Abstract
Inhibitory antibodies to factors VIII or IX represent a serious complication for hemophilia patients. Treatment involves products that bypass the intrinsic pathway and promote thrombin generation. Direct infusion of factor Xa should also restore hemostasis; however, it has a short half-life in plasma and could activate systemic coagulation in an uncontrolled fashion. Here we show that factor Xa mutants with zymogen-like properties (FXaI16L and FXaV17A) circumvent these limitations. In the absence of factor Va, the FXa variants are poor enzymes for a range of physiological ligands and are resistant to inactivation by antithrombin III and tissue factor pathway inhibitor. Notably, assembly of FXaI16L and FXaV17A on activated platelets with factor Va to form prothrombinase completely restores biologic activity. In hemophilic plasma, FXaI16L and FXaV17A have prolonged half-lives compared with wild-type factor Xa (approximately 60 minutes vs approximately 1 minute) and promote robust thrombin generation that bypasses the intrinsic pathway. The variants require factor Va generated in situ for procoagulant function, and cofactor inactivation by the protein C pathway regulates their activity. The efficacy, extended half-life, and mechanism of action suggest that novel zymogen-like forms of factor Xa might prove useful as new therapeutic procoagulants to treat deficiencies upstream of the common pathway.
Introduction
The blood coagulation zymogen factor X (FX) occupies a central position in the coagulation cascade. It is the principal physiological substrate for the extrinsic (tissue factor [TF]/FVIIa) and intrinsic (FVIIIa/FIXa) tenase complexes, thus linking these 2 pathways.1 These enzymes cleave FX at a conserved site (between Arg15 and Ile16; chymotrypsin numbering2), thereby liberating an activation peptide and a new N-terminus (Ile16-Val-Gly-Gly.). The α-amino group from Ile16 in the heavy chain forms a salt bridge with Asp194, which drives a conformational change that leads to maturation of the catalytic domain and imparts function.3,4 For FX/FXa, the zymogen to protease transition not only drives catalytic site activation but also contributes to the formation of the FVa binding site.5,6 Once FXa binds FVa on an anionic membrane surface in the presence of Ca2+ ions to form prothrombinase, the macromolecular enzyme complex rapidly converts prothrombin to thrombin.1 Although free FXa catalyzes this reaction, prothrombinase is considered the physiologically relevant enzyme. In addition to its role in prothrombinase, FXa activates other coagulation factors including FV, FVIII, and FVII and may play a role in activating protease activated receptors (PARs).7–10 Regulation of FXa biologic activity is mediated in part through its tight interaction with tissue factor pathway inhibitor (TFPI) with subsequent inhibition of FVIIa/TF and by its rapid and essentially irreversible inhibition by serpins including antithrombin III (ATIII).11,12
Hemophilia is characterized by a congenital deficiency of FVIII (hemophilia A [HA]) or FIX (hemophilia B [HB]) and results in an inadequate production of FXa and ultimately thrombin.13 The mainstay of hemophilia therapy is intravenous replacement of the missing or dysfunctional FVIII or FIX with either plasma-derived or recombinant protein products. Unfortunately, approximately 20%-30% of patients with FVIII deficiency and approximately 3%-5% of patients with FIX deficiency produce inhibitory antibodies to the infused protein products.14 This has prompted the development of bypass strategies that use other coagulation factors to restore hemostasis in these patients, including recombinant FVIIa and activated prothrombin complex concentrates, which contains a mixture of prothrombin complex zymogens and their activation products.15,16 Although effective in most cases, their efficacy can vary, and in some circumstances these products can increase the morbidity of the disease.17,18 Furthermore, these products are costly, and some are derived from plasma with risk of blood borne disease.
In principle, wild-type (wt)-FXa should be the ideal candidate to bypass deficiencies in the intrinsic pathway because it is the enzyme product that is lacking in hemophilia. However, there are serious limitations inherent to FXa that make it unviable. For example, it is rapidly inactivated by plasma protease inhibitors, resulting in a short half-life.19,20 In addition, the FXa that escapes inhibition could cause excessive activation of systemic coagulation through the direct activation of procoagulant coagulation factors (eg, FV and FVIII), further limiting its utility. Previous attempts using FXa either alone or in combination with phospholipids in a hemophilic dog model have borne out these limitations.21,22 Recently, our laboratory has characterized variants of FXa that could circumvent these associated problems.6 These FXa derivatives have a mutation at either position 16 or 17 at the N-terminus of the heavy chain (eg, FXaI16L and FXaV17A). Biochemical studies revealed that these mutations destabilize the normal zymogen to protease conversion process. As a consequence, these proteins have “zymogen-like” properties characterized by the free enzyme having low activity resulting from altered active site binding and function. Surprisingly, activity can be rescued with saturating concentrations of FVa, and once assembled into prothrombinase, the FXa variants function comparably to wt-FXa. Based on these features, we speculate that zymogen-like forms of FXa should act as long-lived proteases in circulation that are otherwise functionally inert in the absence of FVa but retain the ability to catalyze thrombin formation upon FVa binding on an activated cellular surface. In the current study, we explore this possibility using in vitro systems of increasing complexity and examine the potential of zymogen-like FXa variants to serve as procoagulants to bypass deficiencies in the intrinsic pathway.
Methods
Reagents
The peptidyl substrate methoxycarbonyl-cyclohexylglycyl-glycyl-arginine-ρ-nitroanilide (spectrozyme FXa [SpecXa]) was from American Diagnostica, and H-D-phenylalanyl-pipecolyl-arginine-ρ-nitroanilide (S-2238) was purchased from Diapharma Group Inc. Substrate solutions were prepared in water, and concentrations were verified using E342 = 8270 M−1 cm−1.23 The inhibitors benzamidine and APMSF (4-amidinophenylmethanesulfonyl fluoride hydrochloride) were from Sigma-Aldrich, and DAPA [dansylarginine-N-(3-ethyl-1,5-pentanediyl)amide] was from Haematologic Technologies. All tissue-culture reagents were from Invitrogen except insulin-transferrin-sodium selenite, which was from Roche. Small unilamellar phospholipid vesicles (PCPS) composed of 75% (wt/wt) hen egg L-α-phosphatidylcholine and 25% (wt/wt) porcine brain L-α-phosphatidylserine (Avanti Polar Lipids) were prepared and characterized as described previously.24 Technothrombin thrombin calibrator and thrombin generation assay reagent RB was purchased from Diapharma Group Inc. The fluorogenic substrate Z-Gly-Gly-Arg-AMC was purchased from Bachem Bioscience Inc, prepared in 15mM CaCl2, and its concentration verified using E326 = 17 200 M−1 cm−1. Normal human pooled plasma (NHP) and factor deficient plasmas from individual donors used throughout the study were purchased from George King Biomedical, Inc. Automated activated partial thromboplastin time reagent (aPTT; TriniClot) was from Trinity Biotech.
Proteins
Human prothrombin, FX, and FV were isolated from plasma as described previously.25,26 Thrombin was obtained from Haematologic Technologies and hirudin was from EMD Biosciences. The FX activator from Russell viper venom (RVVX-CP) was purified as described previously.27 Human plasma-derived FVa or recombinant FVa was prepared by proteolytic activation of FV or FV-810 by thrombin and purified as described previously.28 Recombinant wt-FX and 2 zymogen-like variants (FXI16L and FXV17A) were prepared, purified, and characterized as previously described.5,6 Recombinant and plasma-derived FX were activated with RVVX-CP and purified as described previously.5,6 Human activated protein C (APC), ATIII, soluble thrombomodulin (sTM), and 2 domain TFPI (residues 13-161) were obtained from Dr. Sriram Krishnaswamy (Children's Hospital of Philadelphia). Molecular weights and extinction coefficients (E0.1% 280 nm) of the various proteins used were taken as follows: RVVX-CP, 93 000 and 1.18;29 prothrombin, 72 000 and 1.47;30 thrombin, 37 500 and 1.94;31 FVa, 173,000 and 1.78;28 FX, 56 000 and 1.16;32 FXa, 45 300 and 1.16;32 and ATIII, 58 000 and 0.62.33 The concentration of TFPI was determined using Mr = 17 400 and E0.1% 280 nm = 0.45, calculated from the primary structure and by the method of Gill and von Hippel.34 Unless otherwise noted, all functional assays were performed at 25°C in 20mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 0.15M NaCl, 2mM CaCl2, 0.1% polyethylene glycol 8000, pH 7.4 (assay buffer).
Inhibition of FXa by ATIII
The rate of inactivation of FXa by ATIII was measured under pseudo-first order rate conditions at 25°C as described previously.5 Reactions were prepared in assay buffer containing 0-10μM ATIII with 2nM wt-FXa or 10nM variant (FXaI16L or FXaV17A). After 0-90 minutes, residual enzyme activity remaining as a function of time was determined after the addition of 0.1mM SpecXa and monitoring the initial steady state increase in absorbance at 405 nm.
Inhibition of FXa by TFPI
The overall dissociation constant (Ki*) for TFPI binding to FXa were inferred from measurements of residual enzyme amidolytic activity. Reactions were prepared in assay buffer containing either 0.2 or 0.4nM wt-FXa or 2 or 4nM variant (FXaI16L or FXaV17A) with increasing concentrations of TFPI. After incubation for 3 hours at 25°C, residual enzyme activity was determined after the addition of 0.1mM SpecXa and monitoring the initial steady state increase in absorbance at 405 nm.
Prothrombin activation on isolated human platelets
Human platelets were isolated from freshly collected whole blood as previously described.35 Institutional review board approval was given by the Children's Hospital Research Institute for isolating human platelets from blood drawn from subjects. Prothrombinase-catalyzed prothrombin activation using thrombin-activated platelets was determined as previously described.35 Platelets (1 × 108/mL) in 20mM HEPES-Tyrode buffer, pH 7.4, were activated with 20nM thrombin for 3 minutes at 25°C followed by the addition of 30nM hirudin. Activated platelets were then incubated with prothrombin (0.1-1.4μM), DAPA (3.0μM), ± FVa 10nM), and reactions were initiated with FXa (0.1nM). At various time points (0, 0.5, 1.0, 1.5, and 2.0 minutes), aliquots were removed and initial rates of thrombin generation were determined as previously described using S-2238.5
Activation of physiological ligands by FXa
The activation of various physiological ligands by FXa was assessed by gel electrophoresis. Factor V (400nM), B-domainless FVIII (600nM), or rFVII (2μM) were incubated with PCPS (50μM) and increasing concentrations of wt-FXa or variant FXa (0.5-100nM). After 10 minutes incubation at 25°C, samples (4 μg/lane) were prepared for electrophoresis using 4%-12% gradient gels (Invitrogen) under reducing conditions using the MOPS [3-(N-morpholino)propanesulfonic acid] or MES [2-(N-morpholino)ethanesulfonic acid] buffer system followed by staining using Coomassie Brilliant Blue R-250.
FXa-specific clotting assays
FXa procoagulant or bypassing activity was measured using a modified aPTT in hemophilic plasma. In a typical assay, 50 μL of HA, HB, or HA plasma with inhibitors was mixed with an equal volume of aPTT reagent followed by a 180-second incubation period at 37°C. A 50-μL mixture of FXa (0.1nM) with or without FVa (10nM) in assay buffer was then added to the solution and coagulation was initiated after the addition of 50 μL of 25mM CaCl2. Time to clot formation was measured using a Start4 coagulation instrument (Diagnostica Stago Inc). For half-life determinations, FXa (20nM, final) was incubated in hemophilic plasma at room temperature, and at various time points aliquots of the reaction were diluted (0.1nM FXa, final) in assay buffer. Residual FXa bypassing activity was assessed as detailed above in this section.
Thrombin generation assays
Thrombin generation in platelet poor plasma was determined according to methods previously described with some modifications.36 Normal or hemophilic plasma was mixed with a TF-phospholipid reagent (10 μL; Technothrombin RB; 2pM TF/4.0μM phospholipid) in a black microtiter plate (Nunc; F16 black Maxisorp). Where indicated, 5 μL of bypassing protease (FXa, zymogen-like FXa, or FVIIa) in dilution buffer (20mM HEPES, 150mM NaCl, 0.1% PEG-8000, pH 7.4) was added to the microtiter plate followed by the immediate addition of Z-Gly-Gly-Arg-AMC in 15mM CaCl2 (50 μL; 0.5mM, final). For experiments in which the protein C pathway was introduced into the thrombin generation assay, serial dilutions of sTM or APC were prepared in hemophilic plasma just before the addition of the TF-phospholipid reagent. Fluorescence was measured over 90 minutes at 37°C in a Spectramax M2e (Molecular Devices) with excitation and emission wavelengths set at 360 nm and 460 nm, respectively. Raw fluorescence values were compared with a thrombin calibration curve generated using a thrombin calibrator (Technothrombin thrombin generation assay calibrator) to convert the signal to nM of thrombin. Thrombin generation curves (nM thrombin vs. time) were analyzed to extract lag time, peak height, and endogenous thrombin potential (ETP). ETP is the area under the thrombin generation curve and represents a measure of the total amount of thrombin generated in plasma.
Data analysis
Data were analyzed according to the referenced equations by nonlinear least squares regression. Reported estimates of error represent plus or minus 2 standard deviations (SDs). The qualities of the fits were assessed by the criteria described.37 Initial velocity measurements of prothrombin activation by prothrombinase were analyzed by fitting the data to the Henri-Michaelis-Menten equation to yield fitted values for Km and Vmax.38 The rate of inhibition of FXa or prothrombinase by ATIII was measured under pseudo-first order rate conditions, and the second-order rate constant was calculated by dividing the pseudo-first order rate constant (kobs) by the concentration of ATIII as previously described.39 Determination of the overall equilibrium dissociation constant (Ki*) for TFPI binding to FXa was determined according to equations previously described.40
Results
Characterization of FXa variants using physiological ligands
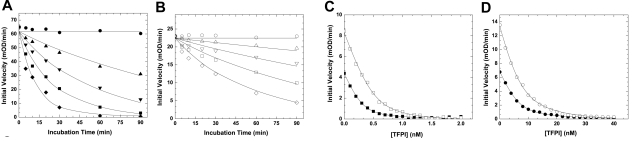
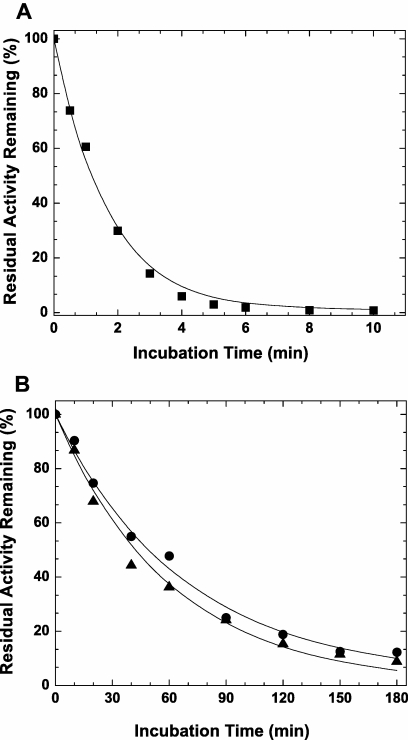
wt-FXa is rapidly inactivated in vivo by ATIII and TFPI, which neutralize the enzyme largely via active site binding interactions.19,20 Kinetic studies using purified proteins showed that the inhibition of FXaI16L by ATIII was substantially altered relative to wt-FXa (Figure 1A-B and Table 1). Analysis of the data for FXaI16L and FXaV17A revealed that the second order rate constant for inhibition was reduced by 25- to 40-fold relative to wt-FXa. Similarly, the variants were also resistant to TFPI resulting from weak binding of the inhibitor to the mutant proteases (Ki* increased by approximately 20-fold; Figure 1C-D and Table 1). Together, these results indicate that the active site of the zymogen-like variants is not readily accessible to the physiological inhibitors, making them partially resistant and potentially protected from inactivation once introduced into the circulation.
Figure 1.
Inhibition of FXa by ATIII and TFPI. The rate of inactivation of FXa by ATIII was measured under pseudo-first order conditions. Antithrombin III (A, ●, 0; ▴, 0.1; ▾, 0.25; ■, 0.50; and ♦, 1.0μM; B, ○, 0; ▵, 1.0; ▿, 2.5; □, 5.0; and ◇, 10μM) was incubated with FXa (A, 2.0 nM wt-FXa; B, 10.0nM FXaI16L) in assay buffer for up to 90 minutes. At various time points, residual enzyme activity was measured using the chromogenic substrate SpecXa. For the inhibition of FXa by TFPI, wt-FXa (C, 0.2 or 0.4nM,) was incubated with increasing concentrations of TFPI (0.1-2.0nM), whereas FXaI16L (D, 2.0 or 4.0nM) was incubated with higher concentration of TFPI (1.0-40nM) for 3 hours at 25°C. Residual enzyme activity was determined after the addition of SpecXa. The lines are drawn following analysis to equations detailed in “Methods,” and the fitted values are given in Table 1. The data are representative of 2 to 3 similar experiments.
Table 1.
Inhibition kinetics of FXa or prothrombinase
| Inhibitor |
||
|---|---|---|
| Antithrombin III, k2 ± SD × 103, M−1 s−1 | TFPI, Ki*, nM | |
| Free FXa | ||
| wt-FXa | 1.28 ± 0.060 | 0.07 ± 0.012 |
| FXaI16L | 0.03 ± 0.006 | 1.86 ± 0.340 |
| FXaV17A | 0.05 ± 0.002 | 1.16 ± 0.120 |
| Prothrombinase | ||
| wt-FXa | 0.18 ± 0.010 | ND |
| FXaI16L | 0.09 ± 0.006 | ND |
| FXaV17A | 0.05 ± 0.003 | ND |
The errors in the fitted constants represent ± 2 SDs. The data are representative of 2-3 independent measurements. Inhibition kinetics of FXa alone or prothrombinase were determined from initial velocity studies conducted with peptidyl substrate, Spec Xa. Details of the experimental design can be found in “Methods.”
ND, not determined.
In contrast to these data with the free enzyme, incorporation of FXaI16L or FXaV17A into prothrombinase altered the active site of the proteins such that the level of inhibition by ATIII was now comparable with wt-FXa (Table 1). However, the data show that the second order rate constants for the inhibition of prothrombinase (with wt or variant FXa) versus free FXa is markedly reduced. These data are in general agreement with prior reports suggesting that prothrombinase is poorly inhibited by ATIII.41,42
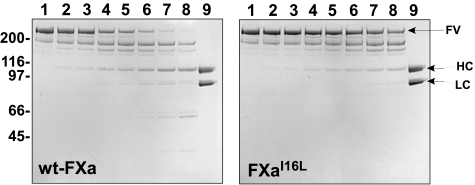
Consistent with the idea that FXaI16L or FXaV17A have altered active site structure and function in the absence of FVa, the ability of these proteins to activate a range of physiological ligands was also suboptimal. Specifically, we found that the rate of FV cleavage with FXaI16L in the presence of phospholipids was markedly reduced compared with wt-FXa (Figure 2). Densitometric analysis after the formation of the heavy and light chains revealed that the rate of FV cleavage by FXaI16L was reduced by approximately 20- to 40-fold. Similarly, the rate of rFVIII, FVII, and prothrombin activation by FXaI16L were all reduced by more than 20-fold (data not shown). These data indicate that in their free states, zymogen-like FXa variants interact poorly with a range of physiological ligands.
Figure 2.
Activation of FV by membrane-bound FXa. Plasma-derived FV (400nM) was incubated with increasing concentrations of wt-FXa or FXaI16L in the presence of PCPS (50μM) for 10 minutes at 25°C. Samples (4 μg/lane) were then resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis and visualized by staining with Coomassie Blue R-250. Lane 1, platelet-derived (pd)-FV, no FXa; lanes 2-8, FXa (wt or variant) at 0.5, 1.0, 5.0, 10, 20, 50, and 100 nM; lane 9, recombinant FVa. The apparent molecular weights of the standards are indicated on the left. HC, heavy chain; LC, light chain. The data are representative of 2 similar experiments.
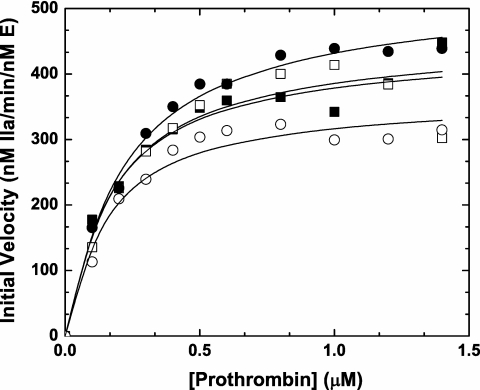
Prothrombin activation on thrombin-activated human platelets
Previous initial velocity studies using synthetic anionic phospholipids vesicles (PCPS) and saturating amounts of FVa revealed that the zymogen-like variants and wt-FXa activate prothrombin with similar kinetics.6 To extend these results to a more physiological surface, we pursued experiments using thrombin activated platelets. Assembly of FXaI16L (or FXaV17A, data not shown) on 1 × 108/mL thrombin activated platelets in the presence of added FVa yielded rates of thrombin generation and kinetic constants that were comparable with wt-FXa (Figure 3 closed symbols). Interestingly, similar results were obtained even in the absence of added FVa (Figure 3 open symbols), indicating that FV/FVa release from the α-granules of thrombin-activated platelets was sufficient to rescue FXaI16L. Although the absolute values differed from donor to donor, comparable results were obtained when using activated platelets (± added FVa) from multiple people with the following average values (± SD; n = 4) for Km and kcat: wt-Xa +FVa: Km = 0.32 ± 0.18μM and kcat = 560 ± 70 minutes−1; wt-Xa: Km = 0.17 ± 0.01μM and kcat = 500 ± 70 minutes−1; FXaI16L +FVa: Km = 0.25 ± 0.04μM and kcat = 560 ± 50 minutes−1; and FXaI16L: Km = 0.35 ± 0.20μM and kcat = 530 ± 160 minutes−1.
Figure 3.
Activation of prothrombin on stimulated platelets. Thrombin-activated human platelets (1 × 108/mL; 20nM thrombin, 3 minutes followed by the addition of 30nM hirudin) were incubated with increasing concentrations of prothrombin (0.1-1.4μM) either in the presence (closed symbols) or absence (open symbols) of 10nM FVa at 25°C. The reaction was initiated with 0.1nM rFXa (squares, wt-FXa; circles, FXaI16L), and aliquots of the reaction mixture were quenched during the initial rate of the reaction (0, 0.5, 1, 1.5, and 2 minutes), and thrombin generation was measured using the chromogenic substrate S-2238. The solid lines were drawn following analysis of all data sets to a rectangular hyperbola with the following fitted parameters: wt-Xa +FVa: Km, 0.18 ± 0.03μM, kcat, 450 ± 21 minutes−1; wt-Xa: Km, 0.17 ± 0.06μM, kcat, 440 ± 36 minutes−1; FXaI16L +FVa: Km, 0.22 ± 0.02μM, kcat, 530 ± 15 minutes−1; FXaI16L: Km, 0.15 ± 0.03μM, kcat, 370 ± 18 minutes−1. The data are representative of 4 similar experiments.
Assessment of coagulation and thrombin generation in hemophilic plasma
Based on the opposing functional states of the free enzyme versus FXaI16L or FXaV17A assembled in prothrombinase, we hypothesized that these variants could be useful to bypass deficiencies in the intrinsic pathway. In control experiments, the addition of wt-FXa (0.1nM) to HA plasma corrected the prolonged aPTT clotting time (from 91 seconds to 29 seconds; normal human plasma, approximately 45 seconds; Table 2). In these experiments, it is important to note that coagulation is immediately initiated after the addition of FXa in an attempt to minimize its inactivation by plasma inhibitors. Introduction of FXaI16L or FXaV17A (0.1nM) to HA plasma shortened the clotting time to approximately 53 seconds and 56 seconds, respectively, and were approximately 20% as effective as wt-FXa. Similar results were obtained using HB plasma and plasma with neutralizing inhibitors against FVIII (Table 2).
Table 2.
Coagulation and thrombin generation assay parameters
| Sample* | aPTT, sec | aPTT + FVa†, sec | ETP, nM min | Time to peak, min | Peak height, nM | t½, min |
|---|---|---|---|---|---|---|
| NHP | 45 ± 1 | 39 ± 1 | 4390 ± 750 | 30 ± 8 | 260 ± 120 | ND |
| HA | 91 ± 2 | 78 ± 2 | NA | NA | NA | ND |
| HA + wt-FXa | 29 ± 1 | 23 ± 1 | 4370 ± 560 | 16 ± 1 | 210 ± 40 | 1.1 ± 0.1 |
| HA + FXaI16L | 53 ± 1 | 32 ± 1 | 4830 ± 400 | 13 ± 1 | 340 ± 30 | 49 ± 5 |
| HA + FXaV17A | 56 ± 2 | 33 ± 1 | 4750 ± 410 | 13 ± 1 | 315 ± 30 | 41 ± 5 |
| HB | 109 ± 3 | 87 ± 2 | NA | NA | NA | ND |
| HB + wt-FXa | 29 ± 1 | 22 ± 1 | 5076 ± 410 | 19 ± 2 | 253 ± 30 | 1.1 ± 0.1 |
| HB + FXaI16L | 55 ± 1 | 31 ± 1 | 5689 ± 250 | 15 ± 1 | 368 ± 20 | 57 ± 7 |
| HB + FXaV17A | 60 ± 1 | 34 ± 1 | 5694 ± 380 | 13 ± 1 | 378 ± 40 | 47 ± 9 |
| HA + I | 125 ± 2 | 105 ± 3 | NA | NA | NA | ND |
| HA + I + wt-FXa | 31 ± 1 | 24 ± 1 | 1930 ± 560 | 28 ± 5 | 70 ± 20 | 0.9 ± 0.1 |
| HA + I + FXaI16L | 68 ± 1 | 35 ± 2 | 3800 ± 300 | 26 ± 6 | 154 ± 5 | 54 ± 7 |
| HA + I + FXaV17A | 72 ± 2 | 37 ± 2 | 3500 ± 280 | 23 ± 4 | 147 ± 6 | 40 ± 9 |
The data are representative of 2-3 independent measurements. The errors in the fitted constants represent ± 2 SDs.
In these experiments 0.1nM FXa was used in all experiments with +FXa.
In these experiments, 10nM FVa was employed.
NHP, normal human plasma; HA, hemophilia A plasma; HB, hemophilia B plasma; HA + I, hemophilia A plasma with inhibitory antibody; NA, not able to determine a value; ND, not determined.
Previous studies indicate that FV activation in hemophilic plasma, depending on reaction conditions, can be delayed.43 If this is the case, it is possible that in our assay system insufficient amounts of activated FV are being generated during the initiation of coagulation leading to suboptimal rescue of the zymogen-like FXa variants. To test this hypothesis, FVa (10nM) was directly introduced into HA plasma along with FXa. As a control, the addition of FVa to HA plasma without FXa had only a minor effect on the clotting time (Table 2); however, the activity of the zymogen-like variants was markedly increased when added with FVa. The aPTT clotting times for FXaI16L and FXaV17A were shortened from approximately 54 seconds to approximately 32 seconds, corresponding to approximately 50% activity relative to wt-FXa (with added FVa) versus approximately 20% in the absence of added FVa; similar results were obtained with HB plasma and HA inhibitor plasma (Table 2). Thus, although the FXa variants have substantial bypassing activity in hemophilic plasma, this can be augmented either by coadministration with exogenous FVa or under conditions that favor enhanced FV activation.
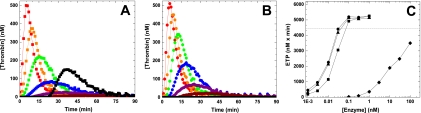
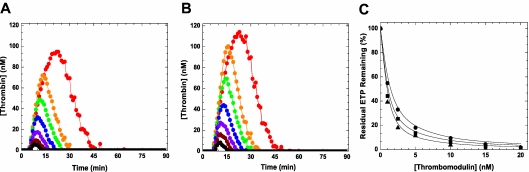
To extend this analysis in another system, we used a thrombin generation assay.36 In this system initiation of coagulation with 2pM TF yields a robust signal with pooled NHP whereas thrombin generation is essentially undetectable using HA plasma (Figure 4). The addition of pM amounts of FXa (wt-FXa, FXaI16L or FXaV17A) was sufficient to restore thrombin generation in HA plasma in a dose-dependent manner (Figure 4 and Table 2). Under these conditions, 0.1nM FXa was able to fully restore the ETP to an extent comparable with NHP. Not surprisingly, the lag time and time to peak were shortened relative to NHP after the addition of FXa. Interestingly, both FXaI16L and FXaV17A appeared to be equally or even slightly more effective on a molar basis than wt-FXa. This could be a result of the possibility that sufficient amounts of FVa are generated during the thrombin generation assay to fully rescue the FXa variants. Alternatively, it could also be related to the different half-lives of the wt and variant FXa in plasma (see below) as there is a time delay (< 1 minute) before the start of the thrombin generation assay. As expected, similar results were obtained using HB plasma and plasma with inhibitors against FVIII (Table 2).
Figure 4.
Influence of FXa on thrombin generation in hemophilic plasma. Thrombin generation was measured for 90 minutes at 37°C in HA plasma supplemented with increasing concentrations (dark red, 0.003; magenta, 0.01; blue, 0.03; green, 0.1; orange, 0.3; and red, 1.0nM) of wt-FXa (A) or FXaI16L (B) in the presence of 2.0pM TF/4μM phospholipid (reagent RB; Technoclone). Thrombin generation was initiated with CaCl2 and a thrombin fluorogenic substrate as detailed in “Methods.” For panel A, the black squares are a representative run of NHP, and for panel B the black circles represent a typical run of HA plasma (essentially undetectable). The endogenous thrombin potential (ETP; C) for each protease is plotted as a function of the protease concentration (wt-FXa (■); FXaI16L (●), rFXaV17A (▴), and rFVIIa (♦). In panel C, the dashed line represents the average ETP obtained for NHP. In each of the panels, the lines are arbitrarily drawn. The data are representative of 3 similar experiments.
Bypass activity of FXa variants compared with recombinant FVIIa
An effective and well-established bypass agent to treat hemophilia patients with inhibitors is recombinant FVIIa (rFVIIa; NovoSeven).15 Direct comparison of wt-FXa and zymogen-like FXa variants with rFVIIa revealed that on a molar basis the zymogen-like FXa variants are at least 1000-fold more effective at restoring thrombin generation in HA plasma (Figure 4C). Like the FXa variants, increasing amounts of rFVIIa shortened the lag time and time to peak (data not shown). These in vitro data provide some measure of how well the FXa zymogen-like variant works, compared with an existing bypass agent, at restoring thrombin generation in hemophilic plasma using a low TF stimulus.
Half-life studies in hemophilic plasma
Because our biochemical data indicate that FXaI16L and FXaV17A are refractory to inhibition by ATIII and TFPI, we evaluated their half-lives in plasma. wt-FXa, FXaI16L, or FXaV17A were incubated in HA plasma for various periods of time, and residual clotting activity was monitored (Figure 5 and Table 2). Consistent with previous reports,19,44 wt-FXa is rapidly inhibited in plasma with a half-life of approximately 1 minute. In contrast, the half-lives of FXaI16L and FXaV17A were markedly prolonged and estimated to be approximately 1 hour. Similar results were obtained using a chromogenic method and a thrombin generation assay to monitor residual FXa activity in hemophilic plasma with half-lives estimated to be approximately 90 minutes for the variants and approximately 1 minute for wt-FXa (data not shown). Taken together, these results suggest that the zymogen-like conformation of FXaI16L and FXaV17A protects the proteins in a plasma environment where there is a high concentration of protease inhibitors.
Figure 5.
Half-life determination of FXa activity in hemophilic plasma. Factor Xa (A, 20nM; wt-FXa, [■]; or B, FXaI16L [●] and FXaV17A [▴]) were incubated at 25°C in HA plasma. At various time points aliquots were removed and diluted to 0.1 nM in assay buffer. Residual FXa activity was determined using an aPTT clotting assay as detailed in the Methods. The solid lines were drawn following analysis of all data sets to an exponential decay function; estimated half-lives are given in the text. The data are representative of 2 to 3 similar experiments.
Regulation of the zymogen-like variants by the PC pathway
Because the FXa zymogen-like variants display significant resistance to plasma inhibitors, an important question that emerges is how would these proteins be regulated in a physiological setting. In vivo, APC cleaves and inactivates FVIIIa and FVa, thereby attenuating the intrinsic and common pathways, respectively. Because our data show that the zymogen-like FXa variants require FVa to be conformationally rescued, we tested the impact of the PC pathway on regulating thrombin generation after a TF-stimulus using FXaI16L and FXaV17A. To reconstitute the PC pathway, either sTM or APC was added directly to HA plasma. Using a FXa (wt or variant) concentration that yielded a robust thrombin generation curve in HA plasma, the addition of increasing amounts of sTM caused a dose-dependent reduction in total thrombin generated (Figure 6). Presumably, these findings result from the ability of sTM to complex with thrombin generated in situ to promote the activation of PC to APC, ultimately leading to the inactivation of FVa. Interestingly, there was minimal influence on the lag time, which is likely because thrombin must first be generated to complex with sTM and yield APC before there is any measurable impact on the further generation of thrombin. Similar results were obtained with the direct addition of APC. APC (1.0-20nM) caused a dose-dependent reduction in total thrombin generation; however, in contrast to sTM, an increase in the lag time was also observed (data not shown). Collectively these data indicate that inactivation of FV/Va by the PC pathway effectively regulates zymogen-like FXa function. In further support of this, thrombin generation could not be rescued with FXaI16L or FXaV17A using FV-deficient plasma, reinforcing the idea that FVa is essential to rescue the procoagulant activity of the zymogen-like variants.
Figure 6.
Influence of the protein C pathway on protease-induced thrombin generation in hemophilic plasma. Protease induced thrombin generation in HA plasma was measured for 90 minutes at 37°C in the presence of increasing amounts of sTM (red, 0; orange, 1.0; green, 2.5; blue, 5.0; purple, 10.0; dark red, 15.0; and black, 20.0nM). In each panel, HA plasma was incubated with the indicated protease (A, wt-FXa, 30pM and B, FXaI16L, 10pM) and 2.0pM TF/4μM phospholipid. Thrombin generation was initiated with CaCl2 and a thrombin fluorogenic substrate as detailed in “Methods.” In panel C, the total ETP remaining for the indicated protease was plotted versus the sTM concentration (wt-FXa [■]; FXaI16L [●], and rFXaV17A [▴]). The results are expressed as percent residual activity where 100% is the maximal ETP in the absence of sTM for a given protease. With use of this experimental setup, the initial ETP for each protease is similar. In each of the panels, the lines are arbitrarily drawn. The data are representative of 3 similar experiments.
Discussion
Over the past 3 decades, substantial progress has been made in managing hemophilia patients with inhibitory antibodies to FVIII or FIX.14 Protein-based products that bypass the intrinsic pathway, such as FVIII bypassing agents (eg, activated prothrombin complex concentrates) and recombinant FVIIa, are effective in treating these patients.15,16 These products reconstitute thrombin generation by enhancing cell-surface FXa production and ultimately prothrombinase levels.43,45 However, their short half-lives, effective dose range, cost, and potential for thrombotic complications indicates that other approaches that enhance thrombin generation should be explored. Several groups have initiated this process and include a full range of strategies such as high-activity FVIIa variants,46 FXIIa implants,47 reversibly acylated FXa,48 and thrombin-activable FX.44 Previous work in the 1980s using a canine hemophilia model examined the possibility of using FXa or FXa in combination with anionic phospholipids as a hemostatic agent to treat hemophilia.22 However, these attempts ultimately proved unsuccessful because of FXa's very short half-life and its potential to induce disseminated intravascular coagulation. Together, these associated problems proved too much to overcome, essentially eliminating the possibility of FXa as a bypass product.
Our recent investigation into the zymogen to protease transition for FX/FXa and the identification of novel zymogen-like FXa variants prompted us to reconsider the utility of an alternative “FXa bypass.” These derivatives of FXa have a mutation at position 16 or 17 (FXaI16L and FXaV17A) that hinders the normal conformational change required for protease formation.6 As a result, these FXa derivatives have poor catalytic function, are ineffectively targeted by small active site probes, and have an altered affinity for FVa and other ligands that target the active protease (ie, they have zymogen-like properties). The reduction in the perceived function of the FXaI16L and FXaV17A represents a change in the ratio between the zymogen-like and protease conformations; stated differently, the equilibrium position between the zymogen and protease states is shifted. Surprisingly, this equilibrium position and thus activity can be rescued with ligands that target the protease conformation. For example, we found that saturating concentrations of FVa not only restored the active site function of FXaI16L and FXaV17A but these proteins also could function in a comparable way to the wt enzyme once fully assembled in prothrombinase.6
Taking into account the differential functional properties of the free versus FVa-bound enzyme, it was evident that the zymogen-like FXa variants may offer a unique opportunity to reevaluate the effectiveness of FXa to bypass deficiencies in the intrinsic pathway. In using a series of in vitro systems of increasing complexity, the current data support the conclusion that zymogen-like forms of FXa may prove useful as therapeutic procoagulants as they are highly effective in promoting thrombin generation in human hemophilic plasma. More importantly, these FXa derivatives overcome several limitations associated with wt-FXa. First, the FXa variants have a prolonged half-life in human plasma because of their zymogen-like conformation. This structural change alters the FXa active site pocket resulting in reduced binding and thus resistance to inactivation by ATIII and TFPI. We speculate that other inhibitors in plasma known to target FXa such as α2-macroglobulin, α1-proteinase inhibitor, and protein Z-dependent inhibitor are also ineffective in inactivating FXaI16L and FXaV17A.19,49 The weak affinity that the variants have for TFPI also minimizes the possibility that the initiation of coagulation would be impaired as the TFPI-FXa complex is highly effective in shutting down FVIIa-TF.11 A second limitation of wt-FXa that the variants overcome is the potential to systemically activate coagulation in an uncontrolled fashion. In their free states, the zymogen-like variants are poor enzymes that are relatively ineffective in activating a range of physiological ligands such as FV, FVIII, prothrombin, and FVII. Thus, before an injury or in the absence of activated cellular surfaces and FVa, the FXa variants are not expected to contribute to the initiation or propagation of coagulation. This is very important from a safety standpoint where activation of these coagulation factors, even in small amounts, would be expected to have undesirable consequences.
A key feature in the effectiveness of the FXa zymogen-like variants in promoting thrombin generation in plasma is their ability to be rescued by FVa generated in situ.6 We have previously shown that these proteins have a modestly reduced affinity for FVa (approximately 10-fold increased Kd) compared with wt-FXa. Therefore, the level of endogenous FVa generated during an assay or in vivo will determine the extent to which the zymogen-like variants are rescued. It was clear that during certain assays, such as the aPTT clotting assay, additional amounts of FVa were needed to fully rescue the zymogen-like variants. This likely reflects the reduced rate of FV activation during this assay and hence the amount of FVa available during the initiation of coagulation in hemophilic plasma. However, this was not the case when using the thrombin generation assay or when assessing the activity of the FXa variants on thrombin activated platelets where no additional FVa was required. The results on thrombin activated platelets using only α-granule released FV/FVa are interesting and suggest that sufficient amounts of FVa would be available at the platelet surface to fully rescue the zymogen-like FXa variants. This is a key point to consider, because the variants would be effectively regulated or turned on by platelet-derived FVa at the activated platelet surface, which is considered to be the primary site for the initial burst of thrombin.45
Although the FXa zymogen-like variants are resistant to plasma protease inhibitors, they are regulated indirectly by the protein C pathway. Introduction of either sTM or APC to plasma attenuated FXa-induced thrombin generation, and neither wt nor zymogen-like FXa proteins could rescue clotting or thrombin generation in FV-deficient plasma. Although these results were to a certain extent expected, they advance our understanding of how the variants may be regulated in vivo. Thus, the generation of thrombin using the FXa zymogen-like variants should be under the same controls as wt-FXa when in prothrombinase (eg, based on availability of active FVa and negatively charged phospholipids), whereas strict regulation of the free enzyme is of less importance as the zymogen-like variants are relatively inert in the absence of FVa.
Finally, it is notable that FXaI16L and FXaV17A restored thrombin generation in hemophilic plasma at much lower concentrations than rFVIIa. Clearly, the magnitude of these differences would be expected to be different if higher concentrations of TF were used or if the phospholipid compositions/concentrations were optimized for FVIIa in this assay. However, from a clinical standpoint, it is well established that high levels of exogenous FVIIa are needed for a full thrombin burst and ultimately hemostatic efficacy. This is thought to be because of its mechanism of action, which principally involves FVIIa activation of FX on activated platelets in a TF-independent fashion, a reaction that is very inefficient. Additional contributions are also derived from a TF-dependent mechanism and a mechanism involving overcoming FVII binding to TF.15,50–52 Like FVIIa, the zymogen-like FXa variants would also be expected to work at the platelet surface; however, the FXa variants would rely on platelet-derived FVa or FVa available from plasma to activate prothrombin. Thus, these differences in mechanism of action may translate into potentially significant dose advantages for the FXa variants compared with rFVIIa while maintaining high hemostatic efficacy. Detailed studies in hemophilic animal models using species specific proteins comparing these bypass agents will assist in deciphering these differences.
In summary, our data show that zymogen-like FXa variants restore thrombin generation and clotting in human hemophilic plasma in a FVa-dependent fashion and importantly have an extended half-life relative to wt-FXa. Before the initiation of coagulation where FVa is essentially absent, the zymogen-like variants will be functionally inert and would not be expected to activate systemic coagulation in an uncontrolled fashion. However, once FVa is generated in situ or released from platelet α-granules after the initiation of the hemostatic process, the FXa variant will find its way unabated to the activated platelet surface, assemble in prothrombinase, and generate a burst of thrombin at the site of injury. Limits on the availability of FVa, or its subsequent inactivation by the PC pathway, effectively regulates zymogen-like FXa function. The efficacy, prolonged half-life, and regulation by cofactor expression suggest that these FXa variants might prove useful and safer as therapeutic procoagulant bypass agents to treat deficiencies upstream of the common pathway. Future in vivo studies will help us further delineate the clinical utility of zymogen-like FXa for this purpose.
Acknowledgments
We are grateful to Drs Sriram Krishnaswamy and Valder R. Arruda for useful suggestions and critical review of the manuscript.
This work was supported in part by National Institutes of Health grants P01 HL-74 124, Project 2 and by research funding from Pfizer (R.M.C.).
Footnotes
Presented in part at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.W.B., R.T., and R.M.C. conducted research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: R.M.C. receives licensing fees and research funding from Pfizer (formerly Wyeth Pharmaceuticals). The remaining authors declare no competing financial interests.
Correspondence: Rodney M. Camire, PhD, The Children's Hospital of Philadelphia, Division of Hematology, 5018 Colket Translational Research Bldg, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: rcamire@mail.med.upenn.edu.
References
- 1.Mann KG, Nesheim ME, Church WR, Haley PE, Krishnaswamy S. Surface dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76(1):1–16. [PubMed] [Google Scholar]
- 2.Bode W, Mayr I, Bauman Y, et al. The refined 1.9 Å crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Trp insertion segment. EMBO J. 1989;8(11):3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AM, James MNG. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Prot Sci. 1998;7(4):815–836. doi: 10.1002/pro.5560070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber R, Bode W. Structural basis of the activation and action of trypsin. Acc Chem Res. 1978:11114–11122. [Google Scholar]
- 5.Camire RM. Prothrombinase assembly and S1 site occupation restore the catalytic activity of FXa impaired by mutation at the sodium-binding site. J Biol Chem. 2002;277(40):37863–37870. doi: 10.1074/jbc.M203692200. [DOI] [PubMed] [Google Scholar]
- 6.Toso R, Zhu H, Camire RM. The conformational switch from the factor X zymogen to protease state mediates exosite expression and prothrombinase assembly. J Biol Chem. 2008;283(27):18627–18635. doi: 10.1074/jbc.M802205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster WB, Nesheim ME, Mann KG. The factor Xa-catalyzed activation of factor V. J Biol Chem. 1983;258(22):13970–13977. [PubMed] [Google Scholar]
- 8.Eaton D, Rodriguez H, Vehar G. Proteolytic processing of human FVIII. Correlation of specific cleavages by thrombin FXa and activated protein C with activation and inactivation of Factor VIII coagulant activity. Biochemistry. 1986;25(2):505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- 9.Butenas S, Mann KG. Kinetics of human factor VII activation. Biochemistry. 1996;35(6):1904–1910. doi: 10.1021/bi951768c. [DOI] [PubMed] [Google Scholar]
- 10.Borensztajn K, Peppelenbosch MP, Spek CA. Factor Xa: at the crossroads between coagulation and signaling in physiology and disease. Trends Mol Med. 2008;14(10):429–440. doi: 10.1016/j.molmed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Broze GJ., Jr Tissue factor pathway inhibitor and the revised theory of coagulation. Annu Rev Med. 1995:46103–46112. doi: 10.1146/annurev.med.46.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Bjork I, Olson ST. Antithrombin. A bloody important serpin. Adv Exp Med Biol. 1997:42517–42533. [PubMed] [Google Scholar]
- 13.Roberts HR, Ma AD. Overview of inherited hemorrhagic disorders. In: Colman RW, Marder VJ, Clowes AW, et al., editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia, PA: Lippincott Williams and Wilkins; 2006. pp. 877–885. [Google Scholar]
- 14.Kessler CM, Mariani G. Clinical manifestations and therapy of the hemophilias. In: Colman RW, Marder VJ, Clowes AW, et al., editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia, PA: Lippincott Williams and Wilkins; 2006. pp. 887–904. [Google Scholar]
- 15.Hedner U. Mechanism of action, development and clinical experience of recombinant FVIIa. J Biotechnol. 2006;124(4):747–757. doi: 10.1016/j.jbiotec.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Turecek PL, Varadi K, Gritsch H, Schwarz HP. FEIBA: mode of action. Haemophilia. 2004;10(suppl 2):3–9. doi: 10.1111/j.1365-2516.2004.00934.x. [DOI] [PubMed] [Google Scholar]
- 17.Hedner U, Lee CA. First 20 years with recombinant FVIIa (NovoSeven) [published online ahead of print June 29, 2010]. Haemophilia. doi: 10.1111/j.1365-2516.2010.02352.x. doi: 10.1111/j.1365-2516.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- 18.Berntorp E. Differential response to bypassing agents complicates treatment in patients with haemophilia and inhibitors. Haemophilia. 2009;15(1):3–10. doi: 10.1111/j.1365-2516.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 19.Gitel SN, Medina VM, Wessler S. Inhibition of human activated Factor X by antithrombin III and alpha 1-proteinase inhibitor in human plasma. J Biol Chem. 1984;259(11):6890–6895. [PubMed] [Google Scholar]
- 20.Jesty J. Analysis of the generation and inhibition of activated coagulation factor X in pure systems and in human plasma. J Biol Chem. 1986;261(19):8695–8702. [PubMed] [Google Scholar]
- 21.Giles AR, Nesheim ME, Mann KG. Studies of Factors V and VIII:C in an animal model of disseminated intravascular coagulation. J Clin Invest. 1984;74(6):2219–2225. doi: 10.1172/JCI111648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giles AR, Mann KG, Nesheim ME. A combination of factor Xa and phosphatidylcholine-phosphatidylserine vesicles bypasses factor VIII in vivo. Br J Haematol. 1988;69(4):491–497. doi: 10.1111/j.1365-2141.1988.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 23.Lottenberg R, Jackson CM. Solution composition dependent variation in extinction coefficients for ρ-nitroaniline. Biochim Biophys Acta. 1983;742(3):558–564. doi: 10.1016/0167-4838(83)90273-x. [DOI] [PubMed] [Google Scholar]
- 24.Higgins DL, Mann KG. The interaction of bovine factor V and factor V-derived peptides with phospholipid vesicles. J Biol Chem. 1983;258(10):6503–6508. [PubMed] [Google Scholar]
- 25.Buddai SK, Toulokhonova L, Bergum PW, Vlasuk GP, Krishnaswamy S. Nematode anticoagulant protein c2 reveals a site on factor Xa that is important for macromolecular substrate binding to human prothrombinase. J Biol Chem. 2002;277(29):26689–26698. doi: 10.1074/jbc.M202507200. [DOI] [PubMed] [Google Scholar]
- 26.Katzmann JA, Nesheim ME, Hibbard LS, Mann KG. Isolation of functional human coagulation Factor V by using a hybridoma antibody. Proc Natl Acad Sci U S A. 1981;78(1):162–166. doi: 10.1073/pnas.78.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kisiel W, Hermodson MA, Davie EW. Factor X activating enzyme from Russell's Viper Venom: Isolation and characterization. Biochemistry. 1976;15(22):4901–4906. doi: 10.1021/bi00667a023. [DOI] [PubMed] [Google Scholar]
- 28.Toso R, Camire RM. Removal of B-domain sequences from factor V rather than specific proteolysis underlies the mechanism by which cofactor function is realized. J Biol Chem. 2004;279(20):21643–21650. doi: 10.1074/jbc.M402107200. [DOI] [PubMed] [Google Scholar]
- 29.Gowda DC, Jackson CM, Davidson EA. Factor X-activating glycoprotein of Russell's viper venom. Polypeptide composition and characterization of the carbohydrate moieties. J Biol Chem. 1994;269(14):10644–10650. [PubMed] [Google Scholar]
- 30.Mann KG. Prothrombin. Methods Enzymol. 1976;45:123–156. doi: 10.1016/s0076-6879(76)45016-4. [DOI] [PubMed] [Google Scholar]
- 31.Lundblad RL, Kingdon HS, Mann KG. Thrombin. Methods Enzymol. 1976;45:156–176. doi: 10.1016/s0076-6879(76)45017-6. [DOI] [PubMed] [Google Scholar]
- 32.Di Scipio RG, Hermodson MA, Yates SG, Davie EW. A comparison of human prothrombin, factor IX (Christmas Factor), factor X (Stuart Factor), and protein S. Biochemistry. 1977;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- 33.Olson ST, Bjork I, Shore JD. Kinetic characterization of heparin-catalyzed and uncatalyzed inhibition of blood coagulation proteinases by antithrombin. In: Lorand L, Mann KG, editors. Methods in Enzymology Part A. San Diego, CA: Academic Press; 1993. pp. 525–559. [DOI] [PubMed] [Google Scholar]
- 34.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 35.Camire RM, Kalafatis M, Simioni P, Girolami A, Tracy PB. Platelet-derived factor Va/VaLeiden cofactor activities are sustained on the surface of activated platelets despite the presence of activated protein C. Blood. 1998;91(8):2818–2829. [PubMed] [Google Scholar]
- 36.Hemker HC, Giesen P, Al DR, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 37.Straume M, Johnson ML. Analysis of residuals: criteria for determining goodness-of-fit. Methods Enzymol. 1992;210:87–105. doi: 10.1016/0076-6879(92)10007-z. [DOI] [PubMed] [Google Scholar]
- 38.Segal IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems. New York, NY: John Wiley & Sons; 1975. [Google Scholar]
- 39.Rezaie AR. Calcium enhances heparin catalysis of the antithrombin-factor Xa reaction by a template mechanism. Evidence that calcium alleviates Gla domain antagonism of heparin binding to factor Xa. J Biol Chem. 1998;273(27):16824–16827. doi: 10.1074/jbc.273.27.16824. [DOI] [PubMed] [Google Scholar]
- 40.Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273(8):4378–4386. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 41.Ellis V, Scully MF, Kakkar VV. Inhibition of prothrombinase complex by plasma proteinase inhibitors. Biochemistry. 1984;23(24):5582–5587. doi: 10.1021/bi00319a030. [DOI] [PubMed] [Google Scholar]
- 42.Rezaie AR. Prothrombin protects factor Xa in the prothrombinase complex from inhibition by the heparin-antithrombin complex. Blood. 2001;97(8):2308–2313. doi: 10.1182/blood.v97.8.2308. [DOI] [PubMed] [Google Scholar]
- 43.Cawthern KM, van't Veer C, Lock JB, et al. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91(12):4581–4592. [PubMed] [Google Scholar]
- 44.Louvain-Quintard VB, Bianchini EP, Calmel-Tareau C, Tagzirt M, Le Bonniec BF. Thrombin-activable factor X re-establishes an intrinsic amplification in tenase-deficient plasmas. J Biol Chem. 2005;280(50):41352–41359. doi: 10.1074/jbc.M507846200. [DOI] [PubMed] [Google Scholar]
- 45.Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22(9):1381–1389. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 46.Tranholm M, Kristensen K, Kristensen AT, et al. Improved hemostasis with superactive analogs of factor VIIa in a mouse model of hemophilia A. Blood. 2003;102(10):3615–3620. doi: 10.1182/blood-2003-05-1369. [DOI] [PubMed] [Google Scholar]
- 47.Ton-That TT, Doron D, Pollard BS, Bacher J, Pollard HB. In vivo bypass of hemophilia A coagulation defect by factor XIIa implant. Nat Biotechnol. 2000;18(3):289–295. doi: 10.1038/73727. [DOI] [PubMed] [Google Scholar]
- 48.Wolf DL, Lin PH, Hollenbach S, et al. Procoagulant activity of reversibly acylated human factor Xa. Blood. 1995;86(11):4153–4157. [PubMed] [Google Scholar]
- 49.Broze GJ., Jr Protein Z-dependent regulation of coagulation. Thromb Haemost. 2001;86(1):8–13. [PubMed] [Google Scholar]
- 50.Monroe DM, Hoffman M, Oliver JA, Roberts HR. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99(3):542–547. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 51.van 't Veer C, Golden NJ, Mann KG. Inhibition of thrombin generation by the zymogen factor VII: implications for the treatment of hemophilia A by factor VIIa. Blood. 2000;95(4):1330–1335. [PubMed] [Google Scholar]
- 52.Butenas S, Brummel KE, Branda RF, Paradis SG, Mann KG. Mechanism of factor VIIa-dependent coagulation in hemophilia blood. Blood. 2002;99(3):923–930. doi: 10.1182/blood.v99.3.923. [DOI] [PubMed] [Google Scholar]