CLINICAL SCENARIO
A 52-year-old male is referred for persistent diarrhea, bloating, weight loss, and iron deficiency anemia following a diagnosis of celiac disease one year ago. At the time of his original diagnosis, serology was notable for a high IgA tissue transglutaminase titer >250 (normal less than 20), intraepithelial lymphocytosis and partial villous atrophy on small intestinal biopsy, and significant steatorrhea.
He reports being compliant with a gluten-free diet, though clinically his symptoms have only marginally improved. Recent laboratories (within a month from referral) show a normal IgA tissue transglutaminase titer, microcytic anemia, low ferritin, and elevated fecal fat (12 grams/24 hours on 100gram daily fat intake). Repeat upper endoscopy shows mucosal scalloping on gross exam and partial villous atrophy on small bowel histology.
What would be the next step in further evaluation of the ongoing symptoms and additional potential etiologies?
THE PROBLEM
This vignette illustrates a case of nonresponsive celiac disease (NRCD). NRCD is defined by a lack of initial response to a prescribed gluten-free diet (GFD), or the recurrence of symptoms despite maintenance of GFD in a patient who responded initially to GFD. The exact prevalence of NRCD is unknown but the presence of symptoms after treatment with a GFD is common in patients with celiac disease (CD). This clinical problem requires a systematic diagnostic and therapeutic approach because of the many distinct underlying etiologies. What are the expected clinical, serological, and histological responses after treatment with a GFD? The amount of time to fell better on a GFD is different for every person but clinical improvement is usually evident within weeks after treatment with a GFD. CD-specific serology, which is gluten-dependent, normalizes by 6-12 months in most patients with strict adherence to GFD. Mucosal recovery after GFD may take longer than 1 year despite strict adherence to the GFD and may be incomplete, especially in adult-onset CD.
MANAGEMENT STRATEGIES AND SUPPORTING EVIDENCE
Does this patient have celiac disease?
The clinical case discussed here concerns a middle-aged man with a clinical diagnosis of CD well supported by typical symptoms (malabsorption), high titers of IgA tissue transglutaminase antibody (overall specificity >95%) and by the presence of partial villous atrophy in the duodenal biopsy. Thus, although the first step when faced to patients with NRCD is to evaluate the certainty of the original diagnosis, an alternative original diagnosis in this case seems unlikely. When the original diagnosis of CD is uncertain, we strongly recommend that the quality of the studies and not just the results should be reviewed. Patients that are self diagnosed or whose initial diagnosis did not follow the usual diagnostic recommendations often do not have CD even if they have clinical improvement after gluten exclusion. Inquiry about the type of serological test(s) that was used to support the diagnosis as well as any dietetic restrictions (e.g., self-prescribed GFD) at the time of initial evaluation may be relevant to refute (or support) the diagnosis of CD because the diagnostic accuracy of serology vary among tests (and centers) and may be affected by a GFD. Anti-gliadin antibodies have a poor specificity for CD and should not be regarded as strong evidence to support CD diagnosis. A positive family history may be supportive evidence for CD, especially if a first-degree family member has biopsy-confirmed CD. A history of biopsy-proven dermatitis herpetiformis confirms CD as the underlying cause of villous atrophy. Re-review of the original intestinal biopsy slides by an expert pathologist may reveal an alternative diagnosis. The use of small-bowel mucosa tissue transglutaminase 2-specific IgA autoantibody deposits may help to distinguish NRCD from other types of enteropathy, especially when the original CD diagnosis is uncertain or for seronegative patients. This method is not widely available, require frozen specimens and experience for the interpretation of results. Finally, the absence of human leukocyte antigens (HLA)-DQ2 or DQ8 alleles may be extremely useful to rule out CD when biopsy results are inconsistent with serology.
What is the differential diagnosis when the original diagnosis of CD is uncertain?
The differential is extensive and should include all other many causes of villous atrophy besides CD. Other causes of villous atrophy with characteristic histology such as Whipple’s disease, collagenous sprue, and eosinophilic enteritis may be excluded by re-review of the intestinal biopsy.
A recent travel or residence history to tropical areas including Southeast Asia, tropical Africa, Central and South America, or the Caribbean islands would increase suspicion of tropical sprue being the underlying cause of persistent symptoms. If additional diseases have been excluded and tropical sprue is a strong consideration, empiric treatment with antibiotics and folate would be indicated.
Autoimmune enteropathy (AIE), collagenous sprue, and Whipple’s disease could be additional potential etiologies, though these diseases are less common. A recent case series on AIE characterized the overlapping clinical and histological features of AIE and CD. The distinguishing features of positive gut epithelial cell antibodies (anti-enterocyte and/or anti-goblet cell antibody) and persistent villous atrophy with associated malabsorption despite gluten restriction are suggestive of AIE. Intraepithelial lymphocytosis could be observed in patients with AIE. Gut epithelial cell antibodies are not specific for AIE but support the diagnosis when other clinical criteria for AIE are present. Anti-goblet cell antibodies may be especially nonspecific and should be interpreted cautiously. There is a need for rigorous studies regarding sensitivity and specificity of gut epithelial cell antibodies. Collagenous sprue is characterized by villous atrophy, severe symptoms, and a thick subepithelial collagen band. Collagenous sprue is a heterogeneous disorder that may or may not be associated with CD. The mainstay of treatment for both starts with either systemic or topical steroids, though experience is limited due to the rarity of these diseases. Immunosuppressive drugs and total parenteral nutrition are often necessary in patients with either AIE or collagenous sprue. The GFD can be dispensed with those patients who do not carry the at-risk HLA alleles associated with CD.
Causes of Nonresponsive Celiac Disease
Intentional or inadvertent gluten contamination is the most common cause of NRCD (Figure 1). Deliberate ingestion of gluten is not often acknowledged but most commonly patients are unknowingly ingesting gluten, highlighting the importance of expert dietary instruction with the initial diagnosis of CD and a detailed dietary review when patients present with a less than adequate response to a GFD. While serological follow-up tests are not especially sensitive to low level gluten intake, a persistently positive test after 1 year of a GFD suggests a high level of gluten ingestion. A normal follow-up serology doesn’t guarantee either adherence to GFD or mucosal recovery of the intestine. The discovery of gluten contamination by dietary review or by inference from serological testing should be broached tactfully with the patients providing them with information and support to assist in identifying and eliminating gluten sources. Membership of a CD support group could be useful for some patients to remain gluten free. If gluten contamination has been reasonably excluded, other additional disorders associated with NRCD must be actively investigated (Table 1). Repeat upper endoscopy with intestinal biopsy may help to differentiate causes of NRCD associated with persistent mucosal damage from those usually associated with normal duodenal mucosa.
Figure 1.
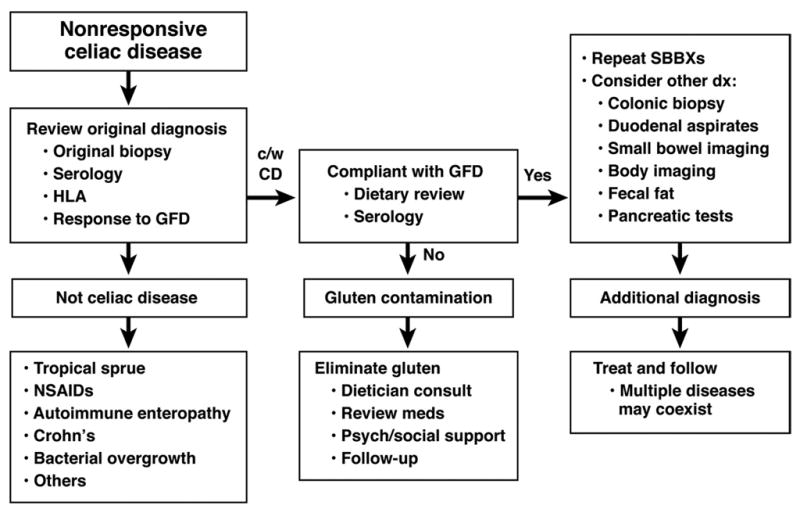
Diagnostic approach in non-responsive celiac disease.
Table 1.
Differential diagnosis of nonresponsive celiac disease
| Etiology | Diagnostic approach | Comments |
|---|---|---|
| Gluten contamination* | Dietary review, celiac serology, intestinal biopsy | Most frequent cause of nonresponsive celiac disease |
| Small intestine bacterial overgrowth* | Breath tests, culture of intestinal fluid, empiric trial with antibiotics | Frequent |
| Exocrine pancreatic insufficiency | Fecal elastase or other indirect pancreatic function tests, empiric trial with pancreatic enzymes | Low fecal elastase observed in up to 30% treated celiacs with diarrhea |
| Microscopic colitis | Colonic biopsies | Frequent, suspect if recurrent watery diarrhea |
| Refractory celiac disease* | Detection of abnormal (clonal) intraepithelial lymphocytes, extensive endoscopic and imaging evaluation | Rare, diagnosis of exclusion that may be supported by positive findings |
| Functional bowel disorders | Clinical criteria | Common, diagnosis should be strongly suspected in patients with normal histology and prominent symptoms |
| Protein-losing enteropathy | Fecal α-1 antitrypsin | Rare, suspect if severe hypoalbuminemia or lymphopenia |
| Lactose and fructose intolerance | Breath tests, food exclusion trial | Prevalence in nonresponsive CD unknown |
| Giardiasis* | Microscopic analysis of stool or intestinal fluid, Giardia antigen in stool | Frequent cause of chronic symptoms and malabsorption. Prevalence of giardiasis in nonresponsive CD unknown |
| Malignancies | Bowel & body imaging, deep- enteroscopy with biopsies | Rare but important especially in old adults |
Disorders associated with abnormal duodenal biopsy on follow-up
Small intestinal bacterial overgrowth and exocrine pancreatic insufficiency are two causes of persistent steatorrhea despite treatment with a GFD. Quantitative cultures of duodenal aspirates or a positive breath test (e.g., lactulose) are characteristic of bacterial overgrowth but clinical response to antibiotics confirms the diagnosis. Low fecal elastase (or other indirect pancreatic function tests) is a common finding in NRCD and may suffice to evaluate for exocrine pancreatic insufficiency, though an empiric trial of pancreatic enzymes supplementation is a useful approach when steatorrhea persists.
The risk for microscopic colitis is increased up to 70-fold in the celiac disease population as compared to the general population. Microscopic colitis is a frequent cause of persistent or more often recurrent watery diarrhea though is less likely in patients with steatorrhea. If other etiologies are excluded, colonic biopsies taken proximal to the rectosigmoid junction are indicated. There is currently no consensus on whether flexible sigmoidoscopy or colonoscopy is the best approach for endoscopic biopsy in microscopic colitis. Most patients with abnormal histology may have evidence of disease in the left colon but colonoscopy may be necessary to unmask patients with isolated right-side disease (~10%).
A subgroup of patients with NRCD may have more than one disorder associated with persistent symptoms (e.g., concurrent microscopic colitis and bacterial overgrowth). Thus, for these patients, specific treatment for all associated disorders is necessary to alleviate symptoms.
Finally, after exclusion of other more frequent causes, refractory celiac disease should be considered in patients with severe symptoms and NRCD.
Does this patient have refractory celiac disease?
Refractory CD (RCD) is a rare complication of CD defined by “persistent or recurrent malabsorptive symptoms and villous atrophy despite strict adherence to a GFD for at least 6–12 months in the absence of other causes of NRCD and overt malignancy.” These patients are at higher risk for T-cell lymphoma than celiacs in remission and a careful search for the complication of lymphoma is necessary. RCD type 1 is characterized by polyclonal T-cell expansion in the mucosa. The presence of abnormal (clonal) expansion of the intraepithelial lymphocytes is the hallmark of RCD type 2. The abnormal intraepithelial lymphocyte phenotype is supported by the loss of normal surface markers CD3, CD4 and CD8 with preserved expression of intracytoplasmic CD3 (e.g., ≥40–50% by immunohistochemistry or >20–25% by flow cytometry) and by T-cell receptor clonal rearrangement by polymerase chain reaction. Interval monitoring of intraepithelial lymphocyte immunophenotype and clonality may be more accurate than a single snapshot evaluation to classify RCD subtype and to predict risk of lymphomagenesis. The abnormal T-cells (clone) may serve as a source of the devastating enteropathy-associated T cell lymphoma. Both RCD type 1 and type 2 RCD can be associated with excess mortality. Mortality in RCD type 1 is more often due to severe malnutrition and/or secondary infection especially early in the course of the disease. RCD type 2 is associated with increased mortality due to an especially high risk of progression to enteropathy-associated T-cell lymphoma. Novel techniques are available to accurately examine either the whole small-bowel or extraintestinal tissues (e.g., wireless capsule endoscopy, balloon enteroscopy, CT- and MRI-enterography, PET scan). A combination of these techniques could be very useful to distinguish between severe CD and complicated CD and to exclude malignancy in RCD. Management of RCD requires careful attention to correcting the fluid and nutritional deficiencies that are common in these patients. Bone disease may be severe and parenteral nutrition may be necessary. The mainstay of treatment for RCD type 1 is steroid therapy with prednisone or more recently, budesonide. Azathioprine either alone or in combination with steroids can be used in those with RCD type 1. Oral cyclosporine may be effective for RCD type 1 but severe side effects require consideration. There is no established treatment for RCD type 2. (Figure 2) Although clinical response can be observed in most patients with RCD type 2 after treatment with steroids, the risk of progression to lymphoma is not affected. Intravenous cladribine can be useful for some patients with RCD type 2 but lymphomagenesis still a concern. Potent chemotherapy followed by autologous hematopoietic stem cell rescue has been explored for treatment of RCD type 2 in a pilot study from a single center. In the near future, interleukin-15 blockade may be a potential therapeutic target for RCD type 2. Long term prognosis of RCD depends in large part on the presence or absence of these aberrant T-cell populations and progression to lymphoma although other clinical factors may be relevant such as older age, low hemoglobin, hypoalbuminemia, and degree of villous atrophy at diagnosis of the refractory state.
Figure 2.
Diagnostic and therapeutic approach in refractory celiac disease
AREAS OF UNCERTAINTY
The prevalence and causes of NRCD in the community are not known yet. The most cost-effective approach for NRCD remains to be determined. The long-term outcomes after intervention in patients with NRCD are incompletely understood. The best therapeutic approach for asymptomatic subjects with persistently abnormal mucosa after GFD is unknown.
PUBLISHED GUIDELINES
The AGA Institute technical review on the diagnosis and management of CD published in 2006 outlines the key aspects of evaluating a celiac patient with persistent symptoms: verification of the original diagnosis and compliance with a GFD, and consideration of either other causes of NRCD or severe complications (e.g., RCD and malignancies).
RECOMMENDATIONS FOR THIS PATIENT
In the patient described in the vignette, an empiric trial with pancreatic enzymes led to complete resolution of his symptoms. Gluten contamination was ruled out by an expert dietitian but adherence to GFD was reinforced. One year later, the patient remains asymptomatic two months after stopping pancreatic enzyme supplementation with both normal serology and histology.
Acknowledgments
Grant support: This work was supported in part by NIH Training Grant in Digestive Diseases T32 DK07198 (to SHB), NIH Training Grant in Gastrointestinal Allergy and Immunology T32AI-07047 (to AR-T) and NIH Grant DK57892 (to JAM).
Abbreviations used in this paper
- AIE
autoimmune enteropathy
- CD
celiac disease
- GFD
gluten-free diet
- NRCD
nonresponsive celiac disease
- RCD
refractory celiac disease
Footnotes
Financial disclosure: Dr. Murray has been a consultant to Astra Zeneca, Alvine, and Novartis, and an investigator for Alba Therapeutics and Dynagen. Dr. Rubio-Tapia and Dr. Barton declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suggested readings
- 1.Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr. 2004;79:669–73. doi: 10.1093/ajcn/79.4.669. [DOI] [PubMed] [Google Scholar]
- 2.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal Recovery and Mortality in Adults With Celiac Disease After Treatment With a Gluten-Free Diet. Am J Gastroenterol. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campanella J, Biagi F, Ilaria Bianchi P, Zanellati G, Marchese A, Roberto Corazza G. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand J Gastroenterol. 2008:1–4. doi: 10.1080/00365520802200036. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Tapia A, Van Dyke CT, Lahr BD, Zinsmeister AR, El-Youssef M, Moore SB, Bowman M, Burgart LJ, Melton LJ, 3rd, Murray JA. Predictors of family risk for celiac disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:983–7. doi: 10.1016/j.cgh.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koskinen O, Lindfors K, Collin P, Peraaho M, Laurila K, Woolley N, Partanen J, Maki M, Kaukinen K. Intestinal transglutaminase 2 specific antibody deposits in non-responsive coeliac disease. Dig Liver Dis. doi: 10.1016/j.dld.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–8. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 8.Owens SR, Greenson JK. The pathology of malabsorption: current concepts. Histopathology. 2007;50:64–82. doi: 10.1111/j.1365-2559.2006.02547.x. [DOI] [PubMed] [Google Scholar]
- 9.Westergaard H. Tropical Sprue. Curr Treat Options Gastroenterol. 2004;7:7–11. doi: 10.1007/s11938-004-0020-6. [DOI] [PubMed] [Google Scholar]
- 10.Akram S, Murray JA, Pardi DS, Alexander GL, Schaffner JA, Russo PA, Abraham SC. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007;5:1282–90. doi: 10.1016/j.cgh.2007.05.013. quiz 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corazza GR, Biagi F, Volta U, Andreani ML, De Franceschi L, Gasbarrini G. Autoimmune enteropathy and villous atrophy in adults. Lancet. 1997;350:106–9. doi: 10.1016/S0140-6736(97)01042-8. [DOI] [PubMed] [Google Scholar]
- 12.Biagi F, Bianchi PI, Trotta L, Corazza GR. Anti-goblet cell antibodies for the diagnosis of autoimmune enteropathy? Am J Gastroenterol. 2009;104:3112. doi: 10.1038/ajg.2009.511. [DOI] [PubMed] [Google Scholar]
- 13.Rubio-Tapia A, Talley NJ, Gurudu SR, Wu TT, Murray JA. Gluten-free diet and steroid treatment are effective therapy for most patients with collagenous sprue. Clin Gastroenterol Hepatol. 8:344–349. e3. doi: 10.1016/j.cgh.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vakiani E, Arguelles-Grande C, Mansukhani MM, Lewis SK, Rotterdam H, Green PH, Bhagat G. Collagenous sprue is not always associated with dismal outcomes: a clinicopathological study of 19 patients. Mod Pathol. 23:12–26. doi: 10.1038/modpathol.2009.151. [DOI] [PubMed] [Google Scholar]
- 15.Pietzak MM. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology. 2005;128:S135–41. doi: 10.1053/j.gastro.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Rubio-Tapia A, Barton SH, Rosenblatt JE, Murray JA. Prevalence of small intestine bacterial overgrowth diagnosed by quantitative culture of intestinal aspirate in celiac disease. J Clin Gastroenterol. 2009;43:157–61. doi: 10.1097/MCG.0b013e3181557e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leeds JS, Hopper AD, Hurlstone DP, Edwards SJ, McAlindon ME, Lobo AJ, Donnelly MT, Morley S, Sanders DS. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther. 2007;25:265–71. doi: 10.1111/j.1365-2036.2006.03206.x. [DOI] [PubMed] [Google Scholar]
- 18.Green PH, Yang J, Cheng J, Lee AR, Harper JW, Bhagat G. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol. 2009;7:1210–6. doi: 10.1016/j.cgh.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Pardi DS. Microscopic colitis: an update. Inflamm Bowel Dis. 2004;10:860–70. doi: 10.1097/00054725-200411000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016–21. doi: 10.1111/j.1572-0241.2002.05917.x. [DOI] [PubMed] [Google Scholar]
- 21.Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 59:547–57. doi: 10.1136/gut.2009.195131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 23.Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5:445–50. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Evans KE, Sanders DS. Joint BAPEN and British Society of Gastroenterology Symposium on 'Coeliac disease: basics and controversies'. Coeliac disease: optimising the management of patients with persisting symptoms? Proc Nutr Soc. 2009;68:242–8. doi: 10.1017/S0029665109001360. [DOI] [PubMed] [Google Scholar]
- 25.Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:413–24. doi: 10.1016/j.bpg.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Biagi F, Corazza GR. Defining gluten refractory enteropathy. Eur J Gastroenterol Hepatol. 2001;13:561–5. doi: 10.1097/00042737-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 28.Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373–8. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cellier C, Cerf-Bensussan N. Treatment of clonal refractory celiac disease or cryptic intraepithelial lymphoma: A long road from bench to bedside. Clin Gastroenterol Hepatol. 2006;4:1320–1. doi: 10.1016/j.cgh.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Brar P, Lee S, lewis S, Egbuna I, Bhagat G, Green PH. Budesonide in the treatment of refractory celiac disease. Am J Gastroenterol. 2007;102:2265–69. doi: 10.1111/j.1572-0241.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 31.Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. quiz 352–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, Bouhnik Y, Colombel JF, Delchier JC, Allez M, Cosnes J, Lavergne-Slove A, Meresse B, Trinquart L, Macintyre E, Radford-Weiss I, Hermine O, Brousse N, Cerf-Bensussan N, Cellier C. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 33.Al-Toma A, Goerres MS, Meijer JW, von Blomberg BM, Wahab PJ, Kerckhaert JA, Mulder CJ. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin Gastroenterol Hepatol. 2006;4:1322–7. doi: 10.1016/j.cgh.2006.07.007. quiz 1300. [DOI] [PubMed] [Google Scholar]
- 34.Al-toma A, Visser OJ, van Roessel HM, von Blomberg BM, Verbeek WH, Scholten PE, Ossenkoppele GJ, Huijgens PC, Mulder CJ. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood. 2007;109:2243–9. doi: 10.1182/blood-2006-08-042820. [DOI] [PubMed] [Google Scholar]
- 35.Verbeek WH, Goerres MS, von Blomberg BM, Oudejans JJ, Scholten PE, Hadithi M, Al-Toma A, Schreurs MW, Mulder CJ. Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in Refractory Celiac Disease. Clin Immunol. 2008;126:48–56. doi: 10.1016/j.clim.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Brais R, Lavergne-Slove A, Jeng Q, Payne K, Ye H, Liu Z, Carreras J, Huang Y, Bacon CM, Hamoudi RA, Save V, Venkatraman L, Isaacson PG, Woodward J, Du MQ. Continual monitoring of intraepithelial lymphocyte immunophenotype and clonality is more important than snapshot analysis in the surveillance of refractory coeliac disease. Gut. 59:452–60. doi: 10.1136/gut.2009.186007. [DOI] [PubMed] [Google Scholar]



