Abstract
Risks of secondary solid cancers among allogeneic hematopoietic cell transplant (HCT) recipients who receive conditioning without total body irradiation are not well known. We evaluated the incidence and risk factors for solid cancers after HCT using high-dose busulfan-cyclophosphamide conditioning in 4318 recipients of first allogeneic HCT for acute myeloid leukemia in first complete remission (N = 1742) and chronic myeloid leukemia in first chronic phase (N = 2576). Our cohort represented 22 041 person-years at risk. Sixty-six solid cancers were reported at a median of 6 years after HCT. The cumulative-incidence of solid cancers at 5 and 10 years after HCT was 0.6% and 1.2% among acute myeloid leukemia and 0.9% and 2.4% among chronic myeloid leukemia patients. In comparison to general population incidence rates, HCT recipients had 1.4× higher than expected rate of invasive solid cancers (95% confidence interval, 1.08-1.79, P = .01). Significantly elevated risks were observed for tumors of the oral cavity, esophagus, lung, soft tissue, and brain. Chronic graft-versus-host disease was an independent risk factor for all solid cancers, and especially cancers of the oral cavity. Recipients of allogeneic HCT using busulfan-cyclophosphamide conditioning are at risk for developing solid cancers. Their incidence continues to increase with time, and lifelong cancer surveillance is warranted in this population.
Introduction
Advances in transplantation have improved outcomes and led to an increasing number of long-term survivors of allogeneic hematopoietic cell transplantation (HCT). Previous research has shown that these survivors are at risk for developing secondary malignancies, including new solid cancers, and that secondary cancers are an important cause of late mortality.1–12
The cumulative incidence of secondary solid cancers has been reported to range from 1%-6% at 10 years and 2%-15% at 15 years after transplantation.1,2,4,6–10 In the largest study performed to date, investigators at the Center for International Blood and Marrow Transplant Research (CIBMTR) and the Fred Hutchinson Cancer Research Center assembled a cohort of 28 874 allogeneic HCT recipients with 189 second solid cancers.9 The cumulative incidence of solid cancers was 1% at 10 years, 2.2% at 15 years, and 3.3% at 20 years after HCT. HCT recipients developed new solid cancers at rates twice that expected for the general population, and this risk continued to increase with time. Age at transplantation, exposure to radiation as part of the conditioning regimen, and chronic graft-versus-host disease (GVHD) were important risk factors for invasive solid cancers. Specifically, total body irradiation (TBI) increased the risks of developing nonsquamous cell carcinomas, while chronic GVHD was associated with an increased risk of developing squamous cell carcinomas.
Although previous studies have included some patients transplanted using conditioning regimens that do not include TBI, the risks and risk factors for new solid cancers among recipients of non-TBI–based conditioning have not been well described. We conducted a retrospective cohort study to evaluate the incidence and risk factors of solid cancers in patients receiving an allogeneic HCT for acute myeloid leukemia (AML) in first complete remission (CR1) and chronic myeloid leukemia (CML) in first chronic phase (CP1) using a high-dose busulfan and cyclophosphamide (Bu-Cy) conditioning regimen.
Methods
Data sources
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry, Autologous Blood and Marrow Transplant Registry, and National Marrow Donor Program (NMDP) established in 2004 that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive HCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee, WI and the NMDP Coordinating Center in Minneapolis, MN. Observational studies conducted by the CIBMTR are performed in compliance with the Health Insurance Portability and Accountability Act Privacy Rule as a Public Health Authority and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Boards of the NMDP and the Medical College of Wisconsin since 1985.
The CIBMTR collects data at 2 levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED data include disease type, age, sex, pretransplant disease stage, date of diagnosis, graft type, conditioning regimen, posttransplant disease progression and survival, development of a new malignancy, and cause of death. All CIBMTR teams contribute TED data. More detailed clinical information is collected on a subset of registered patients selected for CRF data by a weighted randomization scheme. TED and CRF level data are collected before transplant, 100 days and 6 months after transplant, and annually thereafter or until death.
Patients
Our study included patients with CRF data who received a first allogeneic HCT between 1986 and 2005 for AML CR1 or CML CP1 using a high-dose Bu-Cy conditioning regimen.
From the 5931 patients who met our study criteria, 1613 were excluded. These included recipients of syngeneic (N = 73) and umbilical cord blood HCT (N = 50). Patients with secondary AML (N = 130) and a previous history of solid cancer (N = 4) were also excluded; none of the patients included in the final study cohort had received a previous autologous HCT or had underlying Fanconi anemia or bone marrow failure disorder. We also removed 34 patients whose conditioning regimen could not be confirmed. A completeness index of follow-up, which is the ratio of total observed person-time and the potential person-time of follow-up in a study, was computed for each team with potentially eligible patients.13 To avoid bias from inclusion of teams with incomplete follow-up and, consequently, incomplete ascertainment of events in the late posttransplant period, the final dataset excluded 93 teams with completeness index of follow-up < 80% at 5 years after transplantation (N = 1121). Similarly, to avoid potential bias due to centers preferentially following patients at higher risk for second cancers, we excluded 5 teams (N = 152) where the median follow-up of patients with solid cancers was longer than the maximum follow-up of patients without solid cancers. All surviving recipients of unrelated donor HCT included in this analysis were retrospectively contacted and provided informed consent for participation in the NMDP research program. Informed consent was waived by the NMDP Institutional Review Board for all deceased recipients. Approximately 10% of surviving patients did not provide consent for use of research data. To adjust for potential bias introduced by exclusion of nonconsenting surviving patients, a corrective action plan modeling process randomly excluded appropriately the same percentage of deceased patients (N = 22).14 Twenty-seven patients were excluded because information on whether a second cancer had occurred after HCT was missing. Pathology and physician reports of second cancers were reviewed centrally, and if necessary, tumors were reclassified.15
Statistical analysis
For each transplant recipient, the number of person-years at risk was calculated from the date of transplantation until date of last contact, death, or diagnosis of new cancer, whichever occurred first. Incidence rates for all invasive cancers in the general population were obtained from selected registries in the United States, England and Wales, Europe, and Asia.15,16 Age-, sex-, calendar year-, and region-specific incidence rates for all invasive solid cancers combined and for cancers of specific anatomical sites were applied to the appropriate person-years at risk to compute the expected numbers of cancers. Observed–to–expected (O/E) ratios, also called standardized incidence ratios, were calculated, and the exact Poisson distribution was used to calculate 95% confidence intervals (CIs).17 Tests of heterogeneity and of linear trends were conducted using the methods of Breslow.18
Multivariate analyses were conducted using Poisson regression for grouped survival data to compare risks of solid cancers for various subgroups of HCT recipients.17,19 The following risk-factors were considered: age at HCT, sex, recipient race, smoking history, diagnosis, time from diagnosis to HCT, donor source, hematopoietic stem cell source, GVHD prophylaxis regimen, year of HCT, time since HCT, use of hematopoietic growth factors to promote myeloid engraftment, and occurrence of acute (grades 2-4) or chronic GVHD. For patients with AML, number of cycles of induction and consolidation chemotherapy was also evaluated as a risk factor. Acute and chronic GVHD were entered as time-dependent covariates. Multivariate analyses using Cox proportional hazards regression were also performed for evaluating risk factors for solid cancers.20 Both Poisson and Cox regression analyses yielded similar results, and only the former are presented in this article. We also evaluated risk factors for cancer sites with adequate numbers of events for analysis: oral cavity (lip, tongue, and mouth combined), trachea, lung, esophagus, and breast.
The cumulative incidence of solid cancers was estimated taking into account the competing risk of death among patients who did not develop a second malignancy. A second transplant for relapse or graft failure was performed in 299 (7%) patients and 135 (3%) patients received donor lymphocyte infusion; these patients were not censored at the time of second HCT for second cancer analyses. All P values are 2-sided. All analyses were carried out using SAS Version 9.1.3 statistical software (SAS Institute).
Funding
The funding sources of the CIBMTR had no role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Results
Patient characteristics
Our final study population consisted of 4318 patients (AML 1742, CML 2576) and represented 22 041 person-years at risk (Table 1). History of smoking before transplantation was present in 24% of AML and 25% of CML patients; we did not have data on the duration and quantity of smoking. No patient had received radiation therapy as treatment for disease before HCT or in preparation for HCT.
Table 1.
Patient characteristics
| Variable | AML CR1, N (%) | CML CP1, N (%) |
|---|---|---|
| Number of patients | 1742 | 2576 |
| Number of centers | 193 | 188 |
| Geographic region of transplant team | ||
| United States, Canada | 600 (34) | 872 (34) |
| Europe | 589 (34) | 829 (32) |
| Other | 553 (32) | 875 (34) |
| Median age, y (range) | 29 (< 1-60) | 36 (< 1-60) |
| Age, y | ||
| < 10 | 226 (13) | 44 (2) |
| 10-19 | 300 (17) | 199 (8) |
| 20-29 | 340 (20) | 496 (19) |
| 30-39 | 366 (21) | 791 (31) |
| 40-49 | 357 (20) | 737 (29) |
| > 50 | 153 (9) | 309 (12) |
| Male | 915 (53) | 1500 (58) |
| Karnofsky score pretransplant ≥ 90 | 1481 (86) | 2309 (90) |
| History of smoking before transplant | ||
| Yes | 416 (24) | 645 (25) |
| No | 1085 (62) | 1510 (59) |
| Missing | 241 (14) | 421 (16) |
| Interval from diagnosis to transplant, mo | ||
| < 6 | 1191 (68) | 618 (24) |
| ≥ 6 | 549 (32) | 1958 (76) |
| Year of transplant | ||
| 1986-1995 | 871 (50) | 1291 (50) |
| 1996-2005 | 871 (50) | 1285 (50) |
| Donor source | ||
| HLA-matched sibling | 1460 (84) | 1954 (76) |
| Other related | 96 (6) | 180 (7) |
| Unrelated | 186 (11) | 442 (17) |
| Graft type | ||
| Bone marrow | 1404 (81) | 2215 (86) |
| Peripheral blood | 338 (19) | 361 (14) |
| Donor or recipient CMV positive | 1263 (73) | 1801 (70) |
| Cycles of prior chemo induction/consolidation (for AML) | ||
| 1-2 | 617 (35) | |
| > 2 | 526 (30) | |
| Missing | 599 (34) | |
| Pretransplant therapy (for CML) | ||
| Interferon ± other | 919 (36) | |
| Hydroxyurea ± other | 1394 (54) | |
| Other | 168 (7) | |
| None | 55 (2) | |
| Missing | 40 (1) | |
| GVHD prophylaxis | ||
| Ex vivo T-cell depletion | 60 (3) | 60 (2) |
| ATG, campath, or anti-CD3 antibody | 98 (6) | 211 (8) |
| MTX + CSA ± other | 1165 (67) | 1874 (73) |
| Other | 418 (24) | 429 (17) |
| G-CSF/GM-CSF to promote engraftment | ||
| Yes | 564 (32) | 891 (35) |
| No | 1085 (62) | 1500 (58) |
| Missing | 93 (5) | 185 (7) |
| Cumulative incidence of acute GVHD (grades 2-4) at day 100, % (95% CI) | 33 (31-35) | 42 (40-44) |
| Cumulative incidence of chronic GVHD at 3 y, % (95% CI) | 32 (30-35) | 46 (44-48) |
| Median follow-up of survivors, y (range) | 7 (< 1-21) | 8 (< 1-19) |
CMV indicates cytomegalovirus; ATG, antithymocyte globulin; MTX, methotrexate; CSA, cyclosporine; G-CSF, granulocyte colony-stimulating factor; and GM-CSF, granulocyte macrophage colony stimulating factor.
Sixty-six solid cancers (AML 22, CML 44) were reported at median of 6 (range, < 1-17) years after HCT. For the whole cohort, patients with solid cancers were older than patients without solid cancers (median age 44 vs 34 years) and a greater proportion reported pretransplant history of smoking (47% vs 29%). In addition, a greater proportion of patients with solid cancers had a history of chronic GVHD (72% vs 40%). Demographic-, treatment-, and transplant-related characteristics of patients with and without solid cancers were otherwise comparable, including donor source, graft type, previous therapy, GVHD prophylaxis regimen, and occurrence of acute GVHD. Only 1 patient had received a second transplant (for graft failure) before the onset of the solid cancer.
Incidence and types of second solid cancers
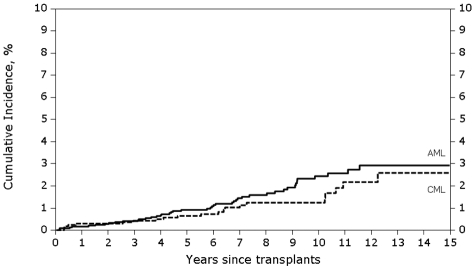
The cumulative incidence of solid cancers was 0.4% (95% CI, 0.2%-0.8%) at 3 years, 0.6% (95% CI, 0.3%-1.1%) at 5 years and 1.2% (95% CI, 0.7%-1.9%) at 10 years after transplantation for AML (Figure 1). The corresponding cumulative incidence of solid cancers in CML patients was 0.4% (95% CI, 0.2%-0.7%), 0.9% (95% CI, 0.6%-1.3%) and 2.4% (95% CI, 1.7%-3.3%), respectively.
Figure 1.
Cumulative incidence of secondary solid cancer by disease group among recipients of allogeneic HCT using high-dose Bu-Cy conditioning for AML in CR1 and CML in CP1. The cumulative incidence of solid cancers at 10 years after transplantation was 1.2% for AML and 2.4% for CML patients.
Among the 66 patients with solid cancers, lung was the most common site (N = 11, 17%; Table 2). Among these 11 patients, 9 patients had non-small cell lung cancer, while lung cancer subtype information was not available for 2 patients. In addition, 9 patients with lung cancer had smoked before transplant, 1 had no history of smoking, and smoking history was missing for 1 patient. Median age at HCT for these 11 patients was 48 years (range, 34-58 years), and lung cancer occurred at a median of 4.5 years after transplantation. Other common sites included breast (N = 9, 14%) and esophagus (N = 6, 9%). Although 18% of patients in our cohort were < 20 years of age at the time of HCT, only 3 solid cancers were reported in this age group; of note, the follow-up of < 20- and ≥ 20-year age groups was comparable (median follow-up 51 and 52 months, respectively).
Table 2.
Ratio of observed (O) to expected (E) cases of new solid cancers among recipients of allogeneic HCT using high-dose Bu-Cy conditioning for AML in CR1 and CML in CP1
| Time since transplantation |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 1 y | 1-4 y | 5-9 y | ≥ 10 y | ||||||||
| Number of patients | 4318 | 3060 | 1982 | 774 | 4318 | ||||||
| Person-years at risk | 3507 | 9902 | 6632 | 2000 | 22 041 | ||||||
| Second cancer site | O | O/E | O | O/E | O | O/E | O | O/E | O | O/E | 95% CI |
| All sites | 9 | 1.77 | 22 | 1.25 | 26 | 1.52* | 9 | 1.21 | 66 | 1.40* | 1.08-1.78 |
| Lip | 0 | 0 | 3 | 54.81† | 1 | 18.32 | 0 | 0 | 4 | 26.00† | 7.08-66.57 |
| Tongue | 0 | 0 | 2 | 12.41* | 2 | 12.82* | 0 | 0 | 4 | 9.25† | 2.52-23.70 |
| Mouth | 0 | 0 | 0 | 0 | 2 | 13.47* | 1 | 15.04 | 3 | 7.32* | 1.51-21.41 |
| Hypopharynx | 0 | 0 | 0 | 0 | 1 | 14.59 | 0 | 0 | 1 | 5.40 | 0.14-30.07 |
| Esophagus | 0 | 0 | 2 | 9.99* | 4 | 18.64† | 0 | 0 | 6 | 10.50† | 3.85-22.85 |
| Colon | 0 | 0 | 0 | 0 | 1 | 1.04 | 2 | 4.34 | 3 | 1.18 | 0.24-3.45 |
| Bronchus and lung | 2 | 5.17 | 4 | 2.73 | 2 | 1.23 | 3 | 3.89 | 11 | 2.59† | 1.29-4.64 |
| Melanoma, skin | 0 | 0 | 1 | 0.89 | 2 | 2.02 | 1 | 2.31 | 4 | 1.38 | 0.38-3.53 |
| Soft tissue | 1 | 17.77 | 1 | 5.81 | 1 | 7.16 | 0 | 0 | 3 | 7.16* | 1.48-20.93 |
| Breast | 0 | 0 | 5 | 1.43 | 4 | 1.23 | 0 | 0 | 9 | 1.00 | 0.46-1.89 |
| Uterine cervix | 1 | 5.69 | 1 | 1.80 | 1 | 2.29 | 0 | 0 | 3 | 2.32 | 0.48-6.77 |
| Prostate | 0 | 0 | 1 | 0.85 | 0 | 0 | 1 | 1.05 | 2 | 0.50 | 0.06-1.81 |
| Testis | 0 | 0 | 0 | 0 | 1 | 3.91 | 0 | 0 | 1 | 1.12 | 0.03-6.25 |
| Kidney | 1 | 6.87 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.73 | 0.02-4.09 |
| Bladder | 1 | 8.05 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.78 | 0.02-4.33 |
| Brain and nervous system | 0 | 0 | 2 | 4.64 | 2 | 5.62 | 0 | 0 | 4 | 3.76* | 1.02-9.62 |
| Adrenal gland | 1 | 148.78* | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 23.17 | 0.59-129.07 |
| Other, unspecified‡ | 2 | 17.74* | 0 | 0 | 2 | 4.84 | 1 | 5.04 | 5 | 4.43† | 1.44-10.33 |
P ≤ .05.
P ≤ .01.
Includes metastatic adenocarcinoma, unknown primary site (N = 2), metastatic squamous cell carcinoma, unknown primary site (N = 2), and metastatic round cell tumor, unknown primary site (N = 1).
Solid cancer risk compared with general population
Transplant recipients had 1.4× higher than expected rate of invasive solid cancers compared with general population incidence rates (O/E 1.40; 95% CI, 1.08-1.79, P = .01). Significantly elevated risks were observed for tumors of the lip (O/E, 26.00, P < .001), tongue (O/E, 9.25, P = .003), mouth (O/E, 7.33, P = .02), esophagus (O/E, 10.50, P < .001), lung (O/E, 2.59, P = .01), soft tissue (O/E, 7.16, P = .02), and brain (O/E, 3.76, P = .05; Table 2). We also explored risks of second cancers compared with the general population by time since transplantation. We did not notice a clear trend, although our analysis was limited by the relatively small number of long-term (≥ 10 years) survivors and the overall small number of second cancers in our study population (Table 2).
Albeit the limitation of small number of long-term survivors and second cancers, we also explored second solid cancer risk in comparison to the general population by diagnosis and age at transplantation (Table 3). The O/E ratio for second solid cancers for patients with AML was 1.61 (95% CI, 1.01-2.44, P = .04) and that for CML patients was 1.31 (95% CI, 0.95-1.76, P = .09). AML patients had higher risks of cancers of the esophagus, lung, and soft tissue, while CML patients had higher risks of cancers of the lip and esophagus. The O/E ratio for patients transplanted at age < 35 years was 1.74 (95% CI, 0.92-2.97, P = .09), age 35-50 years was 1.23 (95% CI, 0.84-1.72, P = .28), and age > 50 years was 1.57 (95% CI, 0.96-2.42, P = .07), respectively (Table 3). Patients < 35 years of age at HCT had greater risks of soft tissue cancers, while older patients (> 50 years) had increased risks of cancers of the esophagus, breast, and brain.
Table 3.
Ratio of observed (O) to expected (E) cases of new solid cancers among recipients of allogeneic HCT using high-dose Bu-Cy conditioning for AML in CR1 and CML in CP1 by diagnosis and age at transplantation
| Cancer site | Diagnosis |
Age at transplantation |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AML (N = 1742) |
CML (N = 2576) |
< 35 y (N = 2264) |
35-50 y (N = 1685) |
> 50 y (N = 369) |
||||||
| O | O/E | O | O/E | O | O/E | O | O/E | O | O/E | |
| All sites | 22 | 1.61* | 44 | 1.31 | 13 | 1.73 | 33 | 1.23 | 20 | 1.57 |
| Lip | 0 | 0 | 4 | 36.58† | 1 | 36.63 | 3 | 36.00† | 0 | 0 |
| Tongue | 1 | 8.83 | 3 | 9.42* | 1 | 19.20 | 2 | 7.36 | 1 | 9.26 |
| Mouth | 1 | 9.48 | 2 | 6.59 | 0 | 0 | 2 | 7.82 | 1 | 9.39 |
| Hypopharynx | 1 | 21.86 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 18.68 |
| Esophagus | 2 | 14.14* | 4 | 9.32† | 1 | 25.86 | 3 | 8.71* | 2 | 10.66* |
| Colon | 1 | 1.44 | 2 | 1.08 | 1 | 4.29 | 2 | 1.38 | 0 | 0 |
| Bronchus and lung | 7 | 6.32† | 4 | 1.28 | 1 | 4.60 | 5 | 2.15 | 5 | 2.95 |
| Melanoma, skin | 2 | 2.17 | 2 | 1.01 | 2 | 2.78 | 2 | 1.22 | 0 | 0 |
| Soft tissue | 2 | 14.73* | 1 | 3.54 | 3 | 20.88† | 0 | 0 | 0 | 0 |
| Breast | 0 | 0 | 9 | 1.44 | 0 | 0 | 3 | 0.49 | 6 | 3.52* |
| Uterine cervix | 1 | 2.48 | 2 | 2.25 | 1 | 2.21 | 2 | 2.68 | 0 | 0 |
| Prostate | 0 | 0 | 2 | 0.66 | 0 | 0 | 0 | 0 | 2 | 0.91 |
| Testis | 0 | 0 | 1 | 1.72 | 1 | 1.75 | 0 | 0 | 0 | 0 |
| Kidney | 0 | 0 | 1 | 1.03 | 0 | 0 | 1 | 1.25 | 0 | 0 |
| Bladder | 0 | 0 | 1 | 1.06 | 0 | 0 | 1 | 1.47 | 0 | 0 |
| Brain and nervous system | 1 | 2.87 | 3 | 4.19 | 0 | 0 | 2 | 3.72 | 2 | 10.70* |
| Adrenal gland | 0 | 0 | 1 | 37.84 | 0 | 0 | 1 | 46.15 | 0 | 0 |
| Other, unspecified‡ | 3 | 9.97† | 2 | 2.42 | 1 | 6.48 | 4 | 6.13* | 0 | 0 |
P ≤ .05.
P ≤ .01.
Includes metastatic adenocarcinoma, unknown primary site (N = 2), metastatic squamous cell carcinoma, unknown primary site (N = 2), and metastatic round cell tumor, unknown primary site (N = 1).
Studies with larger numbers of long-term survivors will be able to better describe the role of time since transplantation, diagnosis, and age on the risks of secondary solid cancers in a similar population.
Risk factors for second solid cancers
On multivariate analyses of our cohort, older age at HCT, Karnofsky performance status score at HCT, and chronic GVHD were independent risk factors for solid cancers (Table 4).
Table 4.
Risk factors for second solid cancers among allogeneic HCT recipients of high-dose Bu-Cy conditioning for AML in CR1 and CML in CP1
| Risk factor | N | Relative risk (95% CI) | P |
|---|---|---|---|
| Age at transplantation | |||
| < 35 y | 2218 | 1.0 | < .001 |
| 35-50 y | 1643 | 3.1 (1.6-5.9) | < .001 |
| > 50 y | 361 | 9.8 (4.8-20.0) | < .001 |
| Karnofsky performance score at HCT | |||
| < 90 | 471 | 1.00 | .026 |
| ≥ 90 | 3713 | 0.4 (0.2-0.8) | .004 |
| Unknown | 38 | 0.7 (0.1-5.6) | .767 |
| Chronic GVHD | |||
| No | 2549 | 1.00 | .001 |
| Yes | 1679 | 2.4 (1.4-4.2) |
We also evaluated risk factors for cancers of the oral cavity (lip, tongue, and mouth), lung, esophagus, and breast (Table 5). Chronic GVHD was the only risk factor significantly associated with cancers of the oral cavity. The risks of lung cancer were increased among older patients and in recipients with a history of smoking before transplantation. Use of growth factors to promote engraftment was associated with increased risks of breast cancer (relative risk 8.3; 95% CI, 1.7-41.0; P = .01). Among the risk factors evaluated, no factor was found to be independently associated with cancer of the esophagus, including chronic GVHD. Of the 5 patients with cancer of the nervous system, none had received craniospinal irradiation or intracranial chemotherapy before transplantation.
Table 5.
Risk factors for selected second solid cancers among allogeneic HCT recipients of high-dose Bu-Cy conditioning for AML in CR1 and CML in CP1
| Risk factor | No. of second cancers | Relative risk (95% CIs) | P |
|---|---|---|---|
| Cancer of trachea, bronchus, and lung | 11 | ||
| Age at transplantation | |||
| < 35 y | 1 | 1.0 | .01 |
| 35-50 y | 5 | 4.03 (0.5-36.4) | .22 |
| > 50 y | 5 | 17.9 (2.0-163.1) | .01 |
| Smoking prior to HCT | |||
| No | 1 | 1.0 | .02 |
| Yes | 9 | 11.6 (1.4-96.0) | .02 |
| Unknown | 1 | 3.8 (0.2-60.64) | .35 |
| Cancer of lip, tongue, and mouth | 11 | ||
| Chronic GVHD | |||
| No | 1 | 1.0 | .02 |
| Yes | 10 | 12.7 (1.6-99.1) |
Discussion
Our study describes the risks of secondary solid cancers among recipients of allogeneic HCT for AML in CR1 and CML in CP1 using a high-dose Bu-Cy preparative regimen. These HCT survivors had greater risks than the general population for cancers of the oral cavity, esophagus, lung, soft tissue, and brain.
Our study highlights similarities and differences in solid cancers that may occur in recipients of myeloablative TBI-based and high-dose Bu-Cy conditioning regimens. The cumulative incidence of new solid cancers appears to be similar regardless of exposure to radiation. The cumulative incidence of solid cancers in a previous CIBMTR study reported by Rizzo et al was 1% at 10 years after HCT.9 Among 28 874 patients included in their study, 70% had received TBI-based conditioning regimen with the majority receiving > 12 Gy in multiple fractions. However, our current cohort was older compared with patients included in the previous study. Similarly, other studies with smaller cohorts of allogeneic HCT recipients and a high representation of TBI-based conditioning regimens have reported 10-year cumulative incidence rates of 2%-4% for solid cancers,1,6,7,10 except for a single institution study by Bhatia et al, in which a 10-year cumulative incidence of 6.4% was observed among 1370 allogeneic HCT recipients. In comparison, the cumulative incidence of solid cancers at 10 years after HCT in our study of allogeneic HCT recipients who did not receive TBI was 1.2% for AML and 2.4% for CML. However, our study included a relatively small number ≥ 10-year survivors, and future studies with a larger number of long-term survivors are still needed to better delineate any differences in risks of second solid cancers with TBI and non-TBI regimens. Such larger studies will also be able to better delineate the impact of age and time since transplantation on second solid cancer risks.
On risk factor analysis that was limited to our cohort of HCT recipients, old age (> 50 years) was an independent risk factor for solid cancers. However, the standardized incidence (O/E) ratios that accounted for the background age-specific incidence of solid cancers were not statistically different for different age groups (< 35, 35-50, and > 50 years) in our cohort and the general population. We did notice a trend toward increased risks of second cancers among young (< 35 years, O/E 1.74, P = .09) and old (> 50 years, O/E 1.57, P = .07) patients in our cohort. Future studies with sufficient number of patients of different age groups are needed to evaluate the impact of age on risks of second cancers among allogeneic HCT recipients using high-dose Bu-Cy regimen.
The types of solid cancers seen after TBI and non-TBI–based conditioning may be different. The use of myeloablative doses of TBI is particularly associated with cancers of the breast and thyroid and melanoma of the skin;4,5,9,12 we did not observe any increased risks for these malignancies in our study. However, we did find higher than usual risks of soft tissue and brain cancers among our cohort, which have also been associated with use of TBI.4,9 The risk of developing cancers of the lung was higher than that seen in the general population, which has not been described in previous series. Busulfan can cause pulmonary injury and fibrosis, but has not been implicated in the causation of lung cancer. We also demonstrate the association between smoking history before transplantation and lung cancer in this population. Exposure to alkylator chemotherapy can increase the risks of lung cancer in Hodgkin lymphoma survivors and smoking has been shown to further increase these risks.21 Hence, there may be a synergistic effect of smoking and Bu-Cy in the causation of lung cancer. The CIBMTR has collected limited data on smoking history, and studies that comprehensively evaluate the association of smoking with risks of lung cancer after HCT are needed. Similar to other studies, we observed chronic GVHD to be an independent risk factor for cancers of the oral cavity.3,9 Human papilloma virus may increase the risks of epithelial cancers among HCT recipients,22 and its association with such cancers in recipients of Bu-Cy conditioning also needs further study. An intriguing observation was the association between use of growth factors to promote engraftment and risk of secondary breast cancer. This finding should be interpreted with caution, since the overall number of patients with breast cancer in our study was small. Myeloid colony stimulating factors have been implicated in carcinogenesis and can promote tumor cell growth and migration, modulate the tumor stroma, and enhance angiogenesis.23,24 The clinical implications, if any, of these biologic mechanisms are not known.
The carcinogenesis of secondary solid neoplasms after chemotherapy is not well understood. Pathogenetic mechanisms such as DNA double-strand break-induced gene translocations and genomic instability due to loss of DNA repair, which have been described in secondary leukemia after chemotherapy and autologous HCT and secondary solid cancers after radiation therapy, may be relevant in this setting as well.25–27 The presence of inherited genetic polymorphisms may also modulate the risks of solid cancers after alkylator-based chemotherapy.28
Our study has the limitations of being a registry-based retrospective cohort study. Although we took several precautions to minimize ascertainment bias, secondary cancers are late posttransplant events, and centers may not be able to capture and report all cases of new solid cancers. The CIBMTR collects comprehensive information on pretransplant chemotherapy and radiation treatments and ensures quality of data by computerized checks for errors, physician reviews of submitted data, and onsite audits of centers; however, we may still not be able to capture all pretransplant treatment exposures. Notwithstanding these limitations, our study shows that the use of chemotherapy-based high-dose Bu-Cy conditioning regimen is associated with higher risks of secondary solid cancers among patients with AML in CR1 and CML in CP1 compared with the general population. Because there does not appear to be a plateau in cumulative-incidence over time since HCT, these patients need lifelong age-appropriate cancer surveillance and screening, as has been recommended by published guidelines.29 Chronic GVHD increases the risks of solid cancers, especially those of the oral cavity. Future larger studies are still needed to better understand the impact of other factors on the risk of second solid cancers.
Acknowledgments
We thank the members of the Writing Committee, including Mutlu Arat, Scott Baker, Smita Bhatia, Linda Burns, Jean-Yves Cahn, David Jacobsohn, James Gajewski, Vikas Gupta, Gregory Hale, Joerg Halter, Hillard Lazarus, Stephanie Lee, David Marks, Richard Maziarz, Vijay Reddy, Olle Ringden, Bipin Savani, Mohamed Sorror, and Jennifer Willert.
This work was supported by the organizations and grants that support the CIBMTR, including Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); 2 grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen Inc; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US Inc; Baxter International Inc; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical Inc; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix GmbH; Centers for Disease Control and Prevention; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc; CytoTherm; DOR BioPharma Inc; Dynal Biotech, an Invitrogen Company; Eisai Inc; Enzon Pharmaceuticals Inc; European Group for Blood and Marrow Transplantation; Gamida Cell Ltd; GE Healthcare; Genentech Inc; Genzyme Corporation; Histogenetics Inc; HKS Medical Information Systems; Hospira Inc; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co Ltd; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma Inc; Michigan Community Blood Centers; Millennium Pharmaceuticals Inc; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics Inc; Otsuka America Pharmaceutical Inc; Pall Life Sciences; Pfizer Inc; Saladax Biomedical Inc; Schering Corporation; Society for Healthcare Epidemiology of America; Soligenix Inc; StemCyte Inc; StemSoft Software Inc; Sysmex America Inc; THERAKOS Inc; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals Inc; ViraCor Laboratories; ViroPharma Inc; and Wellpoint Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.S.M., J.D.R., R.M.S., M.M.H., B.B., J.R.W., and G.S. designed the research, performed the data analysis, and wrote the manuscript; and R.B. and Z.W. performed the statistical analysis and were involved in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Navneet Majhail, Division of Hematology, Oncology, and Transplantation, University of Minnesota, 420 Delaware St Southeast, MMC 480, Minneapolis, MN 55455; e-mail: majha001@umn.edu.
References
- 1.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21(7):1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 3.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105(10):3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DL, Rovo A, Leisenring W, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111(2):939–944. doi: 10.1182/blood-2007-07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher G, Forrest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007;109(1):84–92. doi: 10.1002/cncr.22375. [DOI] [PubMed] [Google Scholar]
- 7.Kolb HJ, Socie G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131(10):738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 8.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24(7):1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socie G, Curtis RE, Deeg HJ, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18(2):348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 11.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A, Rovelli A, Merlo DF, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J Clin Oncol. 2007;25(17):2449–2454. doi: 10.1200/JCO.2006.08.9276. [DOI] [PubMed] [Google Scholar]
- 13.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359(9314):1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 14.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N. SEER Cancer Statistics Review, 1975-2006, based on November 2008 SEER data submission. National Cancer Institute. [Accessed August 1, 2010]. http://seer.cancer.gov/csr/1975_2006/
- 16.Curado MP, Edwards B, Shin HR, et al. Vol. IX. Lyon, France: International Agency for Research on Cancer Scientific Publications No. 160; 2007. Cancer Incidence in Five Continents. [Google Scholar]
- 17.Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol II. Lyon, France: International Agency for Research on Cancer Scientific Publications No. 82; 1987. The design and analysis of cohort studies. pp. 48–81. [PubMed] [Google Scholar]
- 18.Breslow NE. Elementary methods of cohort analysis. Int J Epidemiol. 1984;13(1):112–115. doi: 10.1093/ije/13.1.112. [DOI] [PubMed] [Google Scholar]
- 19.Preston DL, Lubin JH, Pierce DA. Seattle, WA: Hirosoft International; 1993. Epicure User's Guide. [Google Scholar]
- 20.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 21.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94(3):182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 22.Savani BN, Goodman S, Barrett AJ. Can routine posttransplant HPV vaccination prevent commonly occurring epithelial cancers after allogeneic stem cell transplantation? Clin Cancer Res. 2009;15(7):2219–2221. doi: 10.1158/1078-0432.CCR-08-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. 2006;66(16):8026–8036. doi: 10.1158/0008-5472.CAN-06-0158. [DOI] [PubMed] [Google Scholar]
- 24.Tsuruta N, Yatsunami J, Takayama K, Nakanishi Y, Ichinose Y, Hara N. Granulocyte-macrophage-colony stimulating factor stimulates tumor invasiveness in squamous cell lung carcinoma. Cancer. 1998;82(11):2173–2183. [PubMed] [Google Scholar]
- 25.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5(12):943–955. doi: 10.1038/nrc1749. [DOI] [PubMed] [Google Scholar]
- 26.Chakraborty S, Sun CL, Francisco L, et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;27(5):791–798. doi: 10.1200/JCO.2008.17.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynn RF, Cross MA, Hatton C, et al. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet. 1998;351(9097):178–181. doi: 10.1016/S0140-6736(97)08256-1. [DOI] [PubMed] [Google Scholar]
- 28.Fenske TS, McMahon C, Edwin D, et al. Identification of candidate alkylator-induced cancer susceptibility genes by whole genome scanning in mice. Cancer Res. 2006;66(10):5029–5038. doi: 10.1158/0008-5472.CAN-05-3404. [DOI] [PubMed] [Google Scholar]
- 29.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12(2):138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]