Abstract
Recent recommendations for wood dust sampling include sampling according to the inhalable convention of International Organization for Standardization (ISO) 7708 (1995) Air quality—particle size fraction definitions for health-related sampling. However, a specific sampling device is not mandated, and while several samplers have laboratory performance approaching theoretical for an ‘inhalable’ sampler, the best choice of sampler for wood dust is not clear. A side-by-side field study was considered the most practical test of samplers as laboratory performance tests consider overall performance based on a wider range of particle sizes than are commonly encountered in the wood products industry. Seven companies in the wood products industry of the Southeast USA (MS, KY, AL, and WV) participated in this study. The products included hardwood flooring, engineered hardwood flooring, door skins, shutter blinds, kitchen cabinets, plywood, and veneer. The samplers selected were 37-mm closed-face cassette with ACCU-CAP™, Button, CIP10-I, GSP, and Institute of Occupational Medicine. Approximately 30 of each possible pairwise combination of samplers were collected as personal sample sets. Paired samplers of the same type were used to calculate environmental variance that was then used to determine the number of pairs of samples necessary to detect any difference at a specified level of confidence. Total valid sample number was 888 (444 valid pairs). The mass concentration of wood dust ranged from 0.02 to 195 mg m−3. Geometric mean (geometric standard deviation) and arithmetic mean (standard deviation) of wood dust were 0.98 mg m−3 (3.06) and 2.12 mg m−3 (7.74), respectively. One percent of the samples exceeded 15 mg m−3, 6% exceeded 5 mg m−3, and 48% exceeded 1 mg m−3. The number of collected pairs is generally appropriate to detect a 35% difference when outliers (negative mass loadings) are removed. Statistical evaluation of the nonsimilar sampler pair results produced a finding of no significant difference between any pairing of sampler type. A practical consideration for sampling in the USA is that the ACCU-CAP™ is similar to the sampler currently used by the Occupational Safety and Health Administration for purposes of demonstrating compliance with its permissible exposure limit for wood dust, which is the same as for Particles Not Otherwise Regulated, also known as inert dust or nuisance dust (Method PV2121).
Keywords: ACCU-CAP™, Button sampler, CIP10-I sampler, GSP sampler, inhalable sampling, IOM sampler, wood dust
INTRODUCTION
Over a half million US workers (519 651) were employed in wood product industries in 2007 (US Census Bureau) and ∼3.6 million workers in 25 member countries of the European Union are estimated to be exposed to occupational wood dust (Kauppinen et al., 2006). The International Agency for Research on Cancer (IARC) found sufficient evidence of carcinogenicity of wood dust in humans and consequently, wood dust has been classified as a human carcinogen (Group 1; IARC, 1995). The Industrial Injuries Advisory Council (IIAC) in the UK more recently concluded the existence of an association between wood dust exposure and nasopharyngeal cancer based on a literature review (IIAC, 2007). In addition, exposure to wood dust has been implicated in nonmalignant respiratory diseases, including obstructive disease of the lower airways and reactive disease of the upper airways (Whitehead et al., 1981a; Goldsmith and Shy, 1988; Enarson and Chan-Yeung, 1990). However, excess longitudinal decline in lung function with exposure to wood dust was not observed in two recent epidemiological surveys of wood processing industry workers (Innocenti et al., 2006; Glindmeyer et al., 2008). The European Scientific Committee on Occupational Exposure Limits (SCOEL, 2003) concluded that exposure to wood dust >0.5 mg m−3 induces pulmonary effects and should be avoided. The National Institute for Occupational Safety and Health Recommended Exposure Limit for wood dust is 1 mg m−3. In 2005, the American Conference of Governmental Industrial Hygienists (ACGIH) adopted a threshold limit value (TLV)–time-weighted average (TWA) of 1 mg m−3 for all wood species except western red cedar. Oak and beech were classified as confirmed human carcinogen (A1), birch, mahogany, teak, and walnut were classified as suspected human carcinogen (A2), and all other wood dusts were categorized as not classifiable as a human carcinogen (A4), and this remains the current TLV (ACGIH, 2009). The wood dust TLV also calls for sampling according to the inhalable penetration convention of the ISO Standard 7708, (1995) Air quality—particle size fraction definitions for health-related sampling [International Organization for Standardization (ISO)]. The collected sample is known as inhalable particulate matter (IPM) and it includes all particles likely to enter the nose or mouth of a normally breathing worker. In addition, in 2005, French National Decree 2003, 1254 came into force of law requiring exposures be maintained <1 mg m−3 IPM in that country. It should be noted that the French standard currently requires the use of a 37-mm closed-face cassette sampler (CFC—see below), although there has been doubts raised as to whether this correctly represents an IPM sample.
Most airborne wood dust particles are >10 μm in size (Whitehead et al., 1981b; Hinds, 1988; Pisaniello et al., 1991; Tatum et al., 2001; Harper et al., 2004; Verma et al., 2007) and relatively little mass of wood dust is found in smaller particle sizes. Occupational wood dust exposure levels are determined by gravimetric analysis, such as NIOSH method 0500 (NIOSH, 1994) and mass measurements are dominated by larger particles as a result of the cubic relation between diameter and mass. NIOSH Method 0500 does not list a sampler for IPM but instead refers to the 37-mm plastic CFC, which may not have the necessary efficiency for large particle collection to match the ISO inhalable mass fraction convention. Many studies have compared occupational wood dust exposure levels using samplers whose performance is expected to conform to the ISO inhalable convention and the CFC sampler (Martin and Zalk, 1998; Kim and Lee, 1996; Perrault et al., 1996; Tatum et al., 2001; Harper and Muller, 2002; Kauffer et al., 2010) and all studies have confirmed that the CFC collects less sample mass than an IPM sampler sampling the same atmosphere. The numerical value for the current ACGIH IPM limit (ACGIH, 2001) was based on prior workplace studies, which used the CFC but with a correction factor applied to account for the different relative collection efficiencies of the CFC and an inhalable sampler known as the Institute of Occupational Medicine (IOM) sampler. This correction factor, 2.5 or 250%, was developed based on side-by-side studies of the CFC and IOM in a variety of industries (Werner et al., 1996) with a variety of aerosols of different particle size distributions, and woodworking industries represented only a small part of the overall data set. Ratios found in woodworking industries have tended to be >2.5, typically ∼3.5, but this may be due in part to the influence of the orientation of the CFC sampler (Görner et al., 2010; Kauffer et al., 2010) and in part due to particles depositing on the walls of the CFC either during sampling or after sampling in transit to the laboratory (Harper and Demange, 2007). There is no current consensus on which, if any, of the currently available samplers should be recommended for sampling wood dust. While several samplers have laboratory performance approaching the ideal for an ‘inhalable’ sampler, the best choice of sampler for wood dust in field situations is not clear due to other issues including the collection of particles exceeding the upper limit of the inhalable convention and projectile particles that may enter the sampler but not necessarily enter the head airways. The present study aims to compare wood dust personal sampling with five different aerosol samplers in the field, in order to evaluate difference between them and possibly to inform decisions about the most appropriate sampler and sampling method for wood dust collection.
METHODS AND MATERIALS
Sampling sites
Seven different wood product industries of the Southeast USA (AL, KY, MS, and WV) participated in this study, including veneer, plywood, engineered hardwood floor, door skin, shutter, hardwood floor, and kitchen cabinet makers (Table 1). There were a total of 888 individual samples as 444 pairs. Most of the samples were collected where significant wood dust level was anticipated, for example, where the processes involved cutting, drilling, sanding, or sawing.
Table 1.
Wood products of participating companies and collected sample number
| Company | Product | Wood type | Number of samples | Air sampled process |
| A | Veneer | Walnut, cherry, white oak, and maple | 112 | Cutting, debarking, slicing, and sorting |
| B | Plywood | Pine | 106 | Cutting and pressing |
| C | Engineered hardwood flooring | Red oak, maple, walnut, hickory, and poplar | 120 | Cutting, cleaning, and sorting |
| D | Door skins | Pine, oak, and gum | 124 | Chipping, cleaning, and sawing |
| E | Shutters | Western red cedar | 128 | Cutting, drilling, and sanding |
| F | Hardwood flooring | Red oak, white oak, and maple | 154 | Cutting and sorting |
| G | Kitchen cabinetry | Oak, maple, cherry, and MDF | 144 | Cutting and sanding |
MDF, medium-density fibreboard.
Samplers used for this study
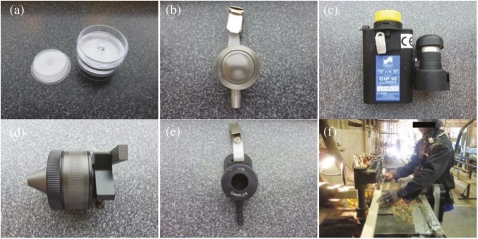
Five different samplers were utilized for this study, including the CFC with ACCU-CAP™, Button, CIP10-I, GSP, and IOM samplers (Fig. 1a–e).
Fig. 1.
Tested aerosol samplers. (a) ACCU-CAP™, (b) Button, (c) CIP10-I, (d) GSP, (e) IOM sampler, and (f) a picture of wood dust sampling (the worker was wearing ACCU-CAP™ and CIP10-I samplers).
ACCU-CAP™ (SKC Inc., Omega Division, Eighty Four, PA, USA) is a 37-mm polyvinyl chloride (PVC) filter with 5-μm pore size sealed to the base of a clear PVC dome with a hole at the vertex. The ACCU-CAP™ is a one-piece filter capsule similar to that used (Moore et al., 1990) in the OSHA method PV2121 (OSHA, 2003). When included in the CFC, the ACCU-CAP™ can prevent losses of sample that would otherwise be deposited on the interior wall of the cassette (Puskar et al., 1992). The ACCU-CAP™ fits inside of the top and bottom pieces of a two-piece 37-mm CFC (SKC Inc.) with back up pad under the filter and with the hole at the vertex of the dome fitting to the entry hole of the top piece of the cassette. A 2 l min−1 flow rate was used. The CFC was located on the body of the wearer at the commencement of sampling with the opening facing at an ∼45° angle to the vertical as previously published (Buchan et al., 1986). However, this position was not fixed with a holder, and at the end of sampling, the CFC was often observed to be pointing face outward from the body in the orientation shown by Kauffer et al. (2010) to be the most appropriate for inhalable sampling.
Button sampler (SKC Inc.) has a spherical shell inlet with numerous holes of nominally 381-μm diameter functioning as orifices. A 25-mm PVC filter was used in the Button for sampling at company A but it resulted in many pump failures because of excessive pressure drop across the filter at the recommended flow rate of 4 l min−1 for sampling in accordance with the inhalable convention, which was consistent with previous studies (e.g. Reynolds et al, 2009). Thus, the remainder of the sampling surveys used a 25-mm glass fiber filter (SKC Inc.) instead. Validation of the gravimetric procedure has shown that glass fiber filters can be used in place of PVC filters with only slightly greater uncertainty in the result (McLister et al., 2001). The uniform distribution of the holes on the curved inlet results in an even distribution of particles on the filter surface (Kalatoor et al., 1995). Aizenberg et al. (2000) investigated the performance of the sampler for particles in the inhalable size range and showed the collection efficiency to lie between that of the CFC and IOM samplers. The Button sampler inlet screen should minimize the collection of particles >100 μm (i.e. those not covered by the ACGIH inhalable convention) in the same manner as the screen proposed for the IOM sampler by Aitken and Donaldson (1996). In laboratory and field studies (Aizenberg et al., 2000), the sampler was shown to be relatively insensitive to wind speed and direction. The performance of the sampler in a second laboratory study was not as good a match to the inhalable convention, but the sampler was shown to have minimal internal wall losses of sample (Li et al., 2000).
CIP10-I (Arelco ARC, Fontenay-sous-Bois, France) is a French sampler (Görner et al., 1999) that also performed well when compared to the inhalable convention (Kenny et al., 1997; Bartley, 1998). It operates at a flow rate of 10 l min−1 by the unusual method of spinning a foam disk. Wood dusts are captured in the polyurethane foam and holder, which are weighed. There are practical issues for this sampler. First, there is an issue concerning the weight stability for foam so that at least equilibration overnight at constant temperature and humidity is recommended by the manufacturer before weighing. Another issue is the large overall mass of the cup and foam combination. Finally, the CIP10-I has to be calibrated in the laboratory using assisted pressure drop compensation and the calibration can only be checked in the field by measuring the revolutions per minute (RPM) of the rotating cup. This situation has the potential for errors.
GSP sampler (Gesamtstaub-Probenahmesystem; GSMGesellschaft für Schadstoffmesstechnik, GmbH, Neuss-Norf, Germany) is a German sampler. Results from the collaborative study previously mentioned (Kenny et al., 1997) suggested that the GSP sampler may operate sufficiently in accordance with the inhalable convention for it to be regarded as a possible inhalable sampling device (Bartley, 1998). These results were confirmed at the University of Cincinnati (Aizenberg et al., 2000). A conductive plastic version of this sampler was evaluated for wood dust (Davies et al., 1999). However, the conductive plastic cassette is also subject to weighing errors (Li et al., 2000). The samplers used in this study were the original cast aluminum samplers. The flow rate was 3.5 l min−1 and PVC filters were used to collect the wood dusts.
IOM sampler (SKC Inc.) was designed to collect the inhalable fraction of an aerosol (Mark and Vincent, 1986). The IOM showed good orientation averaged performance agreement with the ACGIH inhalable convention curve (within 10%) at averaged wind velocities of 0.5 and 1 m s−1 (Bartley, 1998). However, several studies reported that the IOM is susceptible to significant bias in situations where there is a constant directional component to the airflow (Roger et al., 1998; Aizenberg et al., 2000; Li et al., 2000). Both particles collected on the filter as well as the particles collected on the inner inlet surfaces are analyzed since the entire stainless steel cassette is weighed. The IOM is characterized by a large 15-mm entry orifice and is operated facing orthogonally outward from the body at a flow rate of 2 l min−1 loaded with a 25-mm PVC filter. The IOM sampler has been criticized as a sampler for certain dusts because of the large entry inlet, which increases the potential for aspiration of particles >100 μm aerodynamic equivalent diameter (Lidén and Kenny, 1994; Aitken and Donaldson, 1996; Aizenberg et al., 2000; Lidén et al., 2000). Wood dust particles of this size are present in the breathing zone (Vaughan et al., 1990; Martin and Zalk, 1998; Harper et al., 2004) but they are not covered by the inhalable convention because the aspiration efficiency of the human mouth and nose for such large particles has been shown to be low (Breysse and Swift, 1990; Aitken and Donaldson, 1996; Hsu and Swift, 1999; Kennedy and Hinds, 2002; Dai et al., 2006).
Combination of samplers
Workers were asked to wear commercial back braces and the samplers were located on the shoulder straps of these back braces, one on each side, with sides randomized for different pairs. Fifteen possible combination of the samplers were generated: ACCU-CAP™/ACCU-CAP™, ACCU-CAP™/Button, ACCU-CAP™/CIP10-I, ACCU-CAP™/GSP, ACCU-CAP™/IOM, Button/Button, Button/CIP10-I, Button/GSP, Button/IOM, CIP10-I/CIP10-I, CIP10-I/GSP, CIP10-I/IOM, GSP/GSP, GSP/IOM, and IOM/IOM. Combinations were selected at random for each sampling event so that no deliberate bias was introduced by having a large percentage of any one combination being associated with any particular site or task. The sample size was determined by the method used in our previous study (Harper and Muller, 2002). Paired samplers of the identical type were used to calculate environmental variance (true field coefficient of variation) that was then used to determine the number of pairs of samples necessary to detect any difference at a specified level of confidence.
Wood dust sampling
CFC with ACCU-CAP™ samplers were prepared by using a cassette closer (Omega Specialty Instruments; now SKC Inc., Omega Division) connected to a cylinder of nitrogen to apply an even force to close each cassette. Assembled cassettes were wrapped with Omega Gel Bands (Omega Specialty Instruments) to prevent air leaks and leak checked using a field cassette leak tester (Omega Specialty Instruments). The CFC samplers were opened using an EZ Cassette Opener (SKC Inc.). The IOM samplers with stainless steel cassettes, GSP, and Button samplers were prepared in accordance with the manufacturer’s instructions. The pumps were attached to a back belt (Safe-T-Lift, Style No. 70-110543; FLA Orthopedics, Inc.) around the waist of the participants (note that the belts are used in this case only for support of the sampling equipment and an endorsement of such belts for any other purposes is not intended) (Fig. 1f). Aircheck PCXR-4 pumps (SKC Inc.) were connected to the filter holders (other than the CIP10-I) by flexible tubing. The flow rates through the sampling trains were calibrated using a BIOS Dry Cal Meter (Product DCL-MH; BIOS International Corporation, Butler, NJ, USA), using sampler calibration adapters (e.g. IOM Calibration Adapter; SKC Inc.) where necessary. The flow rates were calibrated before and after each day of sampling on-site and to ensure the flow rate did not change significantly. The flow rate of the CIP10-I was calibrated in the NIOSH laboratory with a CIP10 Calibration bench (Arelco, ARC) and an on-site check by measurement of the rotational speed (RPM) of the cup was conducted before and after field sampling. Sampling times were adjusted according to the judgment of the on-site hygienist to obtain optimal particle deposition on the filters and the sampling time was between 1 and 4 h in most of the samples. Most of the wood workshops used kiln-dried wood with very low moisture contents, leading to dust with a very high electrostatic charge, requiring careful handling of the samplers and samples to avoid sample losses. All samples were capped and stored in ziplock bags during transportation from the sampling sites. Samples were taken by hand to the laboratory rather than shipped.
Gravimetric analysis
All filters and foams from the first three sites (A, B, and C) were equilibrated for a minimum 72 h to specific humidity conditions before and after sampling in a cabinet containing a saturated solution of sodium dichromate. Later, a purpose-built weighing room was used for gravimetric analysis for the following four sites (D, E, F, and G). The weighing room maintains constant relative humidity (50% ± 2) and temperature (26°C ± 2). All samples were weighed before and after sampling using a micro Balance (UMT2; Mettler-Toledo, Columbus, OH, USA) and weights were recorded on data sheets. For IOM filter and stainless steel cassette and CIP10-I polyurethane form and rotating cup, measurements were made with a dual range analytical balance (AG245; Mettler-Toledo). A calibration check on the balances was performed and recorded each day of weighing and the balances were zeroed between each weighing. Filters were passed through an electrostatic bar (Mettler-Toledo) before they were weighed to dissipate static charge. Measurements were made after allowing exactly 120 s for balance stabilization. Field blanks were taken but samples were not corrected for field blanks, according to normal practice. Average mass difference from 156 field blank samples was −0.03 mg.
Statistical analysis
Analyses were performed on matched pairs after calculation of air concentrations using SAS 9.2 (SAS Institute, Cary, NC, USA). All variables were tested to ensure that they met statistical assumptions for all analyses. Log-transformed data were used where appropriate. In this sample, there were 995 nonmissing data points. Data were excluded if the mass loading and, therefore, the concentration was less than zero. Data were paired, and if either of the paired sampler concentrations were missing, both pairs were excluded. In the final data set, there were 444 pairs, giving 888 data points, resulting in a loss of 11% of the data.
Associations were performed using correlation analyses to see how well-paired samples correlated with each other. Pearson’s correlation coefficients and their corresponding P-values were generated. Whether or not correlation coefficients were of statistical significance, the size of the coefficient itself may still be meaningful, with values approaching 1 or −1 indicating stronger associations (Table 2).
Table 2.
Correlation coefficient, average difference, and correction factors between pair of the samplers
| Sampler pairs | Sample number | Correlation coefficient (P value) | Average difference between the groups (mg m−3) (P value)a | Correction factorsb |
| ACCU-CAP™/ACCU-CAP™ | 29 | 0.26 (0.264)c | 6.66 (0.324)c | 0.16 ± 0.50c |
| ACCU-CAP™/Button | 30 | 0.94 (<0.001) | 0.10 (0.885) | 0.91 ± 0.46 |
| ACCU-CAP™/CIP10-I | 30 | 0.87 (<0.001) | 1.08 (0.267) | 0.60 ± 1.52 |
| ACCU-CAP™/GSP | 33 | 0.95 (<0.001) | 0.98 (0.709) | 0.77 ± 0.75 |
| ACCU-CAP™/IOM | 32 | 0.46 (0.008) | 0.55 (0.270) | 0.64 ± 0.38 |
| Button/Button | 30 | 0.96 (<0.001) | 0.03 (0.933) | 0.97 ± 0.34 |
| Button/CIP10-I | 32 | 0.22 (0.219) | 0.33 (0.529) | 0.82 ± 1.18 |
| Button/GSP | 30 | 0.32 (0.087) | 0.29 (0.404) | 0.79 ± 0.57 |
| Button/IOM | 24 | 0.80 (<0.001) | 0.05 (0.913) | 0.95 ± 0.52 |
| CIP10-I/CIP10-I | 24 | 0.94 (<0.001) | 0.27 (0.714) | 1.13 ± 1.02 |
| CIP10-I/GSP | 30 | 0.70 (<0.001) | 0.11 (0.871) | 1.08 ± 0.35 |
| CIP10-I/IOM | 28 | 0.99 (<0.001) | 0.22 (0.924) | 1.08 ± 0.39 |
| GSP/GSP | 30 | 0.91 (<0.001) | 0.17 (0.734) | 0.88 ± 0.26 |
| GSP/IOM | 31 | 0.91 (<0.001) | 0.03 (0.982) | 0.99 ± 0.39 |
| IOM/IOM | 31 | 0.74 (<0.001) | 0.75 (0.573) | 0.74 ± 0.74 |
Average difference between the groups was calculated using absolute value difference between the sampler groups.
Correction factors is applied to the second sampler to make average mass concentration same as the first sampler in sampler pairs; ± indicates 95% confidence interval value.
Correlation coefficient becomes 0.83 (P < 0.001), the average difference becomes 0.01 mg m−3 (P = 0.98), and the correction factor becomes 1.02 ± 0.50 with the removal of a single outlier.
Mixed linear models were used to look for statistically significant differences between the paired samples. Random effect models were used to control for and test variation between the different sampling sites. Nonsignificant P-values (P > 0.05) indicate that the samplers were not statistically different, with mean values close to zero indicating similar readings.
Correction factors have also been calculated. These values show what the paired group’s values would have to be multiplied by in order to make them match up as close as possible to each other, with the correction factor being applied to the sampler that comes last alphabetically (i.e. for samplers ACCU-CAP™ and Button, the correction factor would be applied to the Button sampler). Values close to one indicate that the samplers’ data were initially close together. Values for matched samplers (ACCU-CAP™/ACCU-CAP™, Button/Button, CIP10-I/CIP10-I, GSP/GSP, and IOM/IOM) are given to show the potential variability within sampler type, although they may not be meaningful in an applied sense.
RESULTS
Total valid sample number was 888 among 995 nonmissing data. There were originally 1257 data points, including 156 blank samples, but some data were excluded due to the pump failure (62 individual samples), mishandling samples (9 individual samples), and negative mass [35 individual samples, mainly from the CIP10-I sampler in site B, where malfunction possibly related to the weighing or calibration issues referred to previously is suspected since the observed sampling rate, even with outliers omitted, is markedly lower than at any other site (see Fig. 2 below)]. The mass concentration of wood dust ranged from 0.02 to 195 mg m−3 for total data set. Since the mass concentration of wood dust showed log normal distributions, the results can also be described using geometric mean (GM) and geometric standard deviation (GSD) as descriptive statistics. GM (GSD) and arithmetic mean (standard deviation) of wood dust were 0.98 mg m−3 (3.06) and 2.12 mg m−3 (7.74), respectively. One percent of samples exceeded 15 mg m−3, 6% of samples exceeded 5 mg m−3, and 48% of samples exceeded 1 mg m−3. The obtained data were separated by sampling sites, by each sampler, and by pair of sampler for comparison. It should be acknowledged that the data were not 8-h TWA results, but the range of results was consistent with that observed across European countries (Kauppinen, et al., 2006).
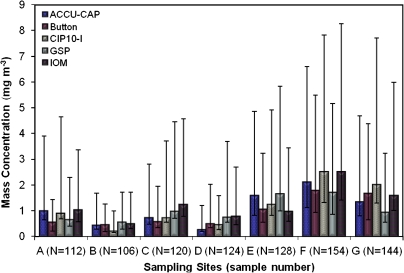
Fig. 2.
GM and GSD wood dust mass concentration at each sampling site by each sampler. N is the combined sample number for all samplers.
Comparison by sampling sites
The GM of mass concentration of wood dust in seven different wood product industries ranged from 0.44 (Site B) to 2.08 mg m−3 (Site F). In accordance with the mixed model analysis of variance, Sites D and B were both significantly different from Site F, but all other pairs were not significantly different at the P < 0.05 level. The GM with GSD for each site by separating with each sampler is shown in Fig. 2. The highest GM of dust concentrations recorded at each site were (A) IOM (1.04 mg m−3), (B) GSP (0.56 mg m−3), (C) IOM (1.25 mg m−3), (D) IOM (0.79 mg m−3), (E) GSP (1.65 mg m−3), (F) CIP10-I and IOM (2.51 mg m−3; although the IOM had a larger variance), and (G) CIP10-I (2.01 mg m−3) samplers.
Comparison by samplers
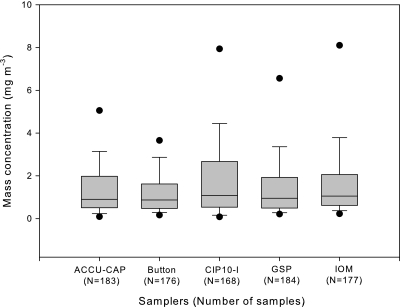
The box plot for wood dust mass concentration collected by each sampler at the seven different wood product industries with total sample number (N) is shown in Fig. 3. The median mass concentration of wood dust for ACCU-CAP™, Button, CIP10-I, GSP, and IOM samplers were 0.90, 0.87, 1.08, 0.95, and 1.06, respectively. The mass concentrations of wood dust collected by ACCU-CAP™ ranged from 0.02 to 195 mg m−3 with GM (GSD) of 1.03 mg m−3 (3.25). The mass concentrations of wood dust collected by Button sampler ranged from 0.03 to 7.68 mg m−3 with GM (GSD) of 0.88 mg m−3 (2.64). The mass concentrations of wood dust collected by CIP10-I sampler ranged from 0.02 to 49.5 mg m−3 with GM (GSD) of 1.01 mg m−3 (3.46). The mass concentrations of wood dust collected by GSP ranged from 0.07 to 29.9 mg m−3 with GM (GSD) of 0.90 mg m−3 (2.94) mg m−3. The mass concentrations of wood dust collected by IOM sampler (filter plus cassette) ranged from 0.14 to 38.7 mg m−3 with GM (GSD) of 1.28 mg m−3 (2.80). The mass concentrations from all five different samplers were not statistically significant different (P > 0.05). GM (GSD) of the IOM sampler with filter-only analysis was 1.06 mg m−3 (2.92) and the range of the concentration was between 0.04 and 37.39 mg m−3. The GM and arithmetic mean difference between the concentrations from IOM samples weighing the filter-only and samples weighing both the filter plus cassette were 0.28 and 0.24 mg m−3, respectively. The two groups are not significantly different (P = 0.78) and the filter-only concentrations were not statistically different from the other samplers.
Fig. 3.
The box plot of wood dust mass concentration (milligrams per cubic meter) by different samplers without outliers. The horizontal lines in the box plot from bottom to top indicate 10th, 25th, 50th (median), 75th, and 90th percentiles. The circles indicate the 5th (lower circle) and 95th (upper circle) percentiles. N is the number of samples for each sampler, and the total sample number is 888 when all samplers combined.
Comparison by pairs of samplers
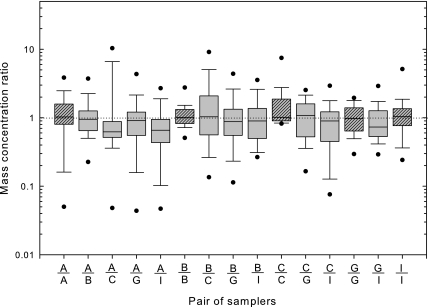
The box plot for wood dust mass concentration ratios between 15 different pairs of the samplers for all sampling sites is shown in Fig. 4. The median mass concentration ratio for all pairs ranged from 0.62 (ACCU-CAP™ and CIP10-I pair) to 1.07 (CIP10-I and GSP pair). Table 2 shows correlation coefficient, average difference in mass concentration (from random effects model), and correction factors for each pair of samplers. All the pairs showed a strong and statistically significant (P < 0.05) correlation between samplers, except for pairs ACCU-CAP™/ACCU-CAP™, Button/CIP10-I, and Button/GSP. Average difference between the groups calculated from GM for each pair ranged from 0.03 (Button/Button pair and GSP/IOM) to 6.66 (ACCU-CAP™/ACCU-CAP™) mg m−3 and all P-values are >0.05, implying that the mass concentrations of wood dust from side-by-side sampling are not significantly different. The average difference between ACCU-CAP™ and ACCU-CAP™ is very large due to single large mass concentration (195 mg m−3). When this value is considered as outlier, the average difference between the samplers drops to just 0.01 mg m−3 and the correlation between pairs becomes significant with a coefficient of 0.83 (P < 0.001). The number of collected pairs is generally appropriate to detect a 35% difference with 80% power at an alpha level of 0.05. A difference of 35% may be considered more appropriate for field studies, as opposed to the NIOSH 25% criterion for laboratory studies, as field studies can have highly variable environmental conditions (Bartley et al., 2007). Statistical evaluation of the nonsimilar sampler pair results produced a finding of no significant difference between any pairing of sampler type. Correction factors ranged from 0.16 (pair of ACCU-CAP™ and ACCU-CAP™ samplers) to 1.13 (pair of CIP10-I and CIP10-I samplers). Correction factor as defined here means that the average level of wood dust measured by the ACCU-CAP™ was the same as that of Button (for ACCU-CAP™ and Button samplers pair) when the stated correction factor (0.91) was applied to the concentration measured by the Button. The average differences between the identical sampler pairs (in Table 2) may reflect the ability of the sampler to collect more or less coarse particles. Variability increases for samplers, such as the IOM, expected to collect coarser particles.
Fig. 4.
Box plot of wood dust mass concentration ratios between the pairs of the samplers without outliers. The horizontal lines in the box plot from bottom to top indicate 10th, 25th, 50th (median), 75th, and 90th percentiles. The circles indicate the 5th (lower circle) and 95th (upper circle) percentiles. A is ACCU-CAP™, B is Button sampler, C is CIP10-I, G is GSP sampler, and I is IOM sampler (total number of pairs is 444).
DISCUSSION
Wood dust exposure levels
The average wood dust exposure levels in the present study were consistent with the previously reported studies, despite the fact that the sampling methods and sites were different in each study and the wood dust concentrations were not always TWA measurements (Moore et al., 1990; Hamill et al., 1991; Pisaniello et al., 1991; Scheeper et al., 1995; Martin and Zalk, 1998; Teschke et al., 1999; Schlünssen et al., 2001; Rando et al., 2005; Scarselli et al., 2007; Kalliny et al., 2008; Galea et al., 2009; Saejiw et al., 2009; Yamanaka et al., 2009; Kauffer et al., 2010). Wood dust exposure levels may have decreased over recent decades possibly due to the changes in equipment, production methods, and upgrading engineering ventilation system for dust control (Teschke et al., 1999; Galea et al., 2009).
Sampler comparison
Many studies have compared and reported ratios between CFC and inhalable samplers. Moore et al. (1990) compared MSA respirable dust cassette that contains an aluminum inner capsule with the filter-only analysis from the standard 37-mm cassette and the mean mass concentration ratio was 2.2 implying that the filter-only analysis was underestimating exposure because of particles lost to the interior surfaces of the cassette. Werner et al. (1996) measured a variety of occupational aerosols including wood dust side-by-side sampling of CFC (filter-only) and IOM and they found that the ratio of IOM and CFC was 2.5. Martin and Zalk (1998) reported ratios of IOM to CFC (filter-only) in carpenter shops ranged from 1.8 to 4.1 (mean ratio 2.8) when the CFC concentrations were >0.5 mg m3. Davies et al. (1999) compared the GSP and CFC (filter-only) and found concentration ratios to be ∼4.0. Tatum et al. (2001) compared wood dust mass concentration between IOM and CFC (filter-only) and the average ratio was 1.85. Harper and Muller (2002) measured wood dust by side-by-side personal sampling using Button, CFC, and IOM samplers and median levels of sampler ratios for IOM/CFC, IOM/Button, and CFC/Button were 3.35, 3.15, and 1.2, respectively. Harper et al. (2004) also reported the ratios between Button, CFC, and IOM samplers with inclusion of particles deposited on the walls of the CFC sampler. The ratios IOM/CFC, IOM/Button, and CFC/Button were 2.16, 3.43, 1.59, respectively, with IOM/CFC and CFC/Button therefore significantly different from the earlier measurements of Harper and Muller (2002) where wall deposits had not been included. Hagström et al. (2008) reported that the ratio between IOM and 25-mm open-face cassette (filter-only) ranged from 0.67 to 17 with an average of 3.2 at wood pellets industries. Kauffer et al. (2010) reported ratios between selected aerosol samplers including ACCU-CAP™, Button, CIP10-I, and IOM samplers and the CFC (filter-only) in wood product industries and the ratios were 1.68, 1.46, 1.84, and 2.00, respectively. They also compared the various samplers to one another and found that concentrations measured by ACCU-CAP™, CIP10-I, and IOM samplers were not statistically different but the Button sampler concentrations were lower than the others, which was consistent with the study from Harper and Muller (2002). The difference ratios between the samplers might be attributed to the different size distribution of wood or occupational dust at sampling sites and different machining processes from each study. It is clear that inclusion of particles collected on the internal surfaces of the CFC provides results that are closer to those of samplers often considered to collect IPM, such as the IOM, the GSP, and the CIP10-I. Thus, the CFC with ACCU-CAP™ might behave more like an inhalable sampler as has previously been noted for samples of airborne metals (Demange et al., 2002; Harper, 2006). Görner et al. (2010) reported that ACCU-CAP™ collected more airborne particles than CFC up to 10 and 20% in wind tunnel and calm air chamber, respectively. In this study, the ratio of ACCU-CAP™ and the IOM sampler was 1.56, which was not significantly different from 1. The closer match between the ACCU-CAP™ and IOM sampler found in this field study as well as the study of Kauffer et al. (2010) is probably because in the field particles are projected toward the worker in calm air rather than falling vertically from above as in a calm air chamber (Lidén and Kenny, 1994). Our findings corroborate these results and show that using the ACCU-CAP™ decreases the difference of mass concentration between CFC and inhalable samplers to statistical nonsignificance. Note that other inserts designed for a similar purpose, such as the Woodchek (MSA, Inc., Pittsburgh, PA, USA) would likely provide the same result, although this was not tested in this study.
Sampler selection for wood dust measurement
While the most important consideration in sampling wood dust is the size fraction (i.e. inhalable, thoracic, or respirable), there are other important considerations, such as cost of sampling and acceptability to workers (comfort and placement of device) and industrial hygienists (ease of calibration and disposability). In the present study, all five samplers tested appear to provide similar results for wood dust measurement. Other factors become important in the final selection of sampler, when the choice is between samplers shown to have acceptably similar performance.
CONCLUSIONS
Wood dusts were collected in seven different wood product industries using five different total and inhalable samplers including the 37-mm CFC with ACCU-CAP™, Button, CIP10-I, GSP, and IOM, with side-by-side personal sampling. Approximately 30 replicates of each of 15 different combinations of samplers were collected as personal sample pairs. Statistical evaluation of the nonsimilar sampler pair results produced a finding of no significant difference between any pairing of sampler type implying that these samplers could be used interchangeably for personal wood dust exposure assessment.
A practical consideration for sampling in the USA is that the ACCU-CAP™ is similar to the sampler currently used by the OSHA for purposes of demonstrating compliance with its permissible exposure limit for wood dust, which is the same as for particles not otherwise regulated, also known as inert dust or nuisance dust (Method PV2121).
FUNDING
National Institute for Occupational Safety and Health [Validation of appropriate wood dust sampling and analysis procedures (CAN# 0927Z6RS)]. Research Settlement Fund for the new faculty of Seoul National University to K.L.
Acknowledgments
Many thanks to wood product industries that participated in this study. Many thanks to Edmond Kauffer and Peter Görner from Institut National de Recherche et de Sécurité, France, and Stephen Reynolds from Colorado State University for reviewing this work. Mark Wilson, Sassan Nikdast, Sasithorn Srimeechai, Soogil Lim, Emily Lee, Madalina Chirila, Sanjeev Kakkar, Yi-Hsuan Wu, Seung Won Kim, and Evan Floyd provided able technical assistance during sample collection in the field.
Disclaimer—The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Mention of commercial equipment should not be construed to imply endorsement.
References
- Aitken RJ, Donaldson R. Large particle and wall deposition effects in inhalable samplers. HSE Contract Research Report No. 117/19996. Sudbury, Suffolk, UK: Health and Safety Executive Books; 1996. [Google Scholar]
- Aizenberg V, Grinshpun SA, Willeke K, et al. Performance characteristics of the button personal inhalable aerosol sampler. Am Ind Hyg Assoc J. 2000;61:398–404. doi: 10.1080/15298660008984550. [DOI] [PubMed] [Google Scholar]
- ACGIH. Threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: ACGIH Worldwide; 2001. [Google Scholar]
- ACGIH. TLVs and BEIs based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: ACGIH; 2009. [Google Scholar]
- Bartley DL. Inhalable aerosol samplers. Appl Occup Environ Hyg. 1998;13:274–78. [Google Scholar]
- Bartley DL, Slaven JE, Rose MC, et al. Uncertainty determination of nondestructive chemical analytical methods using field data and application to XRF analysis for lead. J Occup Environ Hyg. 2007;4:931–42. doi: 10.1080/15459620701712712. Errata. (2008) J Occup Environ Hyg; 5: D72. [DOI] [PubMed] [Google Scholar]
- Breysse DN, Swift DL. Inhalability of large particles into the human nasal passage—in vivo studies in still air. Aerosol Sci Technol. 1990;13:459–64. [Google Scholar]
- Buchan RM, Soderholm SC, Tillery MI. Aerosol sampling efficiency of 37-mm filter cassettes. Am Ind Hyg Assoc J. 1986;47:825–31. doi: 10.1080/15298668691390728. [DOI] [PubMed] [Google Scholar]
- Dai YT, Juang YW, Breysse PN, et al. In vivo measurements of inhalability of ultralarge aerosol particles in calm air by humans. J Aerosol Sci. 2006;37:967–73. [Google Scholar]
- Davies HW, Teschke K, Demers PW. A field comparison of inhalable and thoracic size selective sampling techniques. Ann Occup Hyg. 1999;43:381–92. [PubMed] [Google Scholar]
- Demange M, Görner P, Elcabache J, et al. Field comparison of 37-mm closed-face cassettes and IOM samplers. Appl Occup Environ Hyg. 2002;17:200–08. doi: 10.1080/104732202753438289. [DOI] [PubMed] [Google Scholar]
- Enarson DA, Chan-Yeung M. Characterization of health effects of wood dust exposures. Am J Ind Med. 1990;17:33–8. doi: 10.1002/ajim.4700170107. [DOI] [PubMed] [Google Scholar]
- Galea KS, Tongeren MV, Sleeuwenhoek AJ, et al. Trends in wood dust inhalation exposure in the UK, 1985–2005. Ann Occup Hyg. 2009;53:657–67. doi: 10.1093/annhyg/mep044. [DOI] [PubMed] [Google Scholar]
- Glindmeyer HW, Rando RJ, Lefante JJ, et al. Longitudinal respiratory health study of the wood processing industry. Am J Ind Med. 2008;51:595–609. doi: 10.1002/ajim.20594. [DOI] [PubMed] [Google Scholar]
- Goldsmith DF, Shy CM. Respiratory health effects from occupational exposure to wood dusts. Scand J Work Environ Health. 1988;14:1–15. doi: 10.5271/sjweh.1958. [DOI] [PubMed] [Google Scholar]
- Görner P, Simon X, Wrobel R, et al. Laboratory study of selected personal inhalable aerosol samplers. Ann Occup Hyg. 2010;54:165–87. doi: 10.1093/annhyg/mep079. [DOI] [PubMed] [Google Scholar]
- Görner P, Wrobel R, Roger F, et al. Inhalable aerosol selector for the CIP-10 personal aerosol sampler. J Aerosol Sci. 1999;30:S893–4. [Google Scholar]
- Hagström K, Axelsson S, Arvidsson H, et al. Exposure to wood dust, resin acids, and volatile organic compounds during production of wood pellets. J Occup Environ Hyg. 2008;5:296–304. doi: 10.1080/15459620801957225. [DOI] [PubMed] [Google Scholar]
- Hamill A, Ingle J, Searle S, et al. Levels of exposure to wood dust. Ann Occup Hyg. 1991;35:397–403. doi: 10.1093/annhyg/35.4.397. [DOI] [PubMed] [Google Scholar]
- Harper M. A review of workplace aerosol sampling procedures and their relevance to the assessment of Beryllium exposures. J Environ Monit. 2006;8:598–604. doi: 10.1039/b600924g. [DOI] [PubMed] [Google Scholar]
- Harper M, Akbar MZ, Andrew ME. Comparison of wood-dust aerosol size-distributions collected by air samplers. J Environ Monit. 2004;6:18–22. doi: 10.1039/b312883k. [DOI] [PubMed] [Google Scholar]
- Harper M, Demange M. Analytical performance criteria concerning sampler wall deposits in the chemical analysis of airborne metals. J Occup Environ Hyg. 2007;4:D81–6. doi: 10.1080/15459620701493149. [DOI] [PubMed] [Google Scholar]
- Harper M, Muller BS. An evaluation of total and inhalable samplers for the collection of wood dust in three wood products industries. J Environ Monit. 2002;4:648–56. doi: 10.1039/b202857n. [DOI] [PubMed] [Google Scholar]
- Hinds WC. Basis for particle size-selective sampling for wood dust. Appl Ind Hyg. 1988;3:67–72. [Google Scholar]
- Hsu DJ, Swift DL. The measurements of human inhalablity of ultralarge aerosols in calm air using mannikins. J Aerosol Sci. 1999;30:1331–43. [Google Scholar]
- IIAC. Nasopharyngeal cancer due to exposure to wood dust. London. 2007. [Google Scholar]
- Innocenti A, Ciapini C, Natale D, et al. Longitudinal changes of pulmonary function in workers with high wood dust exposure levels. Med Lav. 2006;97:30–5. [PubMed] [Google Scholar]
- IARC. Monograph on the evaluation of carcinogenic risks to humans. 1995;Volume 62 Wood dust and formaldehyde. Lyon, France: Working Group on Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- ISO Standard 7708. Air quality—particle size fraction definitions for health-related sampling. Geneva, Switzerland: International Organization for Standardization; 1995. [Google Scholar]
- Kalatoor S, Grinshpun SA, Willeke K, et al. New aerosol sampler with low wind sensitivity and good filter collection uniformity. Atmos Environ. 1995;29:1105–12. [Google Scholar]
- Kalliny MI, Brisolaran JA, Glindmeyer H, et al. A survey of size-fractionated dust levels in the U.S. wood processing industry. J Occup Environ Hyg. 2008;5:501–10. doi: 10.1080/15459620802194570. [DOI] [PubMed] [Google Scholar]
- Kauffer E, Wrobel R, Görner P, et al. Site comparison of selected samplers in the wood industry. Ann Occup Hyg. 2010;54:188–203. doi: 10.1093/annhyg/mep078. [DOI] [PubMed] [Google Scholar]
- Kauppinen T, Vincent R, Liukkonen T, et al. Occupational exposure to inhalable wood dust in the member states of the European Union. Ann Occup Hyg. 2006;6:549–61. doi: 10.1093/annhyg/mel013. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Hinds WC. Inhalability of large solid particles. J Aerosol Sci. 2002;33:237–55. [Google Scholar]
- Kenny LC, Aitken R, Chalmers C, et al. A collaborative European study of personal inhalable aerosol sampler performance. Ann Occup Hyg. 1997;41:135–53. doi: 10.1016/S0003-4878(96)00034-8. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee DW. Comparison of area vs. personal total dust concentrations measured by 37 mm closed-face cassette and IPM sampler. Korean Ind Hyg Assoc J. 1996;6:67–76. [Google Scholar]
- Li S, Lundgren DA, Rovell-Rixx D. Evaluation of six inhalable aerosol samplers. Am Ind Hyg Assoc J. 2000;61:506–16. doi: 10.1080/15298660008984562. [DOI] [PubMed] [Google Scholar]
- Lidén G, Juringe L, Gudmundsson A. Workplace validation of a laboratory evaluation test of samplers for inhalable and “Total” dust. J Aerosol Sci. 2000;31:199–219. [Google Scholar]
- Lidén G, Kenny LC. Errors in inhalable dust sampling for particles exceeding 100 μm. Ann Occup Hyg. 1994;38:373–84. [Google Scholar]
- Mark D, Vincent JH. A new personal sampler for airborne total dust in workplaces. Ann Occup Hyg. 1986;30:89–102. doi: 10.1093/annhyg/30.1.89. [DOI] [PubMed] [Google Scholar]
- Martin JR, Zalk DM. Comparison of total dust/inhalable dust sampling methods for the evaluation of airborne wood dust. Appl Occup Environ Hyg. 1998;13:177–82. [Google Scholar]
- McLister J, Stacey PR, Revell G. Round robin filter weighing exercise. Broad Lane, Sheffield, UK: Health and Safety Laboratory; 2001. [Google Scholar]
- Moore L, Dube D, Burk T. Improved sampling and recovery of wood dust using MSA respirable dust cassettes. Am Ind Hyg Assoc J. 1990;51:A475–7. [PubMed] [Google Scholar]
- NIOSH Particulates not otherwise regulated, total. In Clere J, Hearl F, editors. NIOSH manual of analytical methods. 4th edn. Cincinnati, OH. 1994 [Google Scholar]
- OSHA. Method number PV2121. Gravimetric determination. 2003 Available at http://www.osha.gov/dts/sltc/methods/partial/pv2121/pv2121.html. Accessed 1 October 2010. [Google Scholar]
- Perrault G, Drolet D, Cloutier Y. Comparison of total and inhalable sampling of wood dust. Paper presented at the American Industrial Hygiene Conference & Exposition, 1996. Washington, DC: American Industrial Hygiene Association; 1996. [Google Scholar]
- Pisaniello DL, Connell KE, Muriale L. Wood dust exposure during furniture manufacture - results from an Australian survey and considerations for threshold limit value development. Am Ind Hyg Assoc J. 1991;52:485–92. doi: 10.1080/15298669191365090. [DOI] [PubMed] [Google Scholar]
- Puskar MA, Fergon SM, Harkins JM, et al. Gravimetric determination of airborne dust by using a filter cartridge inside a closed-face, 37-mm closed-face cassette. Am Ind Hyg Assoc J. 1992;53:692–8. [Google Scholar]
- Rando R, Poovey H, Mokadam D, et al. Field performance of the RespiCon™ for size-selective sampling of industrial wood processing dust. J Occup Environ Hyg. 2005;2:219–26. doi: 10.1080/15459620590930309. [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Nakatsu J, Tillery M, et al. Field and wind tunnel comparison of four aerosol samplers using agricultural dusts. Ann Occup Hyg. 2009;53:585–94. doi: 10.1093/annhyg/mep021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger F, Lachappelle G, Fabriès J-F, et al. Behaviour of the IOM aerosol sampler as a function of external wind velocity and orientation. J Aerosol Sci. 1998;29(Suppl. 2):S1133–4. [Google Scholar]
- Saejiw N, Chaiear N, Sadhra S. Exposure to wood dust and its particle size distribution in a rubberwood sawmill in Thailand. J Occup Environ Hyg. 2009;6:483–90. doi: 10.1080/15459620902967065. [DOI] [PubMed] [Google Scholar]
- Scarselli A, Binzzi A, Ferrante P, et al. Occupational exposure levels to wood dust in Italy, 1996–2006. Occup Environ Med. 2007;65:567–74. doi: 10.1136/oem.2007.036350. [DOI] [PubMed] [Google Scholar]
- Scheeper B, Kromhout H, Boleij JSM. Wood-dust exposure during wood-working processes. Ann Occup Hyg. 1995;39:141–54. [PubMed] [Google Scholar]
- Schlünssen V, Vinzents PS, Mikkelsen AB, et al. Wood dust exposure in the Danish furniture industry using conventional and passive monitors. Ann Occup Hyg. 2001;45:157–64. doi: 10.1016/s0003-4878(00)00055-7. [DOI] [PubMed] [Google Scholar]
- SCOEL. Recommendation from the scientific committee on occupational exposure limits: risk assessment for wood dust. 2003. Brussels, Belgium. [Google Scholar]
- Tatum VL, Ray AE, Rovell-Rixx DC. The performance of personal inhalable dust sampler in wood-products industry facilities. Appl Occup Environ Hyg. 2001;16:763–9. doi: 10.1080/10473220121612. [DOI] [PubMed] [Google Scholar]
- Teschke K, Marion SA, Vaughan TL, et al. Exposures to wood dust in U.S. industries and occupations, 1979 to 1997. Am J Ind Med. 1999;35:581–9. doi: 10.1002/(sici)1097-0274(199906)35:6<581::aid-ajim5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Vaughan NP, Chalmers CP, Botham RA. Field comparison of personal samplers for inhalable dust. Ann Occup Hyg. 1990;34:553–73. doi: 10.1093/annhyg/34.6.553. [DOI] [PubMed] [Google Scholar]
- Verma DK, Demers C, Finkelstein M, et al. Occupational exposure to chemical, biological, and physical agents in Ontario sawmill and veneer/plywood plants. Research Report Workplace Safety & Insurance Board (WSIB) Research Grant #02001. 2007. Available from DK Verma (McMaster University), vermadk@mcmaster.ca, 102 pp. [Google Scholar]
- Werner MA, Spear TM, Vincent JH. Investigation into the impact of introducing workplace aerosol standards based on the inhalable fraction. Analyst. 1996;121:1207–14. doi: 10.1039/an9962101207. [DOI] [PubMed] [Google Scholar]
- Whitehead L, Ashikaga T, Vacek P. Pulmonary function status of workers exposed to hard wood or pine dust. Am Ind Hyg Assoc J. 1981a;42:178–86. doi: 10.1080/15298668191419541. [DOI] [PubMed] [Google Scholar]
- Whitehead LW, Freund T, Hahn L. Suspended dust concentrations and size distributions, and qualitative analysis of inorganic particles, from woodworking operations. Am Ind Hyg Assoc J. 1981b;42:461–7. [Google Scholar]
- Yamanaka MW, Guidotti TL, Koehncke N, et al. Wood dust levels in Alberta sawmills. Arch Environ Occup Health. 2009;64:270–7. doi: 10.1080/19338240903338247. [DOI] [PubMed] [Google Scholar]