Abstract
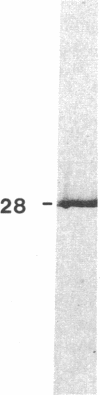
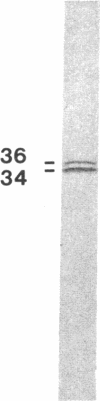
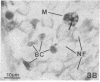
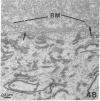
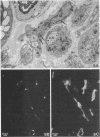
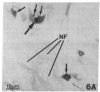
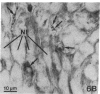
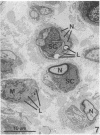
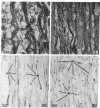
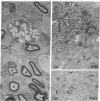
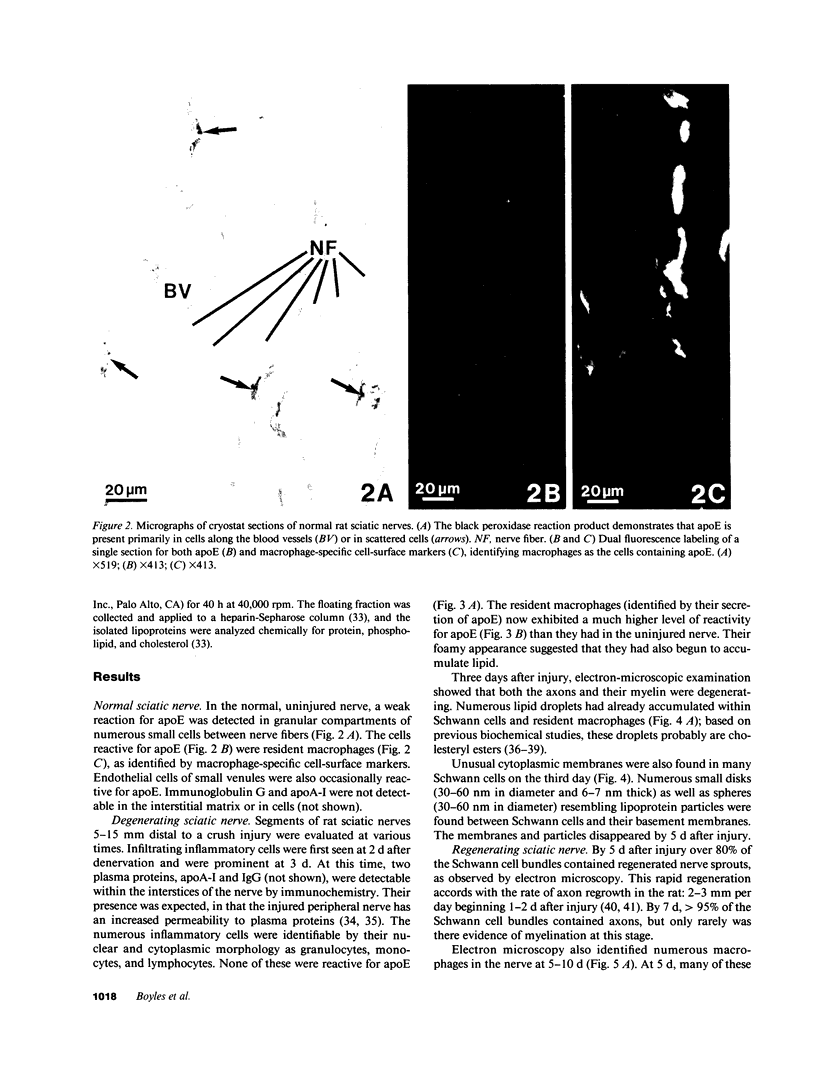
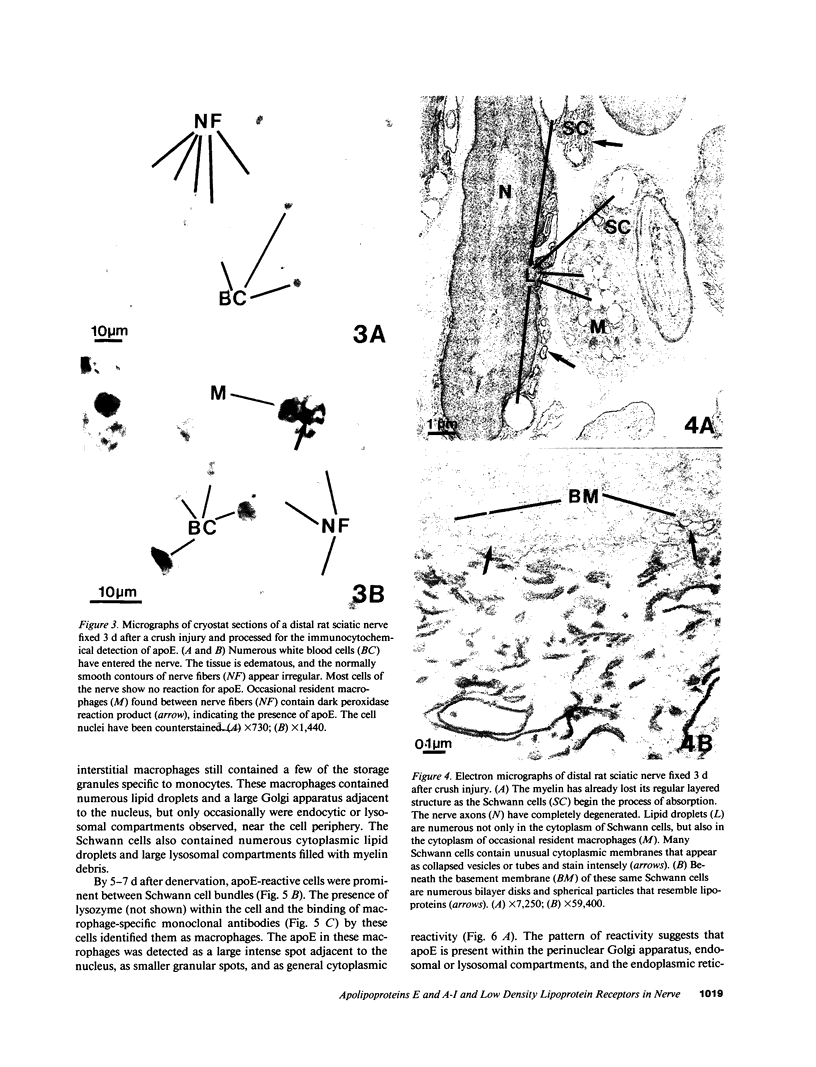
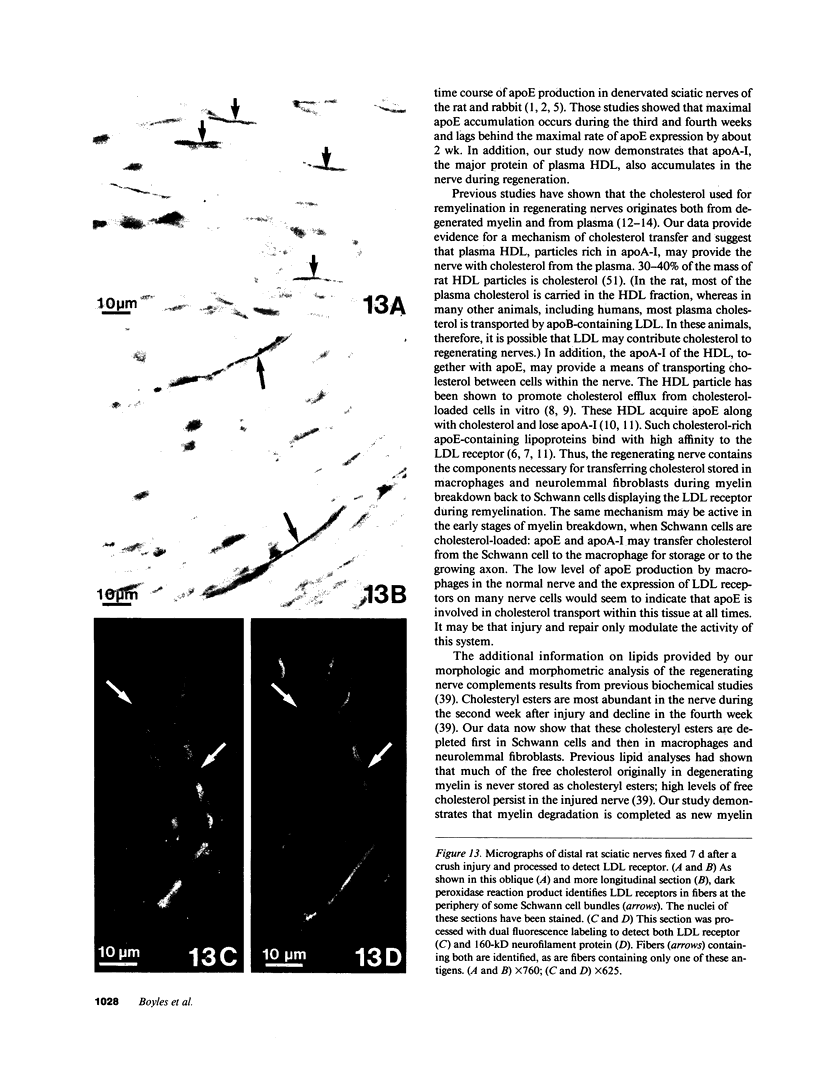
Recent work has demonstrated that apo E secretion and accumulation increase in the regenerating peripheral nerve. The fact that apoE, in conjunction with apoA-I and LDL receptors, participates in a well-established lipid transfer system raised the possibility that apoE is also involved in lipid transport in the injured nerve. In the present study of the crushed rat sciatic nerve, a combination of techniques was used to trace the cellular associations of apoE, apoA-I, and the LDL receptor during nerve repair and to determine the distribution of lipid at each stage. After a crush injury, as axons died and Schwann cells reabsorbed myelin, resident and monocyte-derived macrophages produced large quantities of apoE distal to the injury site. As axons regenerated in the first week, their tips contained a high concentration of LDL receptors. After axon regeneration, apoE and apoA-I began to accumulate distal to the injury site and macrophages became increasingly cholesterol-loaded. As remyelination began in the second and third weeks after injury, Schwann cells exhausted their cholesterol stores, then displayed increased LDL receptors. Depletion of macrophage cholesterol stores followed over the next several weeks. During this stage of regeneration, apoE and apoA-I were present in the extracellular matrix as components of cholesterol-rich lipoproteins. Our results demonstrate that the regenerating peripheral nerve possesses the components of a cholesterol transfer mechanism, and the sequence of events suggests that this mechanism supplies the cholesterol required for rapid membrane biogenesis during axon regeneration and remyelination.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERRY J. F., CEVALLOS W. H., WADE R. R., Jr LIPID CLASS AND FATTY ACID COMPOSITION OF INTACT PERIPHERAL NERVE AND DURING WALLERIAN DEGENERATION. J Am Oil Chem Soc. 1965 Jun;42:492–500. doi: 10.1007/BF02540090. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Bates S. R., Rothblat G. H. Regulation of cellular sterol flux and synthesis by human serum lipoproteins. Biochim Biophys Acta. 1974 Jul 26;360(1):38–55. doi: 10.1016/0005-2760(74)90178-7. [DOI] [PubMed] [Google Scholar]
- Belin J., Smith A. D. Wallerian degeneration of rat sciatic nerve. Changes in cholesteryl ester content and fatty acid composition. J Neurochem. 1976 Oct;27(4):969–970. doi: 10.1111/j.1471-4159.1976.tb05164.x. [DOI] [PubMed] [Google Scholar]
- Boyles J. K., Pitas R. E., Wilson E., Mahley R. W., Taylor J. M. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985 Oct;76(4):1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin A. D., Hirose N., Blankenship D. T., Jackson R. L., Harmony J. A., Sparrow D. A., Sparrow J. T. Binding of a high reactive heparin to human apolipoprotein E: identification of two heparin-binding domains. Biochem Biophys Res Commun. 1986 Jan 29;134(2):783–789. doi: 10.1016/s0006-291x(86)80489-2. [DOI] [PubMed] [Google Scholar]
- De Bruijn W. C., Den Beejen P. Glycogen, its chemistry and morphological appearance in the electron microscope.11. The complex formed in the selective contrast staining of glycogen. Histochem J. 1975 May;7(3):205–229. doi: 10.1007/BF01003591. [DOI] [PubMed] [Google Scholar]
- GUTH L. Regeneration in the mammalian peripheral nervous system. Physiol Rev. 1956 Oct;36(4):441–478. doi: 10.1152/physrev.1956.36.4.441. [DOI] [PubMed] [Google Scholar]
- Gordon V., Innerarity T. L., Mahley R. W. Formation of cholesterol- and apoprotein E-enriched high density lipoproteins in vitro. J Biol Chem. 1983 May 25;258(10):6202–6212. [PubMed] [Google Scholar]
- Ho Y. K., Brown M. S., Goldstein J. L. Hydrolysis and excretion of cytoplasmic cholesteryl esters by macrophages: stimulation by high density lipoprotein and other agents. J Lipid Res. 1980 May;21(4):391–398. [PubMed] [Google Scholar]
- Hoff H. F., Morton R. E. Lipoproteins containing apo B extracted from human aortas. Structure and function. Ann N Y Acad Sci. 1985;454:183–194. doi: 10.1111/j.1749-6632.1985.tb11857.x. [DOI] [PubMed] [Google Scholar]
- Hui D. Y., Brecht W. J., Hall E. A., Friedman G., Innerarity T. L., Mahley R. W. Isolation and characterization of the apolipoprotein E receptor from canine and human liver. J Biol Chem. 1986 Mar 25;261(9):4256–4267. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Gebicke-Härter P. J., Skene J. H., Schilling J. W., Weisgraber K. H., Mahley R. W., Shooter E. M. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M. J., Shooter E. M., Pitas R. E., Mahley R. W. Lipoprotein uptake by neuronal growth cones in vitro. Science. 1987 May 22;236(4804):959–962. doi: 10.1126/science.3576212. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J Biol Chem. 1979 May 25;254(10):4186–4190. [PubMed] [Google Scholar]
- Koo C., Innerarity T. L., Mahley R. W. Obligatory role of cholesterol and apolipoprotein E in the formation of large cholesterol-enriched and receptor-active high density lipoproteins. J Biol Chem. 1985 Oct 5;260(22):11934–11943. [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Rall S. C., Jr, Weisgraber K. H. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984 Dec 1;25(12):1277–1294. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. H., Fry D. L. Canine hyperlipoproteinemia and atherosclerosis. Accumulation of lipid by aortic medial cells in vivo and in vitro. Am J Pathol. 1977 Apr;87(1):205–226. [PMC free article] [PubMed] [Google Scholar]
- Mellick R. S., Cavanagh J. B. Changes in blood vessel permeability during degeneration and regeneration in peripheral nerves. Brain. 1968 Mar;91(1):141–160. doi: 10.1093/brain/91.1.141. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Gebicke-Härter P. J., Hangen D. H., Shooter E. M. A specific 37,000-dalton protein that accumulates in regenerating but not in nonregenerating mammalian nerves. Science. 1985 Apr 26;228(4698):499–501. doi: 10.1126/science.3983637. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Ignatius M. J., Hangen D. H., Shooter E. M. Expression of specific sheath cell proteins during peripheral nerve growth and regeneration in mammals. J Cell Biol. 1986 Feb;102(2):393–402. doi: 10.1083/jcb.102.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson Y. Studies on vascular permeability in peripheral nerves. I. Distribution of circulating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. Acta Neuropathol. 1966 Sep 1;7(1):1–15. doi: 10.1007/BF00686605. [DOI] [PubMed] [Google Scholar]
- Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987 Jan 13;917(1):148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Pitas R. E., Boyles J. K., Lee S. H., Hui D., Weisgraber K. H. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987 Oct 15;262(29):14352–14360. [PubMed] [Google Scholar]
- Rawlins F. A., Hedley-Whyte E. T., Villegas G., Uzman B. G. Reutilization of cholesterol-1,2-H3 in the regeneration of peripheral nerve. An autoradiographic study. Lab Invest. 1970 Mar;22(3):237–240. [PubMed] [Google Scholar]
- Rawlins F. A., Villegas G. M., Hedley-Whyte E. T., Uzman B. G. Fine structural localization of cholesterol-1,2- 3 H in degenerating and regenerating mouse sciatic nerve. J Cell Biol. 1972 Mar;52(3):615–625. doi: 10.1083/jcb.52.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. J., Goldstein J. L., Brown M. S. Purification of the LDL receptor. Methods Enzymol. 1985;109:405–417. doi: 10.1016/0076-6879(85)09106-6. [DOI] [PubMed] [Google Scholar]
- Simon G. Cholesterol ester in degenerating nerve: Origin of cholesterol moiety. Lipids. 1966 Sep;1(5):369–370. doi: 10.1007/BF02532683. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Shooter E. M. Denervated sheath cells secrete a new protein after nerve injury. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4169–4173. doi: 10.1073/pnas.80.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes G. J., McGuire C. B., Norden J. J., Freeman J. A. Nerve injury stimulates the secretion of apolipoprotein E by nonneuronal cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1130–1134. doi: 10.1073/pnas.83.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer B. J., De Bruijn W. C., Van Gent C. M., De Winter C. P. Ultrastructural findings on lipoproteins in vitro and in xanthomatous tissue. Histochem J. 1978 May;10(3):299–307. doi: 10.1007/BF01007561. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber K. H., Newhouse Y. M., Seymour J. L., Rall S. C., Jr, Mahley R. W. Preparative immobiline isoelectric focusing of plasma apolipoproteins on vertical slab gels. Anal Biochem. 1985 Dec;151(2):455–461. doi: 10.1016/0003-2697(85)90204-0. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Bersot T. P., Mahley R. W., Franceschini G., Sirtori C. R. Apolipoprotein A-IMilano. Detection of normal A-I in affected subjects and evidence for a cysteine for arginine substitution in the variant A-I. J Biol Chem. 1983 Feb 25;258(4):2508–2513. [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Mahley R. W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981 Sep 10;256(17):9077–9083. [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Mahley R. W., Milne R. W., Marcel Y. L., Sparrow J. T. Human apolipoprotein E. Determination of the heparin binding sites of apolipoprotein E3. J Biol Chem. 1986 Feb 15;261(5):2068–2076. [PubMed] [Google Scholar]
- Werb Z., Chin J. R. Apoprotein E is synthesized and secreted by resident and thioglycollate-elicited macrophages but not by pyran copolymer- or bacillus Calmette-Guerin-activated macrophages. J Exp Med. 1983 Oct 1;158(4):1272–1293. doi: 10.1084/jem.158.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Chin J. R. Onset of apoprotein E secretion during differentiation of mouse bone marrow-derived mononuclear phagocytes. J Cell Biol. 1983 Oct;97(4):1113–1118. doi: 10.1083/jcb.97.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. L., Hall S. M. Chronic Wallerian degeneration--an in vivo and ultrastructural study. J Anat. 1971 Sep;109(Pt 3):487–503. [PMC free article] [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]
- Wood J. G., Dawson R. M. Lipid and protein changes in sciatic nerve during Wallerian degeneration. J Neurochem. 1974 May;22(5):631–635. doi: 10.1111/j.1471-4159.1974.tb04274.x. [DOI] [PubMed] [Google Scholar]
- Yao J. K., Natarajan V., Dyck P. J. The sequential alterations of endoneurial cholesterol and fatty acid in Wallerian degeneration and regeneration. J Neurochem. 1980 Oct;35(4):933–940. doi: 10.1111/j.1471-4159.1980.tb07092.x. [DOI] [PubMed] [Google Scholar]