain to the C-19 − 25-NH system. The 1H−13C coupling in HMBC from H-14 to the other carbonyl carbon C-13 (δC 201.0) assigned the carbonyl carbon next to C-14, thus establishing the pentaenone system.
Sceliphrolactam’s macrocyclic ring connectivity was determined by the HMBC correlation from 25-NH (δH 7.70) to the carbonyl carbon C-1. The amide functionality was also supported by an IR absorption at 1661 cm−1. The HMBC correlation between H-12 and C-13 was not observed but the connectivity between C-12 and C-13 was deduced based on the molecular formula after connecting other partial structures. This analysis was also supported by a ROESY correlation between H-12 and H-14.
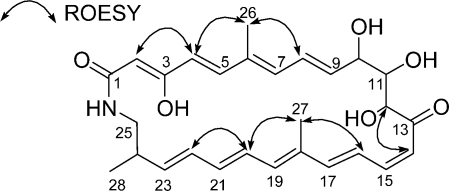
The double bond geometries in 1 were established from the 1H−1H coupling constants and ROESY correlations (Figure 2). A ROESY coupling between H-2 and H-4 established a 2Z geometry. The trans-coupling constant (15.0 Hz) between H-4 and H-5 determined 4E. ROESY correlations between H3-26 and H-4 and H-8 required 6E. Another 1H−1H trans-coupling (14.5 Hz) between H-8 and H-9 revealed 8E. The 14Z geometry was established by the cis-coupling constant (12.0 Hz) of H-14 and H-15. A large coupling constant (15.0 Hz) between H-16 and H-17 determined their 16E geometry. ROESY couplings from H3-27 to H-16 and H-20 secured an 18E geometry. ROESY correlations between H-20 and H-22 and between H-21 and H-23 revealed 20E. The last double bond geometry was determined as 22E by the 15.0 Hz H-22 − H-23 coupling constant. Overall, the structure of 1 turned out to be a previously unreported polyunsaturated and polyoxygenated 26-membered macrocyclic lactam.
Figure 2.
Key ROESY correlations determining the double bond geometries and connectivity of sceliphrolactam (1).
As might be expected from the structure, sceliphrolactam is an extremely fragile molecule, particularly when exposed to light, elevated temperatures, or Lewis acids. Even with extensive precautions, it degrades so quickly that the compound changes from yellow to colorless, which indicates destruction of the extensive conjugated systems, within a few hours. Sceliphrolactam also degrades, albeit slowly, during overnight NMR experiments. All attempts to determine the complete stereochemistry of 1 resulted in degradation and the configurations of the stereogenic centers at C-10, 11, 12, and 24 have not yet determined. There is a small family of related macrocyclic lactams, and the most closely related are salinilactam (2) from the marine actinomycete Salinispora tropica(9) and macromonosporin A (3) from the acidic peat swamp forest actinomycete Micromonospora sp.(10)
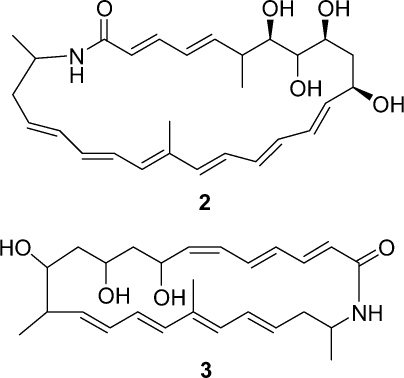
Salinilactam (2) has a 26-membered ring structure analogous to sceliphrolactam (1) but its dienone and hexaene chromophores are quite different from the tetraenone and the pentaenone of 1. In addition to that, salinilactam forms the amide bond with a methine-NH derived from lysine,(9) whereas sceliphrolactam’s amide bond uses a methylene-NH. Macromonosporin A (3) has a 24-membered macrocyclic ring, not a 26-membered ring.(10) No complete stereochemical characterization has been reported for any of these family members.
Because of sceliphrolactam’s lability, its biological activity was evaluated in an antifungal assay that minimized light exposure. Sceliphrolactam inhibited the growth of amphotericin B-resistant Candida albicans with a minimum inhibitory concentration (MIC) of 4 μg/mL (8.3 μM).
The discovery of sceliphrolactam, a previously unreported antifungal polyene macrocyclic lactam, provides additional evidence that insect-associated bacterial communities are a promising source of new bioactive natural products.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2009-0083533) to D.-C.O., the Carlsberg Foundation to M.P., NSF grant DEB-0747002 and NIH grant GM096347 to C.R.C., and CA24487, GM086258, and GM096347 to J.C.
Supporting Information Available
Experimental section and 1H, 13C, gCOSY, TOCSY, ROESY, gHSQC, and gHMBC NMR spectra of 1. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Currie C. R.; Scott J. A.; Summerbell R. C.; Malloch D. Nature 1999, 398, 701. [Google Scholar]; b Oh D.-C.; Poulsen M.; Currie C. R.; Clardy J. Nat. Chem. Biol. 2009, 5, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Scott J. J.; Oh D.-C.; Yuceer M. C.; Klepzig K. D.; Clardy J.; Currie C. R. Science 2008, 322, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Oh D.-C.; Scott J. J.; Currie C. R.; Clardy J. Org. Lett. 2009, 11, 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett J. A. V.; Oh D.-C.; Cao S.; Currie C. R.; Clardy J. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdy J. J. Antibiot. 2005, 58, 1. [DOI] [PubMed] [Google Scholar]
- a Kaltenpoth M.; Goettler W.; Herzner G.; Strohm E. Curr. Biol. 2005, 15, 475. [DOI] [PubMed] [Google Scholar]; b Kroiss J.; Kaltenpoth M.; Schneider B.; Schwinger M.-G.; Hertweck C.; Maddula R. K.; Strohm E.; Svatoš A. Nat. Chem. Biol. 2010, 6, 261. [DOI] [PubMed] [Google Scholar]
- Haeder S.; Wirth R.; Herz H.; Spiteller D. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen M.; Oh D.-C.; Clardy J.; Currie C. R.. PLoS One, accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sceliphrolactam (1): yellow powder; [α]D = −213 (c 0.09, MeOH); IR (neat) νmax 3295, 2955, 1713, 1661, 1555, 1531, 1455 cm−1; UV (MeOH) λmax (log ε) 333 (3.69), 420 (3.16) nm; NMR spectral data, see Table 1; HR-ESI-TOFMS [M + H]+m/z 482.2556 (C28H35NO6) calcd [M + H]+ 482.2543.
- Udwary U. W.; Zeigler L.; Asolkar R. N.; Singan V.; Lapidus A.; Fenical W.; Jensen P.; Moore B. S. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thawai C.; Kittakoop P.; Tanasupawat S.; Suwanborirux K.; Sriklung K.; Thebtaranonth Y. Chem. Biodivers. 2004, 1, 640. [DOI] [PubMed] [Google Scholar]
Table 1. NMR Data for Sceliphrolactam (1) in DMSO-d6.
| C/H | δHa | mult (J in Hz) | δCb | |
|---|---|---|---|---|
| 1 | 173.3 | C | ||
| 2 | 4.97 | s | 94.5 | CH |
| 3 | 166.3 | C | ||
| 3-OH | 13.67 | s | ||
| 4 | 5.83 | d (15.0) | 122.2 | CH |
| 5 | 6.52 | d (15.0) | 138.6 | CH |
| 6 | 134.9 | C | ||
| 7 | 5.85 | d (11.0) | 136.4 | CH |
| 8 | 6.38 | dd (14.5, 11.0) | 129.0 | CH |
| 9 | 5.40 | dd (14.5, 7.5) | 137.1 | CH |
| 10 | 4.04 | dd (9.0, 7.5) | 70.7 | CH |
| 10-OH | 4.75 | s | ||
| 11 | 3.78 | br. d (9.0) | 75.0 | CH |
| 11-OH | 4.71 | s | ||
| 12 | 4.24 | br. s | 80.3 | CH |
| 12-OH | 4.95 | s | ||
| 13 | 201.0 | C | ||
| 14 | 6.20 | m | 121.2 | CH |
| 15 | 6.60 | dd (12.0, 11.5) | 144.3 | CH |
| 16 | 7.38 | dd (15.0, 12.0) | 126.3 | CH |
| 17 | 6.62 | d (15.0) | 147.0 | CH |
| 18 | 135.2 | C | ||
| 19 | 6.22 | m | 136.7 | CH |
| 20 | 6.20 | m | 136.4 | CH |
| 21 | 6.16 | m | 128.0 | CH |
| 22 | 5.80 | dd (15.0, 11.0) | 132.3 | CH |
| 23 | 5.45 | dd (15.0, 8.5) | 138.1 | CH |
| 24 | 2.22 | m | 40.1 | CH |
| 25a | 3.05 | m | 44.4 | CH2 |
| 25b | 2.96 | m | ||
| 25-NH | 7.70 | dd (6.5, 6.0) | ||
| 26 | 1.78 | s | 12.7 | CH3 |
| 27 | 1.58 | s | 12.7 | CH3 |
| 28 | 0.99 | d (6.5) | 16.8 | CH3 |
600 MHz.
150 MHz.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.