Abstract
DAX1/NR0B1 mutations are responsible for X-linked congenital adrenal hypoplasia (AHC) associated with hypogonadotropic hypogonadism (HH). Few data are available concerning testicular function and fertility in men with DAX1 mutations. Azoospermia as well as failure of gonadotrophin treatment have been reported. We induced spermatogenesis in a patient who has a DAX1 mutation (c.1210C>T), leading to a stop codon in position 404 (p.Gln404X). His endocrine testing revealed a low testosterone level at 1.2 nmol/l (N: 12–40) with low FSH and LH levels at 2.1 IU/l (N: 1–5 IU/l) and 0.1 IU/l (N: 1–4 IU/l), respectively. Baseline semen analysis revealed azoospermia. Menotropin (Menopur®:150 IU, three times weekly) and human chorionic gonadotrophin (1500 IU, twice weekly) were used. After 20 months of treatment, as azoospermia persisted, bilateral multiple site testicular biopsies were performed. Histology revealed severe hypospermatogenesis. Rare spermatozoa were extracted from the right posterior fragment and ICSI was performed. Four embryos were obtained and, after a frozen–thawed single-embryo transfer, the patient's wife became pregnant and gave birth to a healthy boy. We report the first case of paternity after TESE–ICSI in a patient with DAX1 mutation, giving potential hope to these patients to father non-affected children. Furthermore, this case illustrates the fact that patients with X-linked AHC have a primary testicular defect in addition to HH.
Keywords: hypogonadotropic hypogonadism, DAX1, assisted reproductive techniques, congenital adrenal hypoplasia (AHC), genetic counselling
Introduction
DAX-1 [Dosage sensitive sex-reversal, Adrenal hypoplasia congenita, on the X chromosome gene 1], alternatively called NR0B1, is a gene that encodes an orphan member of the nuclear hormone receptor family (Zanaria et al., 1994). The protein translated from this gene, called DAX-1, is essentially expressed in tissues involved in steroid hormone production and reproductive function such as the adrenals, hypothalamus and pituitary, as well as the testes (Guo et al., 1995). Clinically, NR0B1/DAX1 mutations are responsible for X-linked congenital adrenal hypoplasia (AHC) (MIM # 300200) associated with hypogonadotropic hypogonadism (HH) (Lin et al., 2006). In mice, Nr0b1 null homozygous male mice have adrenal insufficiency as well as testicular disorganization, dilated seminiferous tubules and failed spermatogenesis (Park et al., 2005). The dilated tubules in the adult reflect impaired testis cord morphogenesis during embryonic development (Meeks et al., 2003). Histological examination revealed a progressive degeneration of seminiferous tubule epithelium, hyperplasia of Leydig cells and sloughing of germ cells. A Sertoli-cell-specific expression of a DAX1 transgene is sufficient to partially rescue the primary testicular defect of the male Dax-1-deficient mouse (Jeffs et al., 2001).
Relatively few data are currently available concerning the testicular function and fertility of men with X-linked AHC harbouring DAX1 mutations. Some cases of azoospermia as well as failure of gonadotrophin treatment have been described (Mantovani et al., 2002; Brown et al., 2003; Ozisik et al., 2003; Seminara et al., 1999). Oligospermia has been reported in a rare case of late-onset AHC associated with a partial loss-of-function mutation (Tabarin et al., 2000). The failure of the gonadotrophin treatment described was judged on semen quality, and open testicular biopsy had not been attempted previously in order to treat male infertility.
We report the first birth of a child after a successful assisted reproductive technique (ART) in a man with classic AHC and HH due to a DAX1 mutation, using TESE–ICSI.
Case report
Our patient presented with primary adrenal insufficiency at the age of 3 weeks, diagnosed on account of major dehydration. He was treated with hydrocortisone and 9-alpha-fludrocortisone in order to restore glucocorticoid and mineralocorticoid deficiencies. He was referred to our unit at the age of 19 years because of hypogonadism. He had no history of cryptorchidism and his testes were small, 6 ml on each side. His penis was 6 cm in length and he had sparse pubic hair. His sense of smell was normal and he was shaving twice a month. His puberty was evaluated at P2 Tanner's stage. Bone densitometry revealed osteopenia with a T-score of −2.5 DS at the lumbar level. His bone age reached 15 years. Hius testosterone level was low 1.2 nmol/l (N: 12–40). Baseline FSH and LH levels were 2.1 IU/l (N: 1–5 IU/l) and 0.1 IU/l (N: 1–4 IU/l), respectively. A GnRH test (100 μg, subcutaneous) was conducted. The FSH and LH peaks reached 3.1 and 0.9 UI/l, respectively. LH and FSH pulsatility was studied over a 4-h period. No LH nor FSH pulses could be detected. Prolactin and FT4 levels were normal at 14.6 pg/ml (N: 5–10) and 17.2 pmol/l (N: 10–23), respectively. Pituitary MRI was normal. An adrenal scan revealed an atrophic left adrenal. The right adrenal could not be visualized. In summary, he presented with adrenal insufficiency associated with HH. He is an only child and a sporadic case in his family.
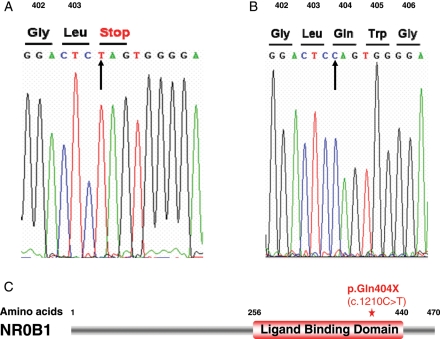
Considering the association of adrenal insufficiency and HH, genetic testing of the NR0B1/DAX1 gene was conducted, after obtaining informed consent according to the French ethics law. As shown in Fig. 1, a change in nucleotide sequence (c.1210C>T) was identified, leading to a stop codon in position 404 (p.Gln404X). This mutation in our patient is found in a large cohort of ACH patients (Lin et al., 2006).
Figure 1.
(A) Nonsense p.Q404X (Gln404Stop) mutation in DAX1. (B) Control sequence (arrow: nucleotide exchange). (C) The mutation, as shown by a red star, is located in the ligand-like binding domain leading to a premature stop codon and predicted non-functional protein.
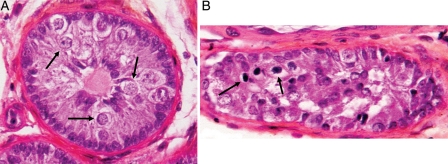
Hypogonadism was treated by testosterone enanthate (Androtardyl®: 250 mg intramuscularly, every 3 weeks). Six years later, as he desired paternity, a semen analysis was performed, which revealed azoospermia. Semen volume and semen pH were found to be normal. Spermatogenesis was therefore induced by gonadotrophins using menotropin (Menopur®:150 IU of FSH and 150 IU of LH, three times weekly) and human chorionic gonadotrophin (hCG: 1500 IU, twice weekly). One month later, his testosterone level reached 4 ng/ml. The treatment doses remained constant. After 20 months of treatment, testis volume reached 11 and 12 ml, for his right and left testis, respectively. However, azoospermia persisted. Consequently, bilateral testicular biopsies were performed. It was a blind, multiple-site biopsy. Very few spermatozoa were extracted from the right posterior fragment. Three straws were cryopreserved, each containing 100 motile spermatozoa. Histological examination revealed severe hypospermatogenesis. The diameter of the seminiferous tubules was either normal or reduced. Tubules were limited by a thickened basement membrane and contained mostly Sertoli cells and a few germ cells arrested at the spermatocyte stage (Fig. 2).
Figure 2.
Seminiferous tubule sections showing (A) Sertoli cells only or an arrest of spermatogenesis at the spermatocyte stage (arrow) in most tubules, and (B) isolated tubules with very rare spermatids (arrow). Few normal Leydig cells were seen. Magnification ×400.
His wife had been stimulated using triptorelin (Decapeptyl®) and recombinant FSH (Gonal-F® 150 IU) for 10 days. As a result, 11 oocytes were obtained, seven of them being mature. ICSI was performed using all the cryoconserved straws. No motile spermatozoa were recovered after thawing, and thus pentoxifylline (1 mg/ml) was used. Indeed, after the freezing–thawing process, the percentage of motile testicular spermatozoa is usually low, with a weak motility making the selection of live spermatozoa difficult. Pentoxifylline was found to significantly increases the motility of spontaneously immotile epididymal and testicular spermatozoa and allows fertilization, pregnancy and birth after ICSI (Terriou et al., 2000). Selection of viable spermatozoa is therefore easier. Four embryos were obtained: two Day-2 embryos were transferred and the remaining two were cryopreserved. No pregnancy followed. The following cycle, only one embryo remained viable after thawing and was used in a single-embryo transfer during a stimulated cycle. After this procedure, the patient's wife became pregnant and gave birth—9 months later, by Caesarean section—to a healthy boy.
Discussion
We report the first case of a child born after TESE–ICSI, with the sperm induced by gonadotrophin treatment, in a man with classic X-linked AHC due to a nonsense mutation in NR0B1/DAX1.
Our patient had a severe clinical phenotype of neonatal adrenal insufficiency associated with hypogonadism, rather than a delayed onset or milder form of AHC (Brown et al., 2003; Ozisik et al., 2003). In this case, the c.1210C>T nucleotide change is predicted to result in a p.Q404X nonsense mutation located in the C-terminal ligand-like binding domain of the protein (Lin et al., 2006). Missense mutations within this domain have already been shown to induce lower activity of DAX1 (Tabarin et al., 2000) and the p.Q404X change is likely to escape nonsense-mediated decay and to result in a truncated protein product lacking an AF-2 domain (Fig. 1C). Therefore, the nonsense mutation in our patient was considered to be responsible for his severe phenotype.
Gonadal defects in men carrying DAX1 mutations have been described in several cases (Caron et al., 1999; Tabarin et al., 2000; Achermann et al., 2001; Mantovani et al., 2002). In our patient, a few testicular spermatozoa were obtained from a testicular biopsy after a long period of treatment using exogenous gonadotrophins. In the past 20 years, such treatments have been used to induce spermatogenesis in men with isolated HH (Finkel et al., 1985; Büchter et al., 1998; European Metrodin HP Study group, 1998; Matsumoto et al., 2008; Liu et al., 2009). Sperm are usually obtained after 12–18 months of treatment. Gonadotrophin treatment has been used to induce or improve fertility in men with X-linked AHC with limited success (Seminara et al., 1999; Mantovani et al., 2002; Brown et al., 2003; Ozisik et al., 2003; Mantovani et al., 2006). A patient with a mild phenotype, described by Tabarin et al. (2000), had oligospermia that did not respond to gonadotrophin treatment. In all the other men treated, persistent azoospermia was observed after 6, 8, 9, 12 and even 36 months of treatment with exogenous gonadotrophins (Seminara et al., 1999; Mantovani et al., 2002; Brown et al., 2003; Ozisik et al., 2003; Mantovani et al., 2006). In our patient, gonadotrophins were administered for 20 months and azoospermia was found to persist upon semen analysis. His FSH, LH and testosterone serum levels were regularly checked and were normal, implying compliance with gonadotrophin injections. This suggests an intrinsic role for DAX1 in the adult human testis resulting in a defect that cannot easily be restored by gonadotrophin treatment. However, the treatment may well have allowed the development of a small number of spermatozoa that were obtained following testicular biopsy and used for ICSI.
In mice, targeted deletion of Dax1 (Ahch) revealed abnormalities in testis development, in rete testis and in spermatogenesis (Park et al., 2005). Efferent duct epithelium was hyperplastic and seminiferous tubules were obstructed by ectopic Sertoli and Leydig cells in adult mice (Jeffs et al., 2001). Therefore, DAX-1 is not needed for initiating spermatogenesis but seems necessary to induce regular spermatogenesis.
In humans, few testicular biopsies have been reported so far (Seminara et al., 1999; Brown et al., 2003; Ozisik et al., 2003). The first biopsy reported in an adult male was performed at the age of 27, following 7 years of low-dose hCG treatment. It revealed Sertoli-cell-only syndrome, rare spermatogonia and no apparent spermatogenesis (Seminara et al., 1999). A second report was of a 20-year-old patient after 6 months of treatment with exogenous gonadotrophins. This patient carried an N-terminal DAX-1 nonsense mutation (p.Q37X) and had a milder phenotype than our patient as he presented with adrenal insufficiency at the age of 20 (Ozisik et al., 2003). The testicular biopsy revealed disorganization of the normal tubule structure and abnormal proliferation of the interstitial tissue as well as moderate Leydig cell hyperplasia. Interestingly, post-mortem testicular histology from a neonate who died at 23 days has revealed normal testicular histology (Brown et al., 2003) with the presence of well-defined seminiferous testis cords containing numerous Sertoli cells and germ cells. This suggests a potential progressive defect in testicular function over the years.
In our patient, histology shows severe hypospermatogenesis with most of the germ cells arrested at the spermatocyte stage. Hypospermatogenesis is characterized by a low, basal level of spermatozoid production, with most of the tubules being depleted of mature forms of spermatozoa (Fig. 2). However, some tubules may contain focal complete spermatogenesis that is not visible in the thin paraffin section stained by hematoxylin and eosin. However, these rare mature spermatozoa may be retrieved from mechanically dilacerated testicular tissue. The testicular phenotype of our patient confirms data obtained in mice, suggesting that patients with DAX1 mutations have a primary testicular defect in addition to central HH. Our case report suggests that a testicular biopsy should be considered if patients remain azoospermic after gonadotrophin treatment.
Genetic counselling in couples where the man is affected by an X-linked condition should mention that every male offspring will be unaffected and that every female offspring will be a heterozygous carrier of the mutation. Consequently, the risk of having an affected male is moved to the second generation. Couples should be aware of sex selection of embryos by preimplantation genetic diagnosis if they wish to avoid this risk.
In conclusion, our case report gives potential hope to patients with DAX1 mutations to father non-affected children after TESE–ICSI.
Funding
J.C.A. is a Wellcome Trust Senior Fellow in Clinical Science (079666). Funding to pay the Open Access publication charges for this article was provided by Wellcome Trust, UK.
Acknowledgements
We would thank Docteur Michèle Guittard for referring the patient to our unit and Prof. Jean-Marie Antoine for performing testicular biopsies
References
- Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185:17–25. doi: 10.1016/s0303-7207(01)00619-0. doi:10.1016/S0303-7207(01)00619-0. [DOI] [PubMed] [Google Scholar]
- Brown P, Scobie GA, Townsend J, Bayne RAL, Seckl JR, Saunders PTK, Anderson RA. Identification of a novel missense mutation that is as damaging to DAX-1 repressor function as a nonsense mutation. J Clin Endocrinol Metab. 2003;88:1341–1349. doi: 10.1210/jc.2002-021560. doi:10.1210/jc.2002-021560. [DOI] [PubMed] [Google Scholar]
- Büchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998;139:298–303. doi: 10.1530/eje.0.1390298. doi:10.1530/eje.0.1390298. [DOI] [PubMed] [Google Scholar]
- Caron P, Imbeaud S, Bennet A, Plantavid M, Camerino G, Rochiccioli P. Combined hypothalamic-pituitary-gonadal defect in a hypogonadic man with a novel mutation in the DAX-1 gene. J Clin Endocrinol Metab. 1999;84:3563–3569. doi: 10.1210/jcem.84.10.6030. doi:10.1210/jc.84.10.3563. [DOI] [PubMed] [Google Scholar]
- European Metrodin HP Study Group. Efficacy and safety of highly purified urinary follicle-stimulating hormone with human chorionic gonadotropin for treating men with isolated hypogonadotropic hypogonadism. Fertil Steril. 1998;70:256–262. doi: 10.1016/s0015-0282(98)00156-3. doi:10.1016/S0015-0282(98)00156-3. [DOI] [PubMed] [Google Scholar]
- Finkel DM, Philipps JL, Snyder PJ. Stimulation of spermatogenesis by gonadotropins in men with hypogonadotropic hypogonadism. N Engl J Med. 1985;313:651–655. doi: 10.1056/NEJM198509123131102. doi:10.1056/NEJM198509123131102. [DOI] [PubMed] [Google Scholar]
- Guo W, Burris TP, McCabe ER. Expression of DAX-1, the gene responsible for X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism, in the hypothalamic-pituitary-adrenal/gonadal axis. Biochem Mol Med. 1995;56:8–13. doi: 10.1006/bmme.1995.1049. doi:10.1006/bmme.1995.1049. [DOI] [PubMed] [Google Scholar]
- Jeffs B, Meeks JJ, Ito M, Martinson FA, Matzuk MM, Jameson JL, Russell LD. Blockage of the rete testis and efferent ductules by ectopic Sertoli and Leydig cells causes infertility in Dax1-deficient male mice. Endocrinology. 2001;142:4486–4495. doi: 10.1210/endo.142.10.8447. doi:10.1210/en.142.10.4486. [DOI] [PubMed] [Google Scholar]
- Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years' experience. J Clin Endocrinol Metab. 2006;91:3048–3054. doi: 10.1210/jc.2006-0603. doi:10.1210/jc.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94:801–808. doi: 10.1210/jc.2008-1648. doi:10.1210/jc.2008-1648. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Ozisik G, Achermann JC, Romoli R, Borretta G, Persani L, Spada A, Jameson JL, Beck-Peccoz P. Hypogonadotropic hypogonadism as a presenting feature of late-onset X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2002;87:44–48. doi: 10.1210/jcem.87.1.8163. doi:10.1210/jc.87.1.44. [DOI] [PubMed] [Google Scholar]
- Mantovani G, De Menis E, Borretta G, Radetti G, Bondioni S, Spada A, Persani L, Beck-Peccoz P. DAX1 and X-linked adrenal hypoplasia congenita: clinical and molecular analysis in five patients. Eur J Endocrinol. 2006;154:685–689. doi: 10.1530/eje.1.02132. doi:10.1530/eje.1.02132. [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Snyder PJ, Bhasin S, Martin K, Weber T, Winters S, Spratt D, Brentzel J, O'Dea L. Stimulation of spermatogenesis with recombinant human follicle-stimulating hormone (follitropin alfa; GONAL-F®): long-term treatment in azoospermic men with hypogonadotropic hypogonadism. Fertil Steril. 2008;92:979–990. doi: 10.1016/j.fertnstert.2008.07.1742. doi:10.1016/j.fertnstert.2008.07.1742. [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Weiss J, Jameson JL. Dax1 is required for testis determination. Nat Genet. 2003;34:32–33. doi: 10.1038/ng1141. doi:10.1038/ng1141. [DOI] [PubMed] [Google Scholar]
- Ozisik G, Mantovani G, Achermann JC, Persani L, Spada A, Weiss J, Beck-Peccoz P, Jameson JL. An alternate translation initiation site circumvents an amino-terminal DAX-1 nonsense mutation leading to a mild form of X-linked adrenal hypoplasia congenital. J Clin Endocrinol Metab. 2003;88:417–423. doi: 10.1210/jc.2002-021034. doi:10.1210/jc.2002-021034. [DOI] [PubMed] [Google Scholar]
- Park SY, Meeks JJ, Raverot G, Pfaff LE, Weiss J, Hammer GD, Jameson JL. Nuclear receptors Sf1 and Dax1 function cooperatively to mediate somatic cell differentiation during testis development. Development. 2005;132:2415–2423. doi: 10.1242/dev.01826. doi:10.1242/dev.01826. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Acherman JC, Genel M, Jameson JL, Crowley WF. X-linked adrenal hypoplasia congenita: a mutation in DAX-1 expands the phenotypic spectrum in males and females. J Clin Endocrinol Metab. 1999;84:4505–4509. doi: 10.1210/jcem.84.12.6172. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Achermann JC, Recan D, Bex V, Bertagna X, Christin-Maitre S, Ito M, Jameson JL, Bouchard P. A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest. 2000;105:321–328. doi: 10.1172/JCI7212. doi:10.1172/JCI7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terriou P, Hans E, Giogetti C, Spach JL, Salzmann J, Urrutia V, Roulier R. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows fertilization, pregnancy and birth after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000;17:194–199. doi: 10.1023/A:1009435732258. doi:10.1023/A:1009435732258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–641. doi: 10.1038/372635a0. doi:10.1038/372635a0. [DOI] [PubMed] [Google Scholar]