Abstract
BACKGROUND
Cancer therapy is one of many conditions which may diminish the ovarian reserve. Banking of human ovarian tissue has become an option for the preservation of female fertility. We have shown that vitrification is an excellent method to cryopreserve ovarian tissue. To carry out vitrification in a clinical setting, we have developed a clinical grade closed system to avoid direct contact of ovarian tissue with liquid nitrogen.
METHODS
Ovarian tissue was obtained by biopsy from 12 consenting women undergoing Caesarean section. Tissues were vitrified in cryotubes, using dimethyl sulphoxide, 1,2-propanediol, ethylene glycol and polyvinylpyrrolidon as cryoprotectants. Non-vitrified and warmed-vitrified tissue was compared by light and electron microscopic morphology of the follicles within the tissues.
RESULTS
We did not see any differences in the light or electron microscopic ultrastructure of oocytes between non-vitrified and vitrified tissues. No irreversible subcellular alterations in vitrified tissues were seen.
CONCLUSIONS
The ultrastructure of follicles within the vitrified human ovarian tissue was well preserved using cryotube in a closed vitrification system to avoid direct contact of liquid nitrogen. The system is compatible with the European tissue directive.
Keywords: vitrification, transmission electron microscopy, ovary
Introduction
The continued progress and enhanced effectiveness of chemotherapy and radiotherapy has increased the survival rate of young female cancer patients (von Wolff et al., 2009) but major side effects of these treatments are premature ovarian failure and loss of fertility. Since many patients are young, they seek the chance to preserve their reproductive potential before treatment. There are a few options available to preserve fertility in these patients. These include cryopreservation of embryos, oocytes (Yang et al., 2007; Porcu et al., 2008) or ovarian tissue (Hovatta et al., 1996; Nugent et al., 1997; Donnez et al., 2000; Kim, 2006).
Parameters such as patient's age, treatment protocol, type of malignancy and the partner's status should be considered when the most suitable strategy for fertility preservation is chosen (Meirow and Nugent, 2001).
Cryopreservation of ovarian tissue is the only option available for prepubertal girls and for women who need immediate chemotherapy (Gosden et al., 1994; Oktay et al., 1998; Donnez et al., 2000, 2005). Auto-transplantation of frozen–thawed cortical fragments has yielded live births in human (Donnez et al., 2004; Meirow et al., 2005; Andersen et al., 2008; von Wolff et al., 2009).
Cryopreservation of ovarian tissue can be performed using slow-rate freezing or vitrification. Slow-rate freezing (Hovatta et al., 1996; Newton et al., 1996) was first applied in human fertility preservation. However, based on animal studies, the vitrification technique seems to have advantages over slow freezing (Kuleshova and Lopata, 2002; Courbiere et al., 2006) as it did not induce apoptosis in mouse and human ovarian tissue after warming (Rahimi et al., 2003; Mazoochi et al., 2008). Vitrification is a rapid and simple technique that preserves large, heterogeneous samples of complex biologic tissue without formation of ice crystals (Pegg, 2001; Kuleshova and Lopata, 2002; Fahy et al., 2004).
Cryoprotectants used in high concentrations in the vitrification procedure include ethylene glycol (EG), dimethyl sulphoxide (DMSO) and propanediol. Adequate penetration of cryoprotectant through the stroma and granulosa cells to the oocytes is necessary, while at the same time avoiding possible cryoprotectant toxicity. Hence, it is a compromise between cell survival and cell death for each of these cell types in the ovary (Hovatta, 2005).
We have earlier shown that vitrification is more efficient than the slow freezing programme particularly in the preservation of ovarian stromal cells (Keros et al., 2009). However, direct contact of the tissue with liquid nitrogen is not optimal for cryopreservation of human ovarian tissue because of the risk of contamination.
In the present study, we used a clinical grade closed system in which the tissue is never in direct contact with liquid nitrogen, neither in the vitrification step nor during storage. The viability and ultrastructural features of the pre-antral follicles in vitrified tissues were studied.
Materials and Methods
Ovarian tissue donors
Small pieces of ovarian cortical tissue, 3–5 × 5–8 mm, with variable thickness, were obtained by biopsy from consenting patients undergoing elective Caesarean section (n = 12). Ovarian tissue obtained from three of these women was only cultured for 24 h as non-vitrified cultured controls. The mean age of the women was 34.1 ± 4.7 with a range between 22 and 40 years. The study was approved by the Ethics committee of the Karolinska Institutet, Karolinska University Hospital Huddinge.
Tissue preparation
Ovarian tissue was collected and transported to the laboratory within 5 min in sterile 50 ml Falcon tubes (Becton Dickinson, Bedford, MA, USA) containing about 20 ml cold (4°C) Flushing medium (MediCult, Jyllinge, Denmark). The tissue was transferred to a culture dish (Becton Dickinson) containing flushing medium and the medullar tissue was removed. The cortical ovarian tissue was then cut into small pieces of about 1–1.5 mm3 with scalpel, keeping the tissue immersed in collection medium while working under a stereomicroscope. Two small pieces of each biopsy were taken as non-vitrified controls and fixed for light microscopy (LM) and transmission electron microscopic (TEM) evaluation. The remaining pieces of the tissue were vitrified. After warming, two pieces were fixed for LM and TEM analysis. The cortical tissue was stored in liquid nitrogen for 6–7 months before it was warmed.
Vitrification
The vitrification procedure was based on a method recently presented by our group (Keros et al., 2009). Ovarian cortical tissue was equilibrated in three incubation steps in solutions with increasing concentrations of DMSO (Sigma-Aldrich, Sweden), 1,2-propanediol (PrOH) and EG supplemented with 10 mg/ml human serum albumin (HSA, Vitrolife, Göteborg, Sweden). After washing for 5 min in Hank's balanced salt solution (HBSS) with 10 mg/ml HSA, cortical tissue pieces were transferred sequentially to vitrification solution VS1, VS2 and VS3 at 2.5, 5 and 10% of each cryoprotectant, respectively. The third solution was also supplemented with (10%w/v) polyvinylpyrrolidon (PVP, Sigma-Aldrich, Sweden). The incubation time in VS1 was 5 min and for both VS2 and VS3 there was 10 min in each. The longer incubation time was applied because in our earlier study we showed that good-quality stroma, composed of collagen fibres and stromal cells was observed in these samples. The first and second incubation steps were performed at room temperature and the third, containing higher concentrations of cryoprotectants, at 4°C.
The vitrification itself was carried out by transferring the tissue pieces from VS3 into 1.8 ml NUNC cryotube (Nunclon, Roskilde, Denmark), with an internal thread cap to make it leak proof, with a minimum volume of the vitrification medium using a small spoon so as not to squeeze the tissue. The cryotube was immersed into liquid nitrogen leaving the screw cap above the surface to avoid leaking.
After vitrification, the tissues were stored in the vapour-phase nitrogen storage freezer (Air liquid-DMC, Espace 151-331-661 Gas, France) to avoid any microbial contamination by liquid nitrogen (Cobo et al., 2010).
In order to fulfil the European tissue directive 2004/23/EG, 2006/17/EG, 2006/86/EG and the Swedish Tissue Law 2008:286 for donation, tissue preparation and banking, the vitrification and warming procedures, were carried out in a closed sterile and traceable system, in a controlled and defined sterile environment.
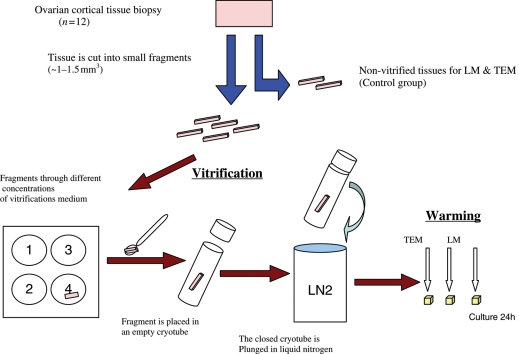
The procedure of tissue handling is shown in Fig. 1. The procedure for vitrification and warming is summarized in a standard operation procedure (SOP) (Supplementary information).
Figure 1.
Schematic experimental setup for the procedure of vitrification in the study.
Warming
The warming procedure was performed in four steps. Vitrified cortical tissue pieces were warmed at room temperature for 30 s and then placed in a 25°C water bath until melted. The contents of each cryotube were transferred into the first pre-warmed, 37°C, solution, consisting of HBSS and 10 mg/ml HSA supplemented with 0.5 M sucrose (Sigma-Aldrich, Sweden), for about 2 min. Then the pieces went through two 5 min steps in warming solutions consisting of HBSS/HSA supplemented with 0.25 and 0.125 M sucrose, respectively.
The incubation time for the fourth step in warming solution, consisting of HBSS supplemented with (10 mg/ml) has, was also 5 min. Some pieces were fixed directly after warming for morphological evaluation and the remaining were cultured by transferring into a dish containing pre-equilibrated culture medium.
Tissue culture
Both non-vitrified and vitrified samples were cultured in 24-well culture plates (Nunclon) in 300 µl of pre-equilibrated culture medium, containing McCoy's 5a medium with bicarbonate supplemented with HEPES (20 mM), l-glutamine (3 mM), penicillin, streptomycin (0.1 mg/ml), transferring (2.5 µg/ml), selenium (4 ng/ml), insulin (10 ng/ml), HSA (0.1%) and FSH all obtain from Sigma-Aldrich, Sweden (Telfer et al., 2008).
Plates were incubated for 24 h in a sterile humidified air atmosphere with 5% CO2 at 37°C.
Light microscopy
The morphology of the follicles, granulosa cells and ovarian stroma after vitrification was analysed by LM.
Vitrified and non-vitrified tissues were fixed in Bouin's solution for 24 h and then dehydrated in 70% alcohol at 4°C. The fixed tissues were embedded in paraffin wax. Serial sections of 4 µm thickness were prepared and every 11th section of each tissue piece was mounted on glass slides and stained with haematoxylin and eosin. To prevent double counting, each follicle was followed through neighbouring sections and counted only once. The development stages of the follicle were classified by the number of layers and shape of granulosa cells surrounding the oocyte, as defined by Gougeon (1996). Briefly, primordial follicles were classified as those containing a single layer of flattened granulosa cells, transitional primary follicles had one or more cuboidal granulosa cells, primary follicles were those with a complete single layer of cuboidal granulosa cells, transitional secondary follicles were where at least part of the follicle had two or more layers of cuboidal granulosa cells, secondary follicles had two complete layers of cuboidal granulosa cells and pre-antral follicles had more than two layers of cuboidal granulosa cells. Atretic follicles were identified as those containing oocyte with eosinophilic cytoplasm; contraction and clumping of the chromatin material were considered as atretic (Gougeon, 1986).
Transmission electron microscopy
Vitrified and non-vitrified tissues were fixed in 2% glutaraldehyde and 0.5% paraformaldehyde in 0.1% mol/ml sodium cacodylate buffer and 0.1 mol/ml sucrose pH 7.4 at room temperature for 2 h and post-fixed in 1% osmium tetroxide.
After dehydration, the samples were embedded in LX-112 (Ladd Research Industries Inc., Burlington, VT, USA). The samples were cut into about 0.5 µm sections and stained with toluidine blue and observed under LM. Then samples with follicles were selected, cut into 50 nm sections and stained with alcoholic uranyl acetate. The ultra-thin sections were then examined using a transmission electron microscope (Tecnai, Fei, Eindhoven, and The Netherlands). Digital images were taken at magnifications of ×1250 to ×30 000 using Megaview III camera.
Ultrastructural evaluation was performed in randomly selected samples. For non-cultured tissue, seven non-vitrified samples and six vitrified samples were used. For cultured tissue, four (out of nine) vitrified samples and three non-vitrified samples were analysed.
The ultrastructural changes in the ovarian tissue after vitrification and 24 h culture were evaluated by assessing the structures of oocytes, granulosa cells and stromal cells separately. The investigator was blinded to the experimental background of the specimens.
In the oocytes, we evaluated the integrity of nuclear organelles and the membranes, cristae of the mitochondria and their density, the density of the cytoplasm, the content of membrane vesicles and attachment to the granulosa cells. The same parameters were judged in granulosa cells. The attachment between granulosa cells and the basement membrane were also evaluated. The oocytes and granulosa cells were considered as intact if all the structures were undamaged. A cell with collapsed and/or disrupted nuclear or plasma membrane was considered as degenerated.
Results
Light microscopy
The morphology and developmental stages of follicles (n = 100) in both non-vitrified and vitrified tissues (before culture) were evaluated using an inverted microscope (Table I). From the follicles found, 37 were at the primordial stage and 42 follicles at the primary stage, comprising 18 transitional follicles and 24 primary follicles. The remaining follicles were 13 secondary and 8 atretic. No clear differences could be found in the structures of the follicles in either group when analysed at the light microscopic level from haematoxylin/eosin stained sections (Fig. 2). No follicles were observed in two patients.
Table I.
Number of follicles analysed in the ovarian tissues from nine women donors assessed by LM.
| Before culture | Total number of follicles | Primordial follicles | Primary follicles | Secondary follicles | Atretic follicles |
|---|---|---|---|---|---|
| Non-vitrified | 48 | 12 | 25 | 7 | 4 |
| Vitrified | 52 | 25 | 17 | 6 | 4 |
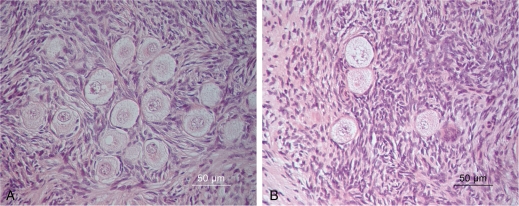
Figure 2.
Morphology of follicles at different developmental stages within human ovarian tissue in non-vitrified (A) and vitrified (B) group. Well-preserved morphology of pre-antral follicles within vitrified tissue (B) was seen, similar to follicles in non-vitrified tissue which showed normal morphology.
The stromal cells and bundles of collagen fibres were as well preserved in the vitrified tissue as it was in the control non-vitrified tissue (Fig. 2). The structure of follicles in both groups, vitrified and non-vitrified tissue was assessed after 24 h culture (Fig. 3). The morphology and developmental stages of follicles (n = 136) (Table II) within the cultured tissues were evaluated by using an inverted microscope. From the follicles found, 15 were at the primordial stage and 66 follicles were at the primary stage; of the remaining follicles 12 were secondary and 43 were atretic. Serial sectioning was provided for all samples in both groups.
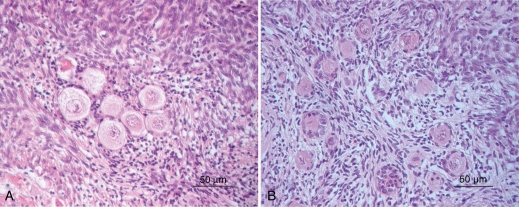
Figure 3.
Follicles within the cultured ovarian tissue in the vitrified group (B) showed similar morphological features as in the non-vitrified tissue (A). Few of pyknotic follicles were seen in both groups.
Table II.
Number of follicles analysed within vitrified human ovarian tissues from three donors assessed after 24 h culture by LM.
| After culture | Total number of follicles | Primordial follicles | Primary follicles | Secondary follicles | Atretic follicles |
|---|---|---|---|---|---|
| Non-vitrified | 84 | 13 | 38 | 5 | 28 |
| Vitrified | 52 | 2 | 28 | 7 | 15 |
No follicles were found in the small vitrified tissue pieces from five patients. In two of the samples from these patients, no follicles were seen in non-vitrified samples either. The variable number of follicles in these tissue pieces is probably due to the normal uneven distribution of follicles in the ovarian cortex as discussed later (Van den Broecke et al., 2001; Schmidt et al., 2003).
Transmission electron microscopy
The total number of follicles analysed from each group was as following: 10 non-vitrified, non-cultured (Figs 4A and 5A), 6 vitrified, non-cultured (Figs 4B and 5B), 16 cultured, non-vitrified (Fig. 6A) and 9 cultured, vitrified (Fig. 6B), respectively.
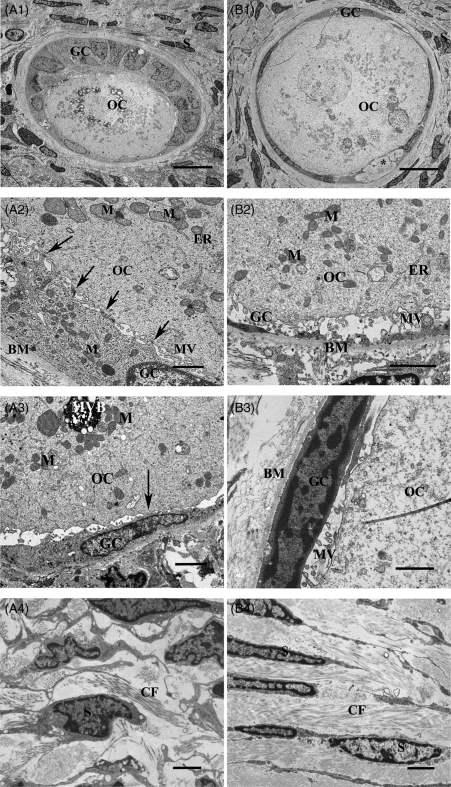
Figure 4.
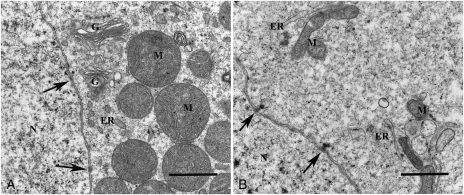
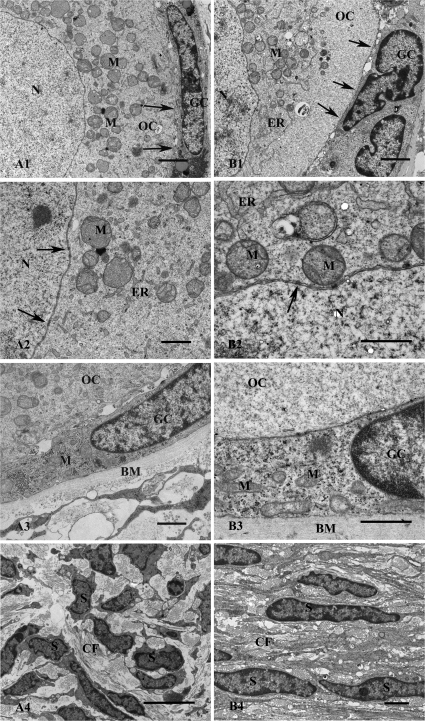
Transmission electron microscopy of follicles within human ovarian tissues in two groups of non-vitrified (A) and vitrified (B) tissues. Overview of non-vitrified tissue showing a transitional primary follicle containing an oocyte (OC) in contact with granulosa cells (GC) surrounded by stromal cells (S) (A1). Higher magnification of the follicle showing the contact between OC and GC and the basement membrane (BM). Gap junctions (arrows) as well as microvilli (MV) are well defined and the contact with BM is uniform. Mitochondria (M) and endoplasmic reticulum (ER) are well defined (A2). An arrow shows the contact between OC and GC (A3). Stroma in non-vitrified tissue contained collagen fibres (CF) and spindle-shaped S (A4). Scale bars; A1 = 10 µm, A2 = 1 µm, A3–A4 = 2 µm. An overview of a primordial follicle within well-preserved ovarian stroma in a vitrified tissue (B1). An irregular pattern seen in the follicles in both vitrified (*) and non-vitrified group (not shown here). Contact between OC and GC and BM in a follicle of vitrified tissue. The homogenous cytoplasm of the oocyte contains uniform M and some ER (B2). Projections of MV are filling the space and connecting the OC and GC (B2), which is seen also in the non-vitrified tissue (A3). Higher magnification of the follicle showing the intact contact between the OC and GC containing gap junctions (arrows). The MV is also clearly seen (B3). Image from the stroma containing abundant CF and spindle-shaped S (B4). Scale bars; B1 = 10 µm, B2 = 2 µm, B3 = 1 µm, B4 = 2 µm.
Figure 5.
Image of the nuclei (N) of the oocytes at a high magnification. The nuclear membranes containing nuclear pores (arrows) are clearly shown in both non-vitrified (A) and vitrified (B) groups. The Golgi apparatus (G) is well defined (A). Scale bars; 5A and 5B = 1 µm.
Figure 6.
Ultrastructure of follicles within human ovarian tissue after culture for 24 h in non-vitrified (A) and vitrified (B) groups. The oocyte nuclei (N) surrounded by nuclear membrane in a primordial follicle is shown (A1). Cytoplasm contains well-defined M. The intact contact between GC and OC is seen (arrows). The N and cytoplasmic organelles, M and ER are well defined. The nuclear pores (arrows) are clearly seen (A2). The contact between GC, OC and BM is shown (A3). GC contains hetero- and euchromatin in their nuclear. Well-defined M scattered in the cytoplasm are seen (A3). Image of stromal tissue composing bundles of CF and S (A4). Scale bars; A1 = 2 µm, A2–A3 = 1 µm and A4 = 5 µm. Overview of a primary follicle with OC and GC showing well-defined M and ER and intact contact between GC and the OC (arrows) (B1). Detail of the border between N and cytoplasm in OC showing well-defined nuclear membrane (arrows), ER and M containing cristae (B2). Granulosa cell in contact with OC and BM is shown (B3). Numerous M are scattered in the cytoplasm of the GC (B3). A sample of stroma containing numerous CF and flattened S is shown (B4). Scale bars; B1 = 2 µm, B2–B3 = 1 µm and B4 = 2 µm.
The ultrastructure of the oocytes was well preserved in vitrified tissues. Ultrastructural evaluation of the pre-antral follicles in both vitrified and non-vitrified control tissues showed similar morphology. The morphology of the oocytes, integrity of the granulosa cells and the ovarian stroma could be analysed in detail.
Ultrastructure of the oocytes
The ultrastructure of the oocytes in vitrified tissues (Fig. 4B) was similar to those in the non-vitrified tissue (Fig. 4A). The nuclei of the oocytes in vitrified tissue were dominated by euchromatin and the inner and outer nuclear membranes of the oocytes were clearly distinguished containing distinct nuclear pores (Fig. 5B). The homogenous cytoplasm of the oocytes was reflected by well-defined structures, without obvious increased vacuolization (Fig. 4B2). The round- or ovoid-shaped mitochondria and endoplasmic reticulum were the most abundant organelles in the oocytes. The endoplasmic reticulum was well defined, and the mitochondria exhibited highly organized structures with a condensed matrix and intact cristae (Figs 4B2 and 5B). The presence of swollen mitochondrial cristae was rarely found. The Golgi complexes were well defined (not shown). The contact between the oocyte and granulosa cells was sharp containing gap junctions and microvilli (Fig. 4B3). There were no noticeable differences between the groups with respect to mitochondria, microvillus and endoplasmic reticulum in oocytes (Fig. 4A2 and B2).
Ultrastructure of granulosa cells
The granulosa cells in vitrified tissues had a normal morphology and organelle distribution (Fig. 4B3). Their nuclei contained euchromatin in the inner part and a small peripheral part of heterochromatin, and no sign of cell death was observed. The intact cytoplasm of the granulosa cells contained abundant typical organelles including mitochondria with cristae, and endoplasmic reticulum. Gap junctions connecting neighbouring cells were clearly seen and microvilli were present. The contact between granulosa cells and basement membrane was also well preserved (Fig. 4B3). The ultrastructure of follicles in the vitrified group was similar to that in the non-vitrified control group, the granulosa cells showed uniform contact with no obvious increase in the level of vacuolization.
A small space (*) with irregular pattern, possibly a lysed cell, was observed in few follicles in both non-vitrified and vitrified tissue (Fig. 4B1). There was no increased number of these cells within follicles either in Group A or B.
Ultrastructure of stromal cells
In the vitrified group, well-preserved stromal tissues contained abundant collagen fibres and spindle-shaped fibroblast like cells. The stromal cells had hetero- and euchromatic containing nuclei, which was homogenously distributed in the nuclear envelope. The stromal cells were surrounded by abundant collagen fibres (Fig. 4B4). The distribution of collagen fibres was uniform and similar to that in control tissue (Fig. 4A4).
Follicular viability after warming
The morphology and ultrastructure of the follicles within the ovarian tissue after warming and culture for 24 h remained mostly normal (Fig. 6B).The ultrastructure of follicles in both non-vitrified (Fig. 6A) and vitrified (Fig. 6B) groups was similar after culture. There were no obvious gross morphological abnormalities or necrosis in the oocytes after culture. The nuclear pores were well defined (Fig. 6B2). Round-shaped mitochondria and a well-preserved cell membrane and endoplasmic reticulum were observed in the oocytes (Fig. 6B1). The structure of the mitochondria was organized and they contained condensed matrix and intact cristae (Fig. 6B2). The granulosa cells had peripheral heterochromatin in their nuclei and numerous rod-shaped mitochondria scattered in their cytoplasm (Fig. 6B3). The stromal cells had hetero- and euchromatic containing nuclei. The stromal tissue contained many collagen fibres and stromal cells (Fig. 6B4). Elongated stromal cells and an abundant amount of dense collagen bundles were seen in the vitrified (Fig. 6B4) group similar to the non-vitrified tissue (Fig. 6A4).
Discussion
Vitrification is a rapid and simple technique which has recently been applied for cryopreservation of ovarian tissues due to its minimal changes in morphology and ultrastructure. It is an ultrarapid cooling process that avoids cellular damage by producing a glass-like solidification of cells by extreme elevation in viscosity (Salehnia et al., 2002; Migishima et al., 2003; Courbiere et al., 2005; Ishijima et al., 2006; Wang et al., 2008).
We show here that vitrification of human ovarian tissue can be carried out in a clinical setting without any direct contact with liquid nitrogen. This enables us to easily comply with the quality requirements of the European tissue directive 2004/23, 2006/17/EG, and 2006/86/EG (www.EU).
Using our closed vitrification system, we can avoid the risk of contamination of the samples during the vitrification and storage procedures. This is a real advantage because ovarian samples for fertility preservation have to be cryo-stored for very long periods.
In a previous study, we compared slow freezing and vitrification of human ovarian tissue, and showed that the morphological integrity of particularly the stromal tissue after vitrification was better than that after slow freezing (Keros et al., 2009). However, the procedure was not carried out in a clinical setting. In this study, we reported an extremely simple and feasible, closed vitrification system which avoids direct contact of ovarian tissue with liquid nitrogen.
The device that we used was a cryotube, which could be closed and then rapidly immersed into liquid nitrogen leaving the cap above the surface of the nitrogen to avoid leaking. It was a non-toxic, sterile cryotube containing internal thread with a silicone gasket to provide the best possible seal. Efficient vitrification requires extremely fast cooling, and apparently this very simple system enables such a rate preserving the morphology of both the pre-antral follicles and stromal tissue. This morphology was maintained following 24 h of culture. It is possible that lysed follicles would not have been identified both after warming and culture; however, this is an error in any assessment of human ovarian tissue following cryopreservation.
Further investigation such as xeno-transplantation may also give information about viability of the follicles within the tissue. Nevertheless, tissue culture is an excellent method to demonstrate the viability of the tissue (Li et al., 2007; Keros et al., 2009). Non-viable follicles do not have intact electron microscopic ultrastructure after 24 h culture (Keros et al., 2009). We did not use any particular apoptosis assays because the most likely consequence of vitrification would be necrosis, which can better be demonstrated using careful morphological and ultrastructural analysis (Hovatta et al., 1996; Keros et al., 2009). An increased number of viable follicles may indicate better survival rate of the follicles during vitrification. Furthermore ultrastructural evaluation by transmission electron microscopy is the best known method to demonstrate the cryodamage (Gook et al., 1999; Hreinsson et al., 2003; Martinez-Madrid et al., 2007).
The final proof of the viability of the tissue would still be a pregnancy and a healthy child, and this remains to be shown.
Cryopreservation of human ovarian tissue using slow-rate freezing with good survival of follicles after thawing was for the first time reported by Hovatta et al. (1996), Hovatta (2005) and Newton et al. (1996). Healthy babies have already been born after transplantation of frozen–thawed human ovarian tissues after slow programmed freezing (Donnez et al., 2004; Meirow et al., 2005; Demeestere et al., 2007; Andersen et al., 2008; Ernst et al., 2010).
The data from comparative studies of vitrification and slow-rate freezing of human ovarian tissue are limited and opposing conclusions have been drawn by investigators. Some results have indicated that vitrification of human ovarian tissue is less efficient than slow-rate freezing (Gandolfi et al., 2006; Isachenko et al., 2009). Some authors concluded that modified vitrification is an effective technique for freezing of ovarian tissue as it showed presence of most normal follicles (80.3%) after warming (Li et al., 2007). Our group showed that vitrification of human ovarian tissue was comparable with slow-rate freezing (Keros et al., 2009). These different results may due to differences in the procedure, for example, cryoprotectant composition and concentrations, exposure times to cryoprotectants and speed of vitrification. The process of slow freezing or vitrification is not the issue. The issue is that the procedures have not been optimized for that particular biological material. The penetration rate of cryoprotectants and the temperature play an important role during the cryopreservations procedure (Newton et al., 1996, 1998). For this reason, it is important to have a sufficient diffusion of cryoprotectant through the stroma and granulosa cells to the oocytes. In order to reach this aim, a compromise between the concentration of cryoprotectants, incubation time and temperature is required (Hovatta, 2005).
The improved stromal morphology reported in the present study confirms our previous observations (Keros et al., 2009) and is in contrast to the poor morphology of human stromal tissue previously reported using slow freezing (Gook et al., 1999). It would appear that the use of the combination of DMSO, PrOH and EG together with the stepwise increase in their concentrations has achieved sufficient dehydration to successfully vitrify all components of human ovarian tissue. In this study, the long incubation time of the cryoprotectants was applied because in our earlier study we showed that good-quality stroma, composed of collagen fibres and stromal cells was observed in the vitrification group with 10 min incubation time (Keros et al., 2009). Stromal cells probably play an important role in the proliferation and differentiation of granulosa cells (Hovatta et al., 1999; Liu et al., 2000). Co-operation between the granulosa cells, the stromal tissue and the oocytes is important for ovarian function (Hovatta, 2005), and preservation of the integrity of these components is necessary (Gook et al., 2004).
Our vitrification protocol can be performed in a clinical setting. We have shown excellent structural preservation of ovarian tissue. Our observations from LM showed similar morphology of the pre-antral follicles in both vitrified and non-vitrified tissue. This observation was confirmed by TEM assesses which revealed that the organelles of granulosa cells and oocytes were well preserved in vitrified tissues. The result of this study suggest that human ovarian tissue can be well preserved using simply a cryotube as a device in a closed system to avoid possible contamination via direct contact with liquid nitrogen.
Authors’ roles
All authors contributed to the study design and to writing the article. M.S. collected the biopsies and took them to the laboratory. She performed the vitrification and thawing and cultures, and light microscopic analyses. O.H. and M.L. lead the study providing expertise on the methodology and advice for the study conduct. K.H. together with M.S. analysed the electron microscopic morphology. B.N. recruited and adviced the ovarian tissue donors.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Funding
The study was supported by grants from the Swedish Research Council and the R&D funds of Karolinska Institutet and Stockholm County (ALF). Funding to pay the Open Access publication charges for this article was provided by the Swedish Research Council/Karolinska Institutet.
Acknowledgements
We thank the doctors (Ingvar Ek, Teija Taimi, Hildur Hadenius, Agneta Blanck, Rebecca Götz- Jänstedt, Emillie Nordenhök and Hedvig Andersson), midwives and nurses at the Department of Obstetrics and Gynaecology and at the operating theatres at Karolinska University Hospital, Huddinge, whose help made this study possible. We also thank Carin Lundmark, Eva Blomén and Ingrid Lindell at the Department of Oral Pathology for the tissue sectioning, slide preparation and staining.
References
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. doi:10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- Cobo A, Romero JL, Pérez S, de Los Santos MJ, Meseguer M, Remohí J. Storage of human oocytes in the vapour phase of nitrogen. Fertil Steril. 2010;94:1903–1907. doi: 10.1016/j.fertnstert.2009.10.042. doi:10.1016/j.fertnstert.2009.10.042. [DOI] [PubMed] [Google Scholar]
- Courbiere B, Massardier J, Salle B, Mazoyer C, Guerin JF, Lornage J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84:1065–1071. doi: 10.1016/j.fertnstert.2005.03.079. doi:10.1016/j.fertnstert.2005.03.079. [DOI] [PubMed] [Google Scholar]
- Courbiere B, Odagescu V, Baudot A, Massardier J, Mazoyer C, Salle B, Lornage J. Cryopreservation of the ovary by vitrification as an alternative to slow-cooling protocols. Fertil Steril. 2006;86(4 Suppl):1243–1251. doi: 10.1016/j.fertnstert.2006.05.019. doi:10.1016/j.fertnstert.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. doi:10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- Donnez J, Godin PA, Qu J, Nisolle M. Gonadal. Cryopreservation in the young patient with gynaecological malignancy. Curr Opin Obstet Gynecol. 2000;12:1–9. doi: 10.1097/00001703-200002000-00001. Review doi:10.1097/00001703-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. doi:10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Martinez-Madrid B, Demylle D, Van Langendonckt A. The role of cryopreservation for women prior to treatment of malignancy. Curr Opin Obstet Gynecol. 2005;17:333–338. doi: 10.1097/01.gco.0000175348.72566.47. Review doi:10.1097/01.gco.0000175348.72566.47. [DOI] [PubMed] [Google Scholar]
- Ernst E, Bergholdt S, Jörgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1281. doi: 10.1093/humrep/deq033. doi:10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- Fahy GM, Wowk B, Wu J, Phan J, Rasch C, Chang A. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology. 2004;48:157–178. doi: 10.1016/j.cryobiol.2004.02.002. doi:10.1016/j.cryobiol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006;85(Suppl 1):1150–1156. doi: 10.1016/j.fertnstert.2005.08.062. doi:10.1016/j.fertnstert.2005.08.062. [DOI] [PubMed] [Google Scholar]
- Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1, 2-propanediol. Hum Reprod. 1999;14:2061–2068. doi: 10.1093/humrep/14.8.2061. doi:10.1093/humrep/14.8.2061. [DOI] [PubMed] [Google Scholar]
- Gook DA, Edgar DH, Stern C. Cryopreservation of human ovarian tissue. Eur J Obstet Gynecol Reprod Biol. 2004;113:S41–S44. doi: 10.1016/j.ejogrb.2003.11.009. doi:10.1016/j.ejogrb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Hovatta O. Methods for cryopreservation of human ovarian tissue. Reprod Biomed Online. 2005;10:729–734. doi: 10.1016/s1472-6483(10)61116-9. Review doi:10.1016/S1472-6483(10)61116-9. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A, Winston RM. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11:1268–1272. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod. 1999;14:2519–2524. doi: 10.1093/humrep/14.10.2519. doi:10.1093/humrep/14.10.2519. [DOI] [PubMed] [Google Scholar]
- Hreinsson J, Zhang P, Swahn ML, Hultenby K, Hovatta O. Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum Reprod. 2003;18:2420–2428. doi: 10.1093/humrep/deg439. doi:10.1093/humrep/deg439. [DOI] [PubMed] [Google Scholar]
- Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, Bader M, Weiss JM. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009;138:319–327. doi: 10.1530/REP-09-0039. doi:10.1530/REP-09-0039. [DOI] [PubMed] [Google Scholar]
- Ishijima T, Kobayashi Y, Lee DS, Ueta YY, Matsui M, Lee JY. Cryopreservation of canine ovaries by vitrification. J Reprod Dev. 2006;52:293–299. doi: 10.1262/jrd.17080. doi:10.1262/jrd.17080. [DOI] [PubMed] [Google Scholar]
- Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–1683. doi: 10.1093/humrep/dep079. doi:10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- Kim SS. Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril. 2006;85:1–11. doi: 10.1016/j.fertnstert.2005.04.071. doi:10.1016/j.fertnstert.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Kuleshova LL, Lopata A. Vitrification can be more favourable than slow cooling. Fertil Steril. 2002;78:449–454. doi: 10.1016/s0015-0282(02)03305-8. doi:10.1016/S0015-0282(02)03305-8. [DOI] [PubMed] [Google Scholar]
- Li Y-B, Zhou C-G, Yang G-F, Wang Q, Dong Y. Modified vitrification method for cryopreservation of human ovarian tissues. Chinese Med J. 2007;120:110–114. [PubMed] [Google Scholar]
- Liu J, Van Der Elst J, Van Den Broecke R, Dumortier F, Dhont M. Maturation of mouse primordial follicles by combination of grafting and in vitro culture. Biol Reprod. 2000;62:1218–1223. doi: 10.1095/biolreprod62.5.1218. doi:10.1095/biolreprod62.5.1218. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril. 2007;87:1153–1165. doi: 10.1016/j.fertnstert.2006.11.019. doi:10.1016/j.fertnstert.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Mazoochi T, Salehnia M, Valojerdi MR, Mowla SJ. Morphologic, ultrastructural, and biochemical identification of apoptosis in vitrified-warmed mouse ovarian tissue. Fertil Steril. 2008;90(4 Suppl):1480–1486. doi: 10.1016/j.fertnstert.2007.07.1384. doi:10.1016/j.fertnstert.2007.07.1384. [DOI] [PubMed] [Google Scholar]
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. doi:10.1093/humupd/7.6.535 Review. [DOI] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar- Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. doi:10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- Migishima F, Suzuki-Migishima R, Song SY, Kuramochi T, Azuma S, Nishijimi M. Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod. 2003;68:881–887. doi: 10.1095/biolreprod.102.007948. doi:10.1095/biolreprod.102.007948. [DOI] [PubMed] [Google Scholar]
- Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11:1487–1491. doi: 10.1093/oxfordjournals.humrep.a019423. [DOI] [PubMed] [Google Scholar]
- Newton H, Fisher J, Arnold JR, Pegg DE, Faddy MJ, Gosden RG. Permeation of human ovarian tissue with cryoprotective agents in preparation for cryopreservation. Hum Reprod. 1998;13:376–380. doi: 10.1093/humrep/13.2.376. doi:10.1093/humrep/13.2.376. [DOI] [PubMed] [Google Scholar]
- Nugent D, Meirow D, Brook PF, Aubard Y, Gosden RG. Transplantation in reproductive medicine: previous experience, present knowledge and future prospects. Hum Reprod Update. 1997;3:267–280. doi: 10.1093/humupd/3.3.267. Review doi:10.1093/humupd/3.3.267. [DOI] [PubMed] [Google Scholar]
- Oktay K, Newton H, Aubard Y, Salha O, Gosden RG. Cryopreservation of immature human oocytes and ovarian tissue: an emerging technology? Fertil Steril. 1998;69:1–7. doi: 10.1016/s0015-0282(97)00207-0. Review doi:10.1016/S0015-0282(97)00207-0. [DOI] [PubMed] [Google Scholar]
- Pegg DE. The current status of tissue cryopreservtion. Cryo Lett. 2001;22:105–114. [PubMed] [Google Scholar]
- Porcu E, Venturoli S, Damiano G, Ciotti PM, Notarangelo L, Paradisi R, Moscarini M, Ambrosini G. Healthy twins delivered after oocyte cryopreservation and bilateral ovariectomy for ovarian cancer. Reprod Biomed. 2008;17:265–267. doi: 10.1016/s1472-6483(10)60204-0. doi:10.1016/S1472-6483(10)60204-0. [DOI] [PubMed] [Google Scholar]
- Rahimi G, Isachenko E, Sauer H, Isachenko V, Wartenberg M, Hescheler J, Mallmann P, Nawroth F. Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reprod Fertil Dev. 2003;15:343–349. doi: 10.1071/RD02063. doi:10.1071/RD02063. [DOI] [PubMed] [Google Scholar]
- Salehnia M, Abbasian Moghadam E, Rezazadeh Velojerdi M. Ultrastructure of follicles after vitrification of mouse ovarian tissue. Fertil Steril. 2002;78:1–2. doi: 10.1016/s0015-0282(02)03287-9. doi:10.1016/S0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Byskov AG, Nyboe Andersen A, Müller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–1164. doi: 10.1093/humrep/deg246. doi:10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. doi:10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- Van den Broecke R, Liu J, Handyside A, Van der Elst JC, Krausz T, Dhont M, Winston RM, Hovatta O. Follicular growth in fresh and cryopreserved human ovarian cortical grafts transplanted to immunodeficient mice. Eur J Obstet Gynecol Reprod Biol. 2001;97:193–201. doi: 10.1016/s0301-2115(00)00507-8. doi:10.1016/S0301-2115(00)00507-8. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, Salle B, Sonmezer M, Andersen CY. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy—a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45:1547–1553. doi: 10.1016/j.ejca.2009.01.029. Review doi:10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;23:2256–2265. doi: 10.1093/humrep/den255. doi:10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- Yang D, Brown SE, Nguyen K, Reddy V, Brubaker C, Winslow KL. Live birth after the transfer of human embryos developed from cryopreserved oocytes harvested before cancer treatment. Fertil Steril. 2007;87:1469e1–1469e2. doi: 10.1016/j.fertnstert.2006.07.1546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.