Abstract
Ghrelin microinjections into discrete regions of the hypothalamus, including the paraventricular nucleus (PVN), stimulate eating and promote carbohydrate oxidation, effects similar to PVN microinjection of neuropeptide Y (NPY). We have also reported that NPY’s orexigenic and metabolic effects are antagonized by pretreatment with 5-hydroxytryptamine (5-HT) or 5-HT receptor agonists. In order to determine whether 5-HT also inhibits ghrelin’s orexigenic and metabolic actions, the present study examined the effects of 5-HT pretreatment on ghrelin-induced alterations in eating and energy substrate utilization following direct injections into the hypothalamic PVN. Both 5-HT (5–20 nmol) and ghrelin (100 pmol) were administered at the onset of the dark cycle. Food intake was measured 2 h postinjection. A separate group of rats (n=8) was injected with 5-HT paired with ghrelin and respiratory quotient (RQ; VCO2/VO2) was measured over 2 h using an open circuit calorimeter. PVN injections of ghrelin increased food intake and increased RQ, reflecting a shift in energy substrate utilization in favor of carbohydrate oxidation. 5-HT effectively blocked the effects of ghrelin on both food intake and RQ. We then administered the 5-HT2A/2C, receptor agonist, DOI, immediately prior to ghrelin. Similar to 5-HT, PVN DOI blocked ghrelin-induced eating and inhibited the peptide’s effect on substrate utilization. These data are in agreement with other evidence suggesting that ghrelin functions as a gut-brain peptide in the control of food intake and energy metabolism, and indicate that 5-HT acts within the PVN to modulate ghrelin’s orexigenic and metabolic signaling.
Keywords: DOI, Energy metabolism, Food intake, Ghrelin, Paraventricular nucleus, Respiratory quotient, Serotonin, 5-HT agonist
I. Introduction
Ghrelin is a 28 amino acid peptide transmitter with a structural homology highly conserved across mammalian species (El-Salhy, 2009; Kojima & Kangawa, 2005). In both rat and human ghrelin the hydroxyl group at serine 3 is modified by n-octanoic acid (Dehlin et al, 2008; Kangawa, 2006; Smith et al., 2001). It is this acylation and conferral of a fatty acid side chain that is a unique biochemical characteristic associated with the peptide’s functional activity (Dehlin et al., 2008; Hosoda et al., 2000). Peripheral ghrelin is synthesized and released primarily by endocrine cells of the gastric oxyntic mucosa (Date et al., 2000; El-Salhy, 2009) and central production of ghrelin has been identified within the hypothalamus where ghrelin receptors have been localized (Cowley et al., 2003; Mondal et al., 2005).
Intracerebroventricular injections of ghrelin increase c-Fos expression in multiple hypothalamic nuclei (Lawrence et al., 2002). Paraventricular nucleus (PVN) injections of the peptide, at orexigenic doses, induce increased c-Fos immunoreactivity in the PVN as well as the arcuate and dorsomedial nuclei (Olszewski et a., 2003). The distribution of ghrelin-producing cell bodies, axons and receptors is consistent with a hypothalamic site of action. Ghrelin interacts with other peptidergic systems within the hypothalamus including neuropeptide Y/agouti-related peptide (NPY/AGRP) neurons (Cowley et al., 2003; Gil-Campos et al., 2006; Hori et al., 2008; Willesen et al., 1999). Hypothalamic PVN microinjection of ghrelin increases food intake and alters energy metabolism specifically by increasing respiratory quotient or carbohydrate oxidation (Currie et al. 2005; Melis et al., 2002; Szentirmai et al., 2007). Ghrelin’s orexigenic and metabolic actions are comparable to NPY as both neuropeptides elicit similar effects on eating and nutrient partitioning (Currie et al., 2003; Currie et al., 2005). These findings are in agreement with the hypothesis that ghrelin, like NPY, alters appetite and energy metabolism by acting on hypothalamic circuits mediating positive energy balance.
We have previously reported that pretreatment with either 5-hydroxtryptamine (5-HT) or DOI, a 5-HT2A/2C receptor agonist, microinjected into the PVN, inhibits feeding stimulated by PVN administration of NPY as well as NPY induced alterations in energy substrate utilization (Currie, 2003; Currie et al., 2002; Currie & Coscina, 1998). A similar effect was not found following DOI pretreatment in the perifornical or ventromedial hypothalamus (Currie & Coscina, 1997) suggesting that NPY-5-HT interactions are specific to the PVN. In order to assess whether serotonergic mechanisms also alter the orexigenic and metabolic action of ghrelin, the present study investigated the effects of 5-HT or DOI pretreatment on ghrelin-stimulated eating and substrate utilization. The results of this study, described recently in preliminary form (Currie et al., 2009), indicate that both 5-HT and DOI, injected into the PVN at the onset of the dark cycle, inhibit the increases in eating and RQ elicited by ghrelin.
2. Materials and methods
2.1. Animals
Adult male Sprague-Dawley rats were purchased from Harlan Laboratories and weighed 275–300 g at the time of surgery. Rats were housed individually in polypropylene cages with free access to standard rodent chow pellets and water. The animal colony room was maintained on a 12 h light/dark cycle (lights on at 0400 h) and at a temperature of 22 ± 2°C. All procedures described below were approved by the Institutional Animal Care and Use Committee of Reed College.
2.2. Apparatus
Oxygen consumption (O2) and carbon dioxide (CO2) production were measured using an Oxyscan open-circuit indirect calorimeter (AccuScan Instruments, Columbus, OH). Detectors measured O2 and CO2 sequentially across each acrylic test chamber. The flow rate was set at 1500 ml/min. Concentrations of the gases were recorded in ml/kg body weight/min. RQ was calculated as the volume of CO2 produced (VCO2) divided by the volume of O2 consumed (VO2). The analysers were calibrated prior to each test using primary gas standards of high purity (Polar Cryogenics, Portland, OR).
2.3. Microinjections
Ghrelin (Research Biopeptides), 5-hydroxytryptamine (Sigma) and DOI (2; 5-dimethoxy-4-iodoamphetamine, Sigma) were dissolved in sterile saline. All injections were administered in a volume of 0.2 µl into the PVN using a microinjector extending 4 mm beyond the permanent guide cannula. Doses were selected based on previous reports examining effects on eating behavior and energy metabolism (Currie, 2003; Currie et al., 2005; Fletcher et al., 1992).
2.4. Design and Procedure
Rats were anesthetized with pentobarbital sodium (50 mg/kg IP) and placed in a Kopf stereotaxic frame with the incisor bar set 3.6 mm below the interaural line. PVN coordinates for the guide cannula relative to bregma were AP −1.8 mm, L −0.3 mm, and V −4.5 mm (Paxinos & Watson, 2007). Unilateral guide cannulae (22-gauge; Plastics One, Roanoke, VA) were implanted 4 mm dorsal to the PVN. Implants were secured with acrylic cement and stainless steel screws penetrating the skull. A 28-gauge stainless-steel inner stylet maintained cannula patency. Behavioral and metabolic testing began following a postoperative recovery period of two weeks.
In feeding tests, separate groups of rats were cannulated to examine the effects of 5-HT (n=10) or DOI (n=10) on ghrelin-stimulated eating. Both 5-HT (5 or 20 nmol) and DOI (2.5 or 5 nmol) were administered 5 min prior to 100 pmol of ghrelin, which itself was injected just before the start of the nocturnal cycle. Under control conditions, two consecutive vehicle injections of sterile saline were administered. Food intake was measured 2 h postinjection. While different groups of animals were used to examine the effects of 5-HT or DOI on ghrelin-induced eating, within a particular group, all rats received each dose of transmitter/drug paired with ghrelin, in a randomized order. At least 4 days separated successive testing.
Similar injection procedures and treatments were followed for metabolic testing using separate groups of rats. Again, 5-HT or DOI (n=8/group) was injected 5 min prior to ghrelin (100 pmol). All injections were administered into the PVN. Respiratory quotient was measured over a 2-h period and testing began immediately after the rats were placed into the apparatus. Food and water were not available during this time.
2.5. Histological and statistical analyses
Cannulae placements were confirmed via histological examination. Sections were examined by light microscopy and viewed relative to the stereotaxic atlas of Paxinos and Watson (Paxinos & Watson, 2007). All rats reported here were found to have injector tracks extending into the PVN.
Data were analyzed by separate analyses of variance (ANOVA) for repeated measures. Specific comparisons between means were evaluated using post hoc Tukey tests. The criterion for statistical significance was p<0.05.
Results
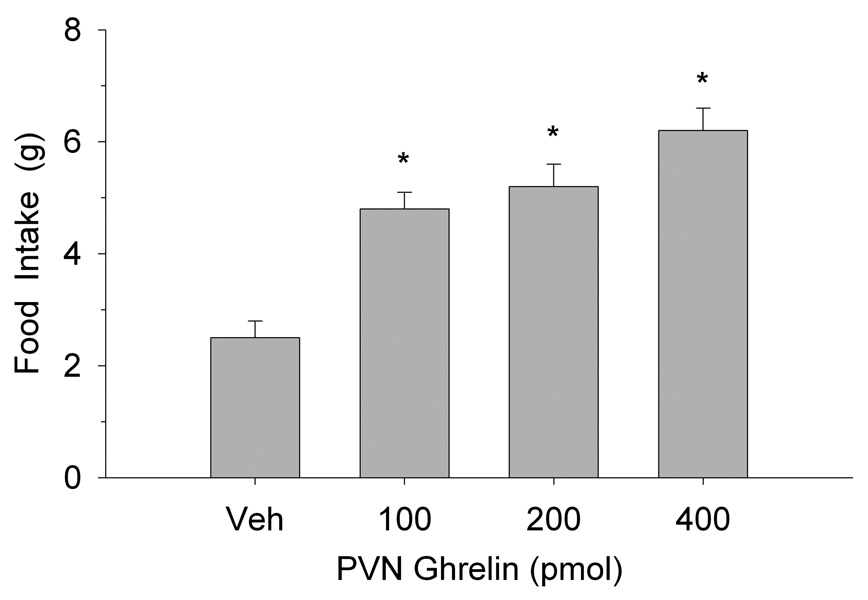
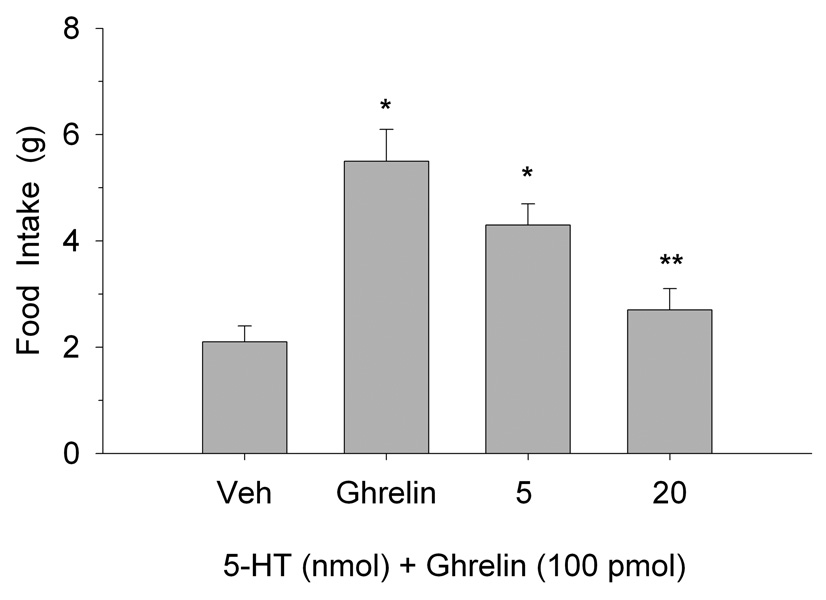
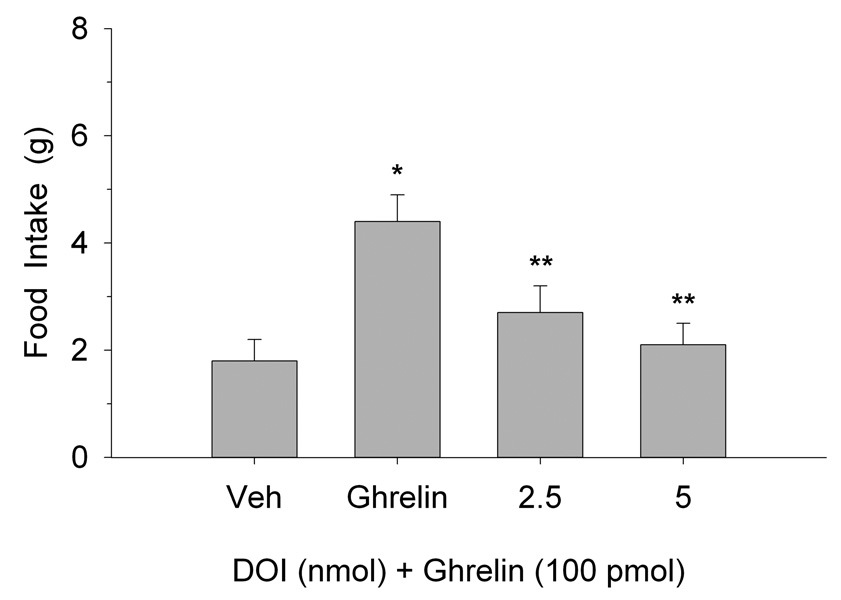
Figure 1 illustrates the effects of PVN ghrelin microinjection on 2-h food intake at the onset of the nocturnal cycle. One-way analysis of variance (ANOVA) indicated that ghrelin increased eating behavior at each dose tested (F(2,18)=13.2, p<0.0002). The effects of PVN administration of 5-HT on ghrelin-stimulated-eating are shown in Figure 2. One-way ANOVA confirmed that PVN ghrelin elicited a significant increase in 2 h food intake and that pretreatment with 5-HT antagonized this effect (F(3,27)=6.65, p<0.002). In fact, the 20 nmol dose completely blocked ghrelin’s orexigenic effect. Similarly, eating resulting from ghrelin administration was also attenuated by PVN pretreatment with both doses of DOI (F(3,27)=89.3, p<0.0001, as depicted in Figure 3.
Fig. 1.
Food intake after ghrelin microinjection into the hypothalamic PVN. Values represent mean intakes (±S.E.M.) measured over the initial 2 h of the nocturnal cycle. *P<0.05 compared to saline vehicle (Veh).
Fig. 2.
Effects of PVN 5-HT administration on the feeding-stimulant action of ghrelin. Values are represented as mean intakes (±S.E.M.) over 2 h. *P<0.05 compared to Veh; **P<0.05 compared to ghrelin.
Fig. 3.
Effects of pretreatment with the 5-HT2A/2C receptor agonist, DOI, injected into the PVN, on eating elicited by PVN injection of ghrelin. Values are mean intakes (±S.E.M.) over 2 h. *P<0.05 compared to Veh; **P<0.05 compared to ghrelin.
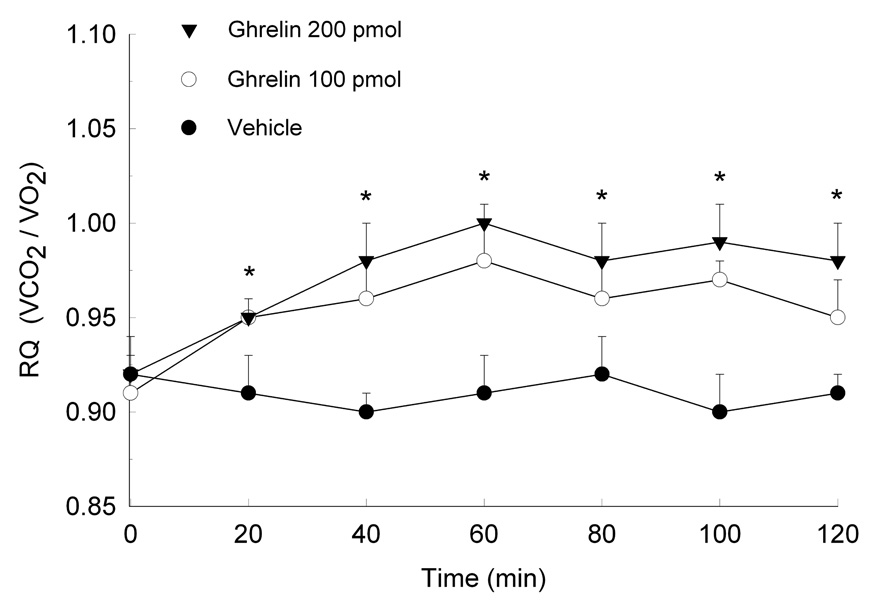
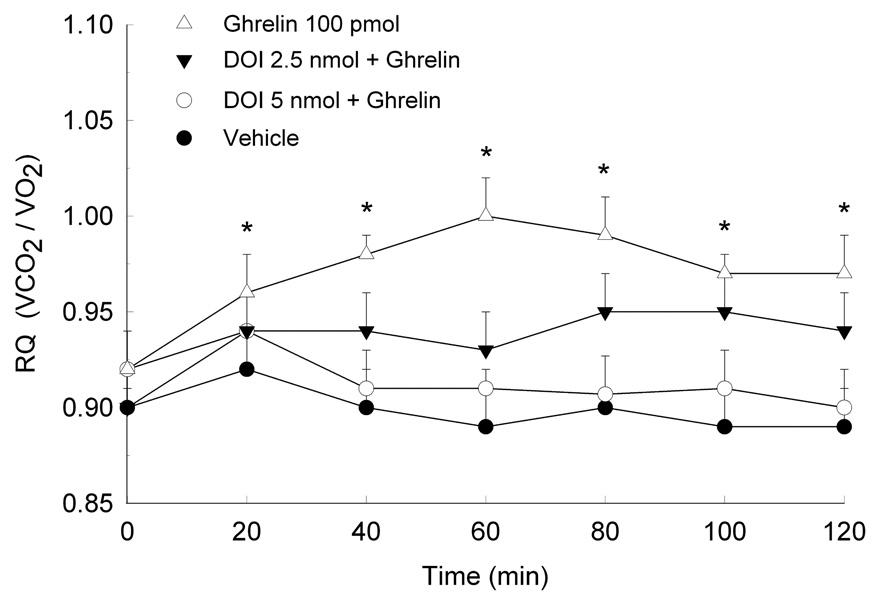
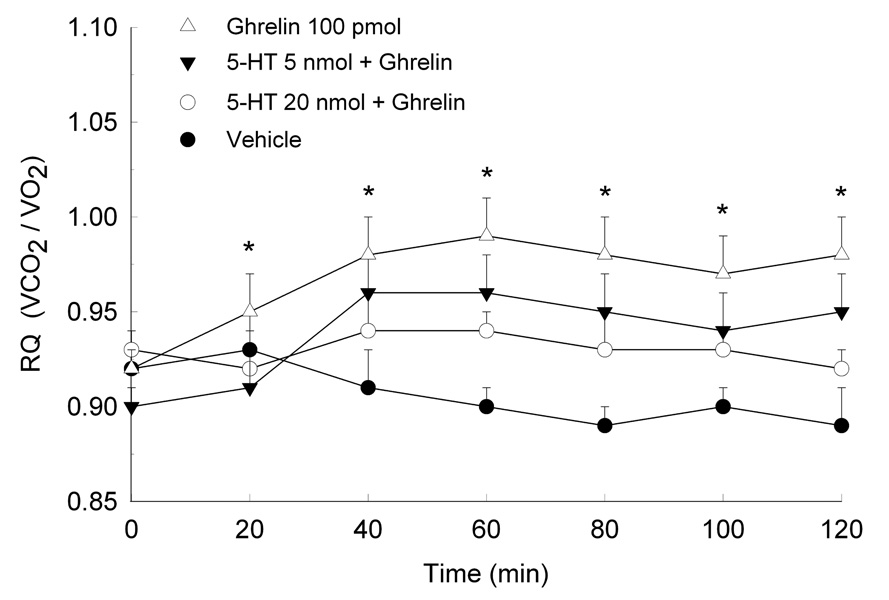
The effects of ghrelin on RQ and the impact of 5-HT and DOI on ghrelin-induced alterations in substrate utilization are shown in Figures 4–6. Data are presented as mean RQ (±SEM) values over the initial 2 h of the dark period and are shown in 20-min intervals. Two-way ANOVA for repeated measures showed that ghrelin reliably increased RQ over the entire 2-h test period (Treatment × Time interaction, (48,336)=78.3, p<0.0001). The increase in RQ was observed within 20 minutes of testing and remained for the duration of the 2 hours. When rats were pretreated with either 5-HT (F(72,504)=21.5, p<0.001) or DOI (F(72,504)=131.6, p<0.0001), ghrelin’s metabolic effects were attenuated. Both doses of 5-HT and DOI were effective in reducing ghrelin’s effects on substrate utilization. Indeed, the higher DOI dose of 5 nmol completely reversed ghrelin’s effects, as RQ values in these rats were comparable to control.
Fig. 4.
Mean (±S.E.M.) RQ following PVN administration of ghrelin. Energy substrate utilization was measured over the 2 h period following treatment. *P<0.05 compared to vehicle.
Fig. 6.
Mean (±S.E.M.) RQ values in rats pretreated with the 5-HT2A/2C receptor agonist, DOI, and then microinjected with ghrelin. *P<0.05 compared to Veh.
4. Discussion
The PVN is one of several hypothalamic sites responsive to the feeding-stimulant effects of ghrelin (Currie et al., 2005; Melis et al., 2002; Szentirmai et al., 2007). This suggests that ghrelin-releasing neurons innervate multiple hypothalamic systems implicated in energy intake and metabolism. However, additional evidence indicates that the PVN, in comparison with other hypothalamic sites, may function uniquely to coordinate various aspects of energy metabolism and appetite control (Currie, 2003; Currie et al., 1996). In the present study both 5-HT and DOI attenuated the effects of ghrelin on food intake and RQ. These findings are consistent with the role of the PVN in mediating the action of orexigenic and metabolic signaling peptides. PVN ghrelin, as shown here, elicited a robust effect on food intake and shifted RQ in the direction of enhanced carbohydrate oxidation. Such eating and metabolic effects are similar to those observed following NPY administration (Currie, 2003; Currie et a., 1996) indicating that both NPY and ghrelin may contribute to similar homeostatic controls. In fact ghrelin is reported to potentiate the effects of NPY on eating and RQ (Currie et al., 2005) suggesting a possible interaction between these systems.
We have previously demonstrated that PVN injections of 5-HT and the 5-HT2A/2C agonist, DOI, suppress eating elicited by NPY as well as NPY-induced alterations in RQ (Currie, 2003; Currie et al., 2002; 1999; 1997). DOI’s effects were blocked by 5-HT2A receptor antagonism providing evidence that the activation of PVN 5-HT2A receptors inhibits NPY’s effects on eating and substrate utilization (Currie et al., 2002). The present study indicates that DOI inhibits the orexigenic and metabolic effects of PVN ghrelin. In particular, the current findings show that both 5-HT and DOI effectively reverse ghrelin’s actions. Taken together, these studies provide evidence in support of a localized interaction of PVN ghrelin, NPY and 5-HT with respect to eating and metabolic function. At the same time it remains unclear whether the inhibitory effects of 5-HT on ghrelin are mediated exclusively by 5-HT2A receptors. As DOI also shows affinity for 5-HT2C receptors, we are currently investigating the role of this receptor subtype specifically in relation to ghrelin’s orexigenic and metabolic activity.
The PVN plays an important role in autonomic and metabolic control via the integration of various neural signals. For example, NPY-producing neurons within the PVN express ghrelin receptors, specifically the GHS-R1a (Shuto et al., 2002). GHS-R1a receptors are localized on the presynaptic terminals of NPY neurons and stimulate NPY release (Cowley et al., 2003). These same NPY neurons receive ghrelin neuronal projections from the arcuate nucleus (Cowley et al., 2003). While our current understanding of the hypothalamic systems in metabolic homeostasis suggests that a complex network of neuropeptide and transmitter systems are involved, the link between brain 5-HT systems and neuropeptides, including ghrelin, remains to be elucidated.
Extensive evidence over the past several decades has established a role for brain 5-HT in the regulation of eating behavior and energy homeostasis. 5-HT1 and 5-HT2 receptors have been shown to regulate the anorexigenic effects of various 5-HT compounds (Vickers et al., 2001; 2000; Lee et al., 2004). Studies examining c-Fos immunoreactivity provide further support for the importance of the hypothalamus in the observed effects of 5-HT and 5-HT receptor agonists. 5-HT reuptake inhibitors and direct agonists at 5-HT receptors have been found to induce c-Fos expression in discrete hypothalamic nuclei, including the PVN, when administered at eating-inhibitory doses (Lee et al., 2004; Singewald et al, 2003). Like ghrelin-containing axons, 5-HT axon terminals establish synaptic contacts with NPY-containing neurons in the arcuate nucleus and 5-HT receptor expression occurs on PVN NPY neurons (Heisler et al., 2006).
Recent work suggests that there is a negative feedback system between brain 5-HT and plasma ghrelin levels. In one study hypothalamic 5-HT1/2 receptor mRNA levels were significantly increased in 24-h fasted animals, as were plasma ghrelin levels (Nonogaki et al., 2006). The increases in expression of hypothalamic serotonergic receptors were proportional to increases in plasma ghrelin. Moreover, elevations in ghrelin were inhibited by administration of direct and indirect 5-HT agonists. These data provide compelling support for an interaction between 5-HT and ghrelin systems.
In conclusion, our findings indicate that ghrelin alters appetite and energy metabolism, including the promotion of carbohydrate oxidation and the preservation of fat stores, by an action on hypothalamic PVN circuits mediating positive energy balance. We propose that the PVN may respond uniquely to the metabolic and orexigenic effects of ghrelin wherein serotonergic control over ghrelin-stimulated eating and energy substrate utilization occurs via 5-HT receptor activation. Specifically, increased PVN 5-HT2A/2C receptor signaling, at the onset of the nocturnal cycle, may exert physiological antagonism or inhibitory control over PVN ghrelin, thereby promoting satiety, decreasing ghrelin-stimulated food intake, and reducing the initial increase in carbohydrate oxidation resulting from enhanced ghrelin production and release.
Fig. 5.
Mean (±S.E.M.) RQ values in rats pretreated with 5-HT and then microinjected with ghrelin. *P<0.05 compared to Veh.
Acknowledgments
Supported by NIH 070496-01A1 to PJC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Currie PJ. Integration of hypothalamic feeding and metabolic signals: Focus on Neuropeptide Y. Appetite. 2003;41:335–337. doi: 10.1016/j.appet.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Coiro CD, Niyomchai T, Lira A, Farahmand F. Hypothalamic paraventricular 5-hydroxytryptamine: receptor specific inhibition of NPY-stimulated eating and energy metabolism. Pharm Biochem Behav. 2002;71:709–716. doi: 10.1016/s0091-3057(01)00671-2. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV. 5-Hydroxytryptaminergic receptor agonists: Effects on neuropeptide Y potentiation of feeding and respiratory quotient. Brain Res. 1998;803:212–217. doi: 10.1016/s0006-8993(98)00643-x. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV. Regional differences in neuropeptide Y-induced feeding and energy substrate utilization. Brain Res. 1996;737:238–242. doi: 10.1016/0006-8993(96)00738-x. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV. Stimulation of 5-HT2A/2C receptors within specific hypothalamic nuclei differentially antagonizes NPY-induced feeding. NeuroReport. 1997;8:3759–3762. doi: 10.1097/00001756-199712010-00020. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol. 2005;289:R353–R358. doi: 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Nicholson ML, Conrad VK, Gottschlich A, Leora KE, John CS, Chapman CD. Program No. 470.4. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, CD-ROM; 2009. Hypothalamic serotonin microinjections inhibit the effects of ghrelin on eating and energy substrate utilization. [Google Scholar]
- Currie PJ, Saxena N, Tu AY. 5-HT2A/2C antagonists in the paraventricular nucleus attenuate the action of DOI on NPY-stimulated eating. NeuroReport. 1999;10:3033–3036. doi: 10.1097/00001756-199909290-00029. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove K, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganama T, Matsukura S, Kanagawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Dehlin E, Liu J, Yun S, Fox E, Snyder S, Gineste C, Willingham L, Geysen M, Gaylinn BD, Gaylinn, Sando JJ. Regulation of ghrelin structure and membrane binding by phosphorylation. Peptides. 2008;54:904–911. doi: 10.1016/j.peptides.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M. Ghrelin in gastrointestinal diseases and disorders: A possible role in the pathophysiology and clinical implications. Internat J Mol Medicine. 2009;24:727–732. doi: 10.3892/ijmm_00000285. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Ming ZH, Zack MH, Coscina DV. A comparison of the effects of the 5-HT1 agonists TFMPP and RU 24969 on feeding following peripheral or medial hypothalamic injection. Brain Res. 1992;580:265–272. doi: 10.1016/0006-8993(92)90953-7. [DOI] [PubMed] [Google Scholar]
- Gil-Campos M, Aguilera CM, Canete R, Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Bri J Nutrition. 2006;96:201–226. doi: 10.1079/bjn20061787. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou LG, Borok E, Thornton-Jones Z. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kanagawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Bichem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Hori Y, Kageyama H, Guan Jian-L, Kohno D, Yada T, Takenoya F, Nonaka N, Kangawa K, Shioda S, Yoshida T. Synaptic interaction between ghrelin and ghrelin-containing neurons in the rat hypothalamus. Reg Peptides. 2008;145:122–127. doi: 10.1016/j.regpep.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Kangawa K. Discovery of ghrelin: It’s structure and physiological significance. Acta Pharmacol Sinica. 2006;27:14–14. [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: Structure and function. Physiological Reviews. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FMH, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centres. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- Lee MD, Somerville EM, Kennett GA, Dourish CT, Clifton PG. Reduced hypophagic effects of d-fenfluramine and 5-HT2C receptor agonists in 5-HT1B receptor knockout mice. Psychopharmacology. 2004;176:39–49. doi: 10.1007/s00213-004-1864-0. [DOI] [PubMed] [Google Scholar]
- Lee MD, Somerville EM, Kennett GA, Dourish CT, Clifton PG. Tonic regulation of satiety by 5-HT1B receptors in the mouse: Converging evidence from behavioural and c-Fos immunoreactivity studies. European J Neurosci. 2004;19:3017–3025. doi: 10.1111/j.0953-816X.2004.03406.x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Mascia MS, Succu S, Torsello A, Muller EE, Deghenghi R, Argiolas A. Ghrelin injected into the paraventricular nucleus of the hypothalamus of male rats induces feeding but not penile erection. Neuroscience Letters. 2002;329:339–343. doi: 10.1016/s0304-3940(02)00673-0. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, Nakazato M. Identification of ghrelin and its receptors in neurons of the rat arcuate nucleus. Regulatory Peptides. 2005;126:55–59. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Ohashi-Nozue K, Yoshitomo O. A negative feedback system between brain serotonin systems and plasma active ghrelin levels in mice. Biomed Biophys Res Comm. 2006;341:703–707. doi: 10.1016/j.bbrc.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: Effect on feeding and c-Fos immunoreactivity. Peptides. 2003;24:919–923. doi: 10.1016/s0196-9781(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 2007. [Google Scholar]
- Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamara H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion feeding and adiposity. J Clin Invest. 2002;109:1429–1436. doi: 10.1172/JCI13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biolog Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Kapas L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- Smith RG, Leonard R, Bailey AR, Palyha O, Feighner S, Tan C, McKee KK, Pong SS, Griffin P, Howard A. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001;14:9–14. doi: 10.1385/ENDO:14:1:009. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Benwell KR, Porter RH, Bickerdike MJ, Kennett GA, Dourish CT. Comparative effects of continuous infusion of mCPP, Ro 60-0175 and d-fenfluramine on food intake, water intake, body weight and locomotor activity in rats. Brit. J. Pharmacol. 2000;130:1305–1314. doi: 10.1038/sj.bjp.0703443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Dourish CT, Kennett GA. Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors. Neuropsychopharm. 2001;41:200–209. doi: 10.1016/s0028-3908(01)00063-6. [DOI] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]