Abstract
The administration of exogenous stem cells offers promise to regenerate many damaged organs. However, failures of these cellular therapies could be related to many issues, such as the type of stem cell, the dose of cellular therapeutic, dosing regime, and mode of delivery. The recent ability to directly label stem cells with magnetic resonance (MR) contrast agents provides a simple, straight-forward manner to monitor accurate cell delivery and track stem cells non-invasively in a serial manner. Provided here is an overview of the currently available MR-labeling methods, including direct non-specific labeling with contrast agents, indirect specific labeling with contrast agents, labeling with MRI reporter genes, and fluorine hot spot labeling. Several of these approaches have now been applied successfully in preclinical animal models of cardiovascular disease. Once properly implemented, future clinical trials may benefit greatly from imaging stem cells with MRI.
Keywords: stem cells, magnetic resonance imaging, superparamagnetic iron oxides, myocardial infarction, reporter gene
Introduction
After initial early observations in preclinical studies of the benefits of cellular myoplasty in acute ischemic disease, the first patient studies using bone-marrow-derived stem cells were initiated in 2000 and 2001 [26, 44]. This has now been followed by many clinical cardiac cellular therapy trials [5, 19, 31, 36, 38, 53] culminating with randomized, double-blinded, placebo controlled trials [41]. However, the results to-date have, at best, shown modest benefit to the patient raising questions about the efficacy of cellular therapy and the optimal treatment regime. At the same time, the first clinical studies using cells labeled with superparamagnetic iron oxides for cell tracking by magnetic resonance imaging (MRI) have been recently reported for neurological and oncological patients [17, 54]. The adaptation of these MR labeling techniques to cardiovascular cellular therapy clinical trials could help shed light on the apparent conflicting results from current clinical trials in several ways. In particular, labeled stem cells could provide a means of determining whether the stem cells reach the heart and, if so, how long they remain in the heart. Furthermore, tracking of labeled stem cells with MRI can be used to ensure correct cell delivery and provide insight into the preferred sites of engraftment. From this information, the most favorable dosing and timing of stem cell injections can be determined to optimize the cellular therapy based on the pathology of the individual patient.
In this review, we will describe several methods to label stem cells for MRI with an emphasis on techniques that show promise for rapid translation to the clinical realm. The advantages and disadvantages of these techniques will be discussed. Seminal preclinical studies using labeled stem cells in the heart that have been performed will be described. Finally, the future directions of stem cell labeling that are on the horizon will be discussed.
MR labeling strategies
The three major ways to label cells are: (1) non-specific direct cell labeling, (2) indirect specific (i.e., receptor-mediated) cell labeling; and (3) reporter gene probe labeling. For MRI cell labeling, direct and receptor-based labeling have been most extensively used. In addition, there are several considerations that must be taken into account besides the route of cell labeling when choosing a labeling compound for stem cells. In general, the compound must be biocompatible with minimal toxicity when released by the stem cell. The agent must not interfere with normal regulatory or differentiation pathways of the cells. Moreover, the imaging technique must be sensitive enough to the label such that it can still be detected, at least following initial stem cell division and replication.
Non-specific direct cell labeling
Direct cell labeling is the most straight-forward and simplest route for cell labeling. Because, with MRI, we are imaging the effect of the label on body water rather than the label directly, special consideration of the reduced sensitivity of the label when internalized must be recognized when the direct cell labeling is used. This issue is encountered with paramagnetic metal chelates or “T1 agents”, which usually induce hyperintense contrast. Agents with higher relaxivity (the effectiveness of contrast agent in increasing the relaxation rate per mmole of metal) are more amenable for direct cell labeling. At higher magnetic field strengths, due to the reduced water exchange across the membrane, which leads to less efficient inner sphere dipole–dipole interactions, internalized paramagnetics can often only induce hypointense contrast through outer sphere magnetic susceptibility effects [39]. In addition, the metabolic fate and excretion pathway of paramagnetics have not been investigated, and with dechelated metals, such as gadolinium, being toxic, prompt clinical translation is questionable. In contrast, superparamagnetic iron oxide (SPIO) compounds are widely used within the stem cell labeling arena due to their ability to create large “blooming” hypointensities in images, which becomes more effective upon cellular internalization and particle clustering. Early in the development of SPIOs, it was recognized that these agents were readily taken up by phagocytic cells, such as macrophages. Thus, after intravenous injection of SPIOs, macrophage-rich atherosclerotic plaques become laden with SPIOs resulting in hypointensities on T2* weighted imaging. However, non-phagocytic cells, such as stem cells, must be induced to internalize SPIOs. Furthermore, for cardiac regenerative therapy, stem cells are typically harvested and potentially expanded or enriched prior to administration. Consequently, exogenous labeling of the stem cells can be performed if the technique is fast enough, affording the ability to label cells with a high concentration of the contrast agent.
Two methods have emerged to directly and non-specifically label stem cells with SPIOs. The first method, “magnetofection”, combines the SPIOs with a transfection agent, such as poly-L-lysine or prot-amine sulfate [21]. The SPIO becomes coated with the transfection agent through electrostatic interactions, with the SPIO being anionic and the transfection agent cationic. The SPIO–PLL complex is typically incubated with the cells in normal media for approximately 24 h. Numerous stem and progenitor lines have been effectively labeled using magnetofec-tion. Many studies [7–9, 20, 21] have shown that cell viability and metabolism is unaltered by magneto-fection, with most reporting no inhibiting effect on cell function or differentiation. However, the in vitro differentiation capacity of mesenchymal stem cells along a chondrocytic lineage was reduced after SPIO labeling in a dose-dependent manner, whereas osteogenic and adipogenic differentiation was not [33]. One study suggested that the transfection agent, poly-L-lysine, was responsible for the negative effects on differentiation [4], but another study showed that poly-L-lysine alone enhanced chondrogenic differentiation and increased pellet size [10]. One disadvantage of magnetofection is the relatively long incubation time, which would not be possible in many of the bone marrow mononuclear stem cell trials that have been performed. A second drawback is that transfection agents are not clinically approved for use in cell labeling. However, one of the first cardiovascular applications of magnetofection was to label mesenchymal stem cells for tracking in a swine model of acute myocardial infarction (Fig. 1) [34].
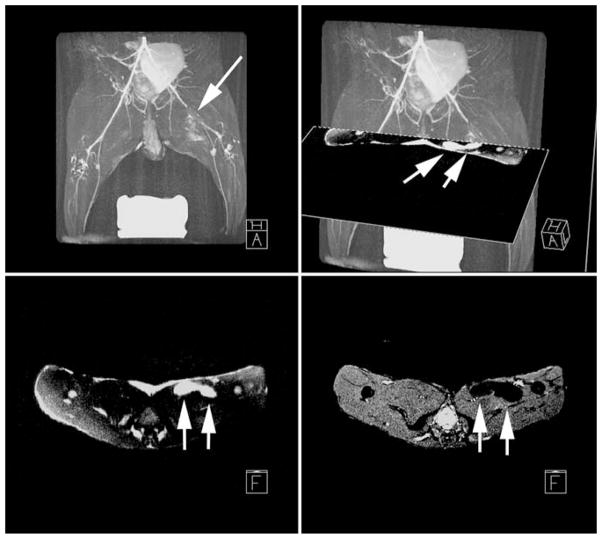
Fig. 1.
In vivo, high resolution, fast gradient echo image [left 6 ms repetition time (TR); 1.6 ms echo (TE); 20° flip angle (FA); 512 × 512 image matrix; 5 mm slice thickness (ST); 32 kHz bandwidth (BW); 28 cm2 field-of-view (FOV); and four number of signal averages (NSA)] acquired on a 1.5 T clinical scanner demonstrate hypointensities (arrows) in the long-axis imaging plane at the location of two injection sites of superparamagnetic iron oxide (SPIO)-labeled mesenchymal stem cells (MSCs). A delayed contrast-enhanced MRI (right 7.8 ms TR; 3.4 ms TE; 25° FA; 256 × 92 image matrix; 5 mm ST; 32 kHz BW; 28 cm2 FOV; two slice averages NSA; and 250 ms inversion delay) acquired in the same MRI examination at 24 h after creation of a reperfused myocardial infarction in a pig in which the myocardial infarction (MI) appears hyperintense, shows that the injections were placed in the infarcted tissue of the left ventricle (LV). RV right ventricle. (Adapted from [34])
A second method, “magnetoelectroporation” (MEP), offers a more rapid method to label stem cells [15, 50]. MEP is based on the technique of electro-poration used to transfect cells with proteins or DNA. With MEP, lower voltage pulses are used to induce the cells to endocytose contrast agents rather than creating channels or pores as typically occurs with higher voltage, higher duration pulses of electroporation. Thus, several million cells can easily be labeled in a matter of minutes. MEP has been used to label a wide variety of stem and progenitor cells without loss of viability or changes to metabolism [47, 50]. After both magnetofection and MEP, the contrast agent is stably maintained in endosomes. Walczak et al. [50] have shown that the amount of uptake of SPIOs using MEP is related to the cell size. For instance, larger mesen-chymal stem cells (MSCs) have more cytoplasm and can accumulate a several-fold higher number of SPIOs as compared to smaller neural stem cells [50], In addition to the ability to rapidly label cells, MEP does not require the use of a transfection agent and, thereby, eliminates another potential regulatory hurdle for clinical acceptance of the labeling technique. For cardiovascular applications, MEP was first used to label bone marrow-derived stem cells in a rabbit model of peripheral arterial disease (Figs. 2) [32]. In this application, the proximity of the SPIO-labeled stem cells could be determined by displaying intrinsically registered MR angiography and high-resolution images of the labeled cell injections (Fig. 2).
Fig. 2.
A maximum intensity projection of a 3D T2-prepared MR angiogram (top left gradient echo imaging of 44 coronal slices with a slice-thickness of 1.5 mm, 14 ms TR, 3.8 ms TE; fractional echo; 20° FA; 270 × 216 mm FOV; 64.7 Hz/pixel bandwidth; T2-prep echo time of 50 ms; and a 800 × 800 image acquisition matrix reconstructed to 1,024 × 1,024) acquired on a 3T MR clinical scanner shows the region of superficial femoral artery occlusion (arrow) at 24 h post-occlusion in a rabbit model of peripheral arterial disease. Mesenchymal stem cells (MSCs) that were labeled using magnetoelectroporation with SPIOs were injected into the medial thigh. Two injection sites imaged immediately after injection are shown with positive contrast imaging (arrows, bottom left) and conventional gradient echo imaging (arrows, bottom right). Since MRI is a tomographic technique, fusion of the positive contrast images and MRA can be used to demonstrate the location of the stem cells (arrows) relative to collateral vessel formation (top right)
A primary disadvantage of the direct labeling techniques is that the label may still remain even after the cell has died. Fortunately, SPIOs are seldom taken up by non-phagocytic cells. Thus, the concern that the MRI signal represents cells other than the exogenously labeled stem cells is largely limited to extracellular SPIO complexes or SPIOs engulfed by macrophages. In practice, it appears that the myocardium is extremely efficient at removing at SPIOs such that the hypointense MRI signal appears to be largely related to the originally labeled stem cells [46]. Another issue with direct stem cell labeling is the dilution of the label as the cell replicates. Therefore, if cardiac cellular therapy was successful at regenerating myocytes, then the labeled cells may no longer be detectable after several initial cell divisions.
A final concern that is intrinsic to any labeling method is the ability to distinguish hypointensities from SPIOs from other causes of negative image contrast artifacts, e.g., hemorrhage, air, metallic devices, etc. Van den Bos et al. [49] demonstrated that SPIO-labeled human umbilical vein endothelial cells that were injected post-mortem could not be distinguished from areas of hemorrhage/microvascular obstruction in the infarcted myocardium of swine. Using an imaging scheme comparing T2*-weighted and proton density-weighted imaging, Kustermann et al. [35] were able to differentiate ultrasmall SPIO-labeled embryonic ventricular cardiomyocytes from the infarcted myocardium (Fig. 4). An alternative approach has been to develop methods to obtain positive contrast from SPIO labeled cells [14, 37, 45] rather than negative contrast (Fig. 2).
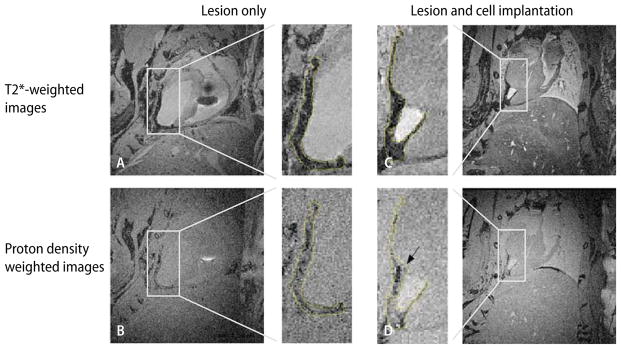
Fig. 4.
In-situ MRIs acquired on a 7T horizontal bore animal scanner of mouse hearts with transmural cryo-lesion. Left column (A, B) lesion only. Corresponding slices of a heart 2 weeks after cryo-lesion without implantation of cardiomyocytes. Right column (C, D) corresponding slices of a cryoinjured heart 2 weeks after implantation of embryonic cardiomyocytes prelabeled with USPIOs. Top row (A, C) slices of a three-dimensional data set acquired with a T2*-weighted gradient echo sequence (60 ms TR and 22 ms TE). Bottom row (B, D) slices of a three-dimensional data set acquired with a proton density-weighted gradient echo sequence (200 ms TR and 3.2 ms TE). The subpanels, inserted in the center of the figure, depict the regions of interest, selectively zoomed from the individual four main panels to better demonstrate the affected region-of-interest. The hypointense areas on the T2*-weighted images (subpanels of A and C) are encircled and the shapes are projected onto the proton density-weighted images (subpanels of C and D) to demonstrate the change in hypointense areas. (From [35] with permission)
Indirect specific (receptor-mediated) cell labeling
Receptor-mediated cell labeling is accomplished by complexing an MR-visible contrast agent with a ligand that will be bound by a stem cell-specific receptor. One example is the use of biotin–avidin interactions to link a monoclonal antibody to a contrast agent, typically for imaging specific vascular targets [12]. There can be several advantages to this technique. If the receptor-contrast agent complex remains on the cell surface, one can reduce the sensitivity problem that is created by internalization of the contrast agent as is seen with direct labeling, in particular when using paramagnetic chelates. In addition, the safety profile of the contrast agent may be enhanced if it is not internalized. If a receptor unique to the stem cell can be found, then potentially the stem cell can be detected by injection of the contrast agent alone. Frequently with receptor-mediated cell labeling, the receptor may be species-specific. Thus, the cell-labeling compound may need to be modified between preclinical and clinical testing. Moreover, the labeling method is more likely to be cell-specific. As a result, a receptor-based cell labeling is more complicated to manufacture and test. At present, no receptor-based cell labeling has been developed for cardiovascular stem cell applications. While receptor-mediated cell targeting has been performed using gadolinium-based agents to enhance the detection of various components of the atherosclerotic plaque [52], the development of such unique, specific targets on the stem cell that are readily accessible through vascular delivery is unlikely.
Reporter gene cell labeling
The third method of cellular labeling is transfection with a reporter gene that expresses either an enzyme/ protein product or receptor that can be detected using MRI. While transfection of a cell with long-term expression of the gene production is the most complex method for cell labeling, the advantages are that (1) the reporter gene does not dilute out with cell division, in principle allowing indefinite cell tracking regarding of the number of cell divisions; (2) the reporter gene is only expressed by viable cells; and (3) the reporter gene can be inserted under a specific promotor that is only switched on if the stem cell differentiates into a particular phenotype (e.g., MSC differentiation into smooth muscle cells). Reporter gene methods have been most extensively developed for radionuclide applications with recent extension to cardiovascular applications (Fig. 3) [11]. This particular example using murine embryonic stem cells transfected with a triple fusion reporter gene demonstrates the fact that cell division results in increasing signal rather than the signal decrease, which would occur with most direct labeling techniques. Potentially, one can tailor the reporter gene to signal an event such as stem cell differentiation down a cardiac or hematopoietic lineage by the choice of a specific promoter such as myosin heavy chain or Tie-2.
Fig. 3.
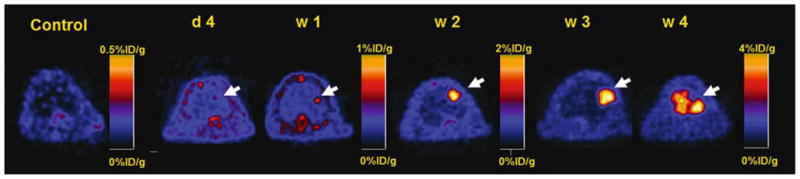
Positron emission tomographic (PET) images acquired at day 4 and 1, 2, and 3 weeks after transplantation of murine embryonic stem cells transfected with a triple fusion reporter gene. There is a substantial increase in activity from 2 to 4 weeks that corresponds to ES cell division and replication. (Adapted from [11])
Several novel reporter genes have been developed for MRI, although none as yet for cardiovascular specific applications [13, 23, 25, 48, 51]. One approach is to engineer cells with overexpression of the ferritin receptor. These cells will then sequester endogenous iron to enable visualization using MRI. While an attractive approach, since it removes the need to administer exogenous contrast agents, the effect on the cells of increase iron internalization is unclear. Another approach is to create a reporter gene that produces an artificial protein [25] or enzyme [51] that will then produce a unique signature that can be detected with chemical exchange saturation transfer or MR spectroscopy. One inherent problem with imaging reporter gene expression is whether individual cells can produce enough receptors, proteins, enzymes, etc. to enable detection by MRI or other non-invasive imaging techniques. This is in contrast with iron oxide labeling, where the sensitivity of detection is at the single cell level [27, 42].
Current status of MR-labeled stem cells in cardiovascular applications
The first studies using MR-labeled stem cells in cardiovascular applications employed direct labeling of the stem cells with iron oxide compounds. Delivery of the progenitor or stem cells was primarily via direct intramyocardial injection either under direct visualization [35, 46, 49], using X-ray fluoroscopic guidance [2, 28, 34], or electromechanical mapping [22]. Serial MR imaging allowed tracking of the stem cell injections to determine stem cell engraftment and persistence [2, 28, 34, 46]. Subsequently, several groups [18, 43] realized two potential benefits of MR fluoroscopic delivery of MR-labeled stem cells: (1) the success of the injections could be immediately recognized; and (2) the targeting of the stem cell injections to the peri-infarcted tissue could be guided by delayed contrast-enhanced MRI viability maps. In most studies, in order to be able to visualize the injection catheter with a high level of confidence, active MR-compatible devices were designed that yield an extremely high signal intensity that when combined with the soft tissue detail of MRI can be used to guide injections (Fig. 5). While presenting a promising platform for optimizing stem cell therapeutic protocols [18, 43], there remain many hurdles to overcome for clinical adoption. Concerns remain about the ability to monitor acute cardiac patients in the high field environment during a cardiac intervention. In addition, user-friendly, graphical interfaces with real-time image acquisition are less developed than the X-ray fluoroscopic environment that results in some resistance to adoption by the interventional cardiologist. While SPIO-labeled cells have been shown to be safe in patients, regulatory hurdles still remain for approval of SPIO-labeled stem cells and MR-compatible injection devices.
Fig. 5.
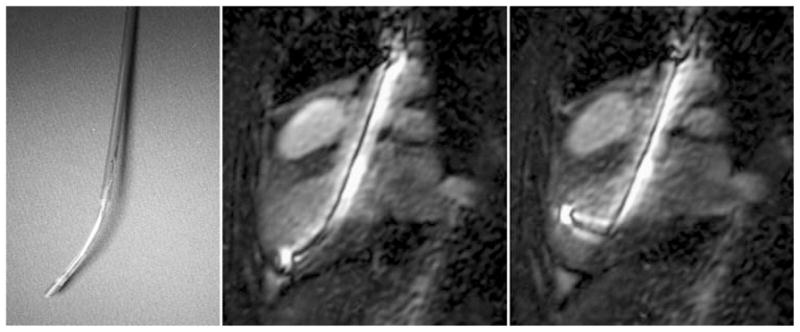
A digital image of the tip of a custom MR-compatible injection catheter for transmyocardial stem cell delivery is flexible and can be guided to all regions of the endocardial surface (left). This active MR delivery device produces a high signal intensity for easy visualization during real-time MR imaging using a steady-state free precession imaging technique (middle, right). The device is steerable and shown in two different degrees of flexion for transmyocardial injections in the anteroseptal myocardium
Future directions
Several new agents have been developed recently that may represent the next generation of candidates for direct labeling of stem cells to succeed SPIO labeling methods. Gadolinium-based agents that have higher relaxivities even when internalized have been used to label stem cells [3, 24]. Intrinsically, gadolinium-based labeled stem cells will appear hyperintense on T1-weighted images, and thereby circumvent some of the concerns about detecting hypointensities from SPIO-labeled stem cells.
Another candidate, perfluorocarbons, have recently been used to label stem cells [40]. Because there is no native fluorine signal in the body, MRI of perfluoro-carbon-labeled stem and progenitor cells allow “hot spot” interpretation [6] analogous to radionuclide imaging where the signal is solely from the administered radioisotopes, although the sensitivity of detection is several orders of magnitude lower for 19F imaging. However, using conventional proton MRI, exquisite anatomical localization can be obtained without exposure of the patient to ionizing radiation, and when the 19F and 1H images are superimposed, the cellular hot spots can be interpreted within their anatomical context.
Akin to combining radionuclide imaging with computed tomography, many institutions are installing combined X-ray angiographic and MR imaging suites (Fig. 6). The advantages of each imaging technology for imaging the heart—the high temporal resolution of X-ray fluoroscopy and the high anatomical and functional detail of MRI—can be exploited [1, 29, 30]. Since the images from both modalities are intrinsically registered, the fusion of the two imaging modalities is simplified and can be more rapidly updated. X-ray fusion with MRI in such a suite has recently been used to use X-ray fluoros-copy to target transmyocardial iron-oxide labeled mesenchymal stem cells in infarcted swine to avoid thinned or non-viable myocardium [16]. Thus, the risk of perforation during these procedures can also be reduced. Moreover these suites can be used to minimize the radiation exposure to the patient and operators.
Fig. 6.
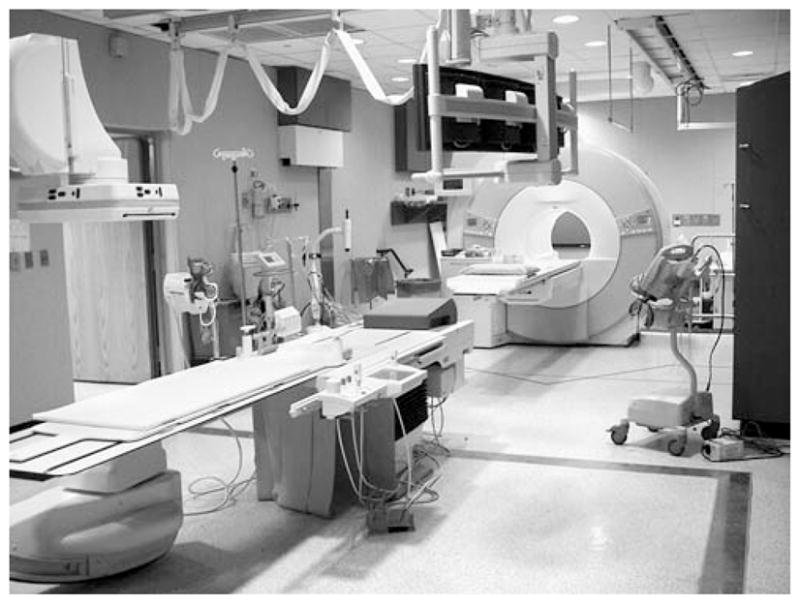
Digital image of a combined X-ray angiographic and MR imaging suite with a flat-panel angiographic display and 1.5T clinical MRI scanner (Siemens Medical Systems). The table top can be transferred between MRI and X-ray to allow registration of the two imaging modalities. Such suites offer the advantages of high temporal resolution from X-ray fluoroscopy and high anatomical detail from MRI for cardiac interventions. Similarly configured suites are available from all major MRI vendors
Conclusions
Numerous studies using MR-labeled stem cells in cardiovascular preclinical applications have now been performed and demonstrate the feasibility and safety of these techniques. Because many of these studies have been performed on clinical MRI scanners using clinically-approved labeling agents, the likelihood of translation of these techniques to ongoing clinical cardiovascular stem cell trials is high.
Footnotes
Conflict of Interest The authors had a grant support from Siemens AG. They did receive research material by Bayer Schering Pharma AG and by Boston Scientific Corporation.
Contributor Information
Dara L. Kraitchman, Email: dara@mri.jhu.edu, Russell H. Morgan Dept. of Radiology and Radiological Science, Johns Hopkins University, School of Medicine, Baltimore (MD), USA. Johns Hopkins University, 601 N. Caroline St., JHOC 4231, Baltimore (MD) 21287-0845, USA. Institute of NanoBiotechnology, Institute for Computational Medicine, Johns Hopkins University, School of Medicine, Baltimore (MD) USA.
Jeff W. M. Bulte, Russell H. Morgan Dept. of Radiology and Radiological Science, Johns Hopkins University, School of Medicine, Baltimore (MD), USA. Dept. of Chemical and Biomolecular, Engineering, Johns Hopkins University, School of Medicine, Baltimore (MD), USA. Institute for Cell Engineering, Cellular Imaging Section and Vascular, Biology Program, Johns Hopkins University, School of Medicine, Baltimore (MD), USA.
References
- 1.Albert TS, Kim RJ, Judd RM. Assessment of no-reflow regions using cardiac MRI. Basic Res Cardiol. 2006;101:383–390. doi: 10.1007/s00395-006-0617-0. [DOI] [PubMed] [Google Scholar]
- 2.Amado LC, Salrais AP, Schuleri KH, St John ME, Xie J, Cattaneo SM, Durand DJ, Fitton T, Kuang JQ, Stewart GC, Lehrke S, Baumgartner WA, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson SA, Lee KK, Frank JA. Gadolinium-fullerenol as a paramagnetic contrast agent for cellular imaging. Invest Radiol. 2006;41:332–338. doi: 10.1097/01.rli.0000192420.94038.9e. [DOI] [PubMed] [Google Scholar]
- 4.Arbab AS, Yocum GT, Rad AM, Khakoo AY, Fellowes V, Read EJ, Frank JA. Labeling of cells with ferumoxides–protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 5.Bartunek J, Vanderheyden M, Van-dekerckhove B, Mansour S, De Bruyne B, De Bondt P, Van Haute I, Lootens N, Heyndrickx G, Wijns W. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112:I178–I183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 6.Bulte JW. Hot spot MRI emerges from the background. Nat Biotechnol. 2005;23:945–946. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- 7.Bulte JW, Arbab AS, Douglas T, Frank JA. Preparation of magnetically labeled cells for cell tracking by magnetic resonance imaging. Methods Enzymol. 2004;386:275–299. doi: 10.1016/S0076-6879(04)86013-0. [DOI] [PubMed] [Google Scholar]
- 8.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 9.Bulte JW, Kraitchman DL. Monitoring cell therapy using iron oxide MR contrast agents. Curr Pharm Biotechnol. 2004;5:567–584. doi: 10.2174/1389201043376526. [DOI] [PubMed] [Google Scholar]
- 10.Bulte JW, Kraitchman DL, Mackay AM, Pittenger MF, Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, Read EJ, Frank JA. Chondrogenic differentiation of mesenchymal stem cells is inhibited after magnetic labeling with ferumoxides. Blood. 2004;104:3410–3413. doi: 10.1182/blood-2004-06-2117. [DOI] [PubMed] [Google Scholar]
- 11.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Con-nolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caruthers SD, Neubauer AM, Hockett FD, Lamerichs R, Winter PM, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. In vitro demonstration using 19F magnetic resonance to augment molecular imaging with paramagnetic perfluorocarbon nanoparticles at 1.5 Tesla. Invest Radiol. 2006;41:305–312. doi: 10.1097/01.rli.0000199281.60135.6a. [DOI] [PubMed] [Google Scholar]
- 13.Cohen B, Ziv K, Plaks V, Israely T, Kalchenko V, Harmelin A, Benjamin LE, Neeman M. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 2007;13:498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham CH, Arai T, Yang PC, McConnell MV, Pauly JM, Conolly SM. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med. 2005;53:999–1005. doi: 10.1002/mrm.20477. [DOI] [PubMed] [Google Scholar]
- 15.Daldrup-Link HE, Meier R, Rudelius M, Piontek G, Piert M, Metz S, Settles M, Uherek C, Wels W, Schlegel J, Rummeny EJ. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol. 2005;15:4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- 16.de Silva R, Gutierrez LF, Raval AN, McVeigh ER, Ozturk C, Lederman RJ. X-ray fused with magnetic resonance imaging (XFM) to target endomyocardial injections: validation in a swine model of myocardial infarction. Circulation. 2006;114:2342–2350. doi: 10.1161/CIRCULATIONAHA.105.598524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boez-eman JB, Adema GJ, Bulte JW, Schee-nen TW, Punt CJ, Heerschap A, Figdor CG. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 18.Dick AJ, Guttman MA, Raman VK, Peters DC, Pessanha BS, Hill JM, Smith S, Scott G, McVeigh ER, Lederman RJ. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in swine. Circulation. 2003;108:2899–2904. doi: 10.1161/01.CIR.0000095790.28368.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Pen-arrubia MJ, de la Fuente L, Gomez-Bueno M, Cantalapiedra A, Fernandez J, Gutierrez O, Sanchez PL, Hernandez C, Sanz R, Garcia-Sancho J, Sanchez A. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 20.Frank JA, Miller BR, Arbab AS, Zy-wicke HA, Jordan EK, Lewis BK, Bryant LH, Jr, Bulte JWM. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 21.Frank JA, Zywicke H, Jordan EK, Mitchell J, Lewis BK, Bryant LH, Jr, Bulte JWM. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad Radiol. 2002;9:S484–S487. doi: 10.1016/s1076-6332(03)80271-4. [DOI] [PubMed] [Google Scholar]
- 22.Garot J, Unterseeh T, Teiger E, Champagne S, Chazaud B, Gherardi R, Hitt-inger L, Gueret P, Rahmouni A, Sonnet C, Le Corvoisier P, Benhaiem-Sigaux N, Su J, Merlet P. Magnetic resonance imaging of targeted catheter-based implantation of myogenic precursor cells into infarcted left ventricular myocardium. J Am Coll Cardiol. 2003;41:1841–1846. doi: 10.1016/s0735-1097(03)00414-5. [DOI] [PubMed] [Google Scholar]
- 23.Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 24.Giesel FL, Stroick M, Griebe M, Troster H, von der Lieth CW, Requardt M, Rius M, Essig M, Kauczor HU, Hennerici MG, Fatar M. Gadofluorine m uptake in stem cells as a new magnetic resonance imaging tracking method: an in vitro and in vivo study. Invest Radiol. 2006;41:868–873. doi: 10.1097/01.rli.0000246147.44835.4c. [DOI] [PubMed] [Google Scholar]
- 25.Gilad AA, McMahon MT, Walczak P, Winnard PT, Jr, Raman V, van Laarhoven HW, Skoglund CM, Bulte JW, van Zijl PC. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol. 2007;25:217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 26.Hamano K, Nishida M, Hirata K, Mikamo A, Li TS, Harada M, Miura T, Matsuzaki M, Esato K. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: clinical trial and preliminary results. Jpn Circ J. 2001;65:845–847. doi: 10.1253/jcj.65.845. [DOI] [PubMed] [Google Scholar]
- 27.Heyn C, Bowen CV, Rutt BK, Foster PJ. Detection threshold of single SPIO-labeled cells with FIESTA. Magn Reson Med. 2005;53:312–320. doi: 10.1002/mrm.20356. [DOI] [PubMed] [Google Scholar]
- 28.Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, Pessanha BS, Guttman MA, Varney TR, Martin BJ, Dunbar CE, McVeigh ER, Lederman RJ. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito H, Komura N, Iwakura K, Kawano S, Okamura A, Fujii K. Combination study of myocardial perfusion and left ventricular filling provides an excellent prediction of clinical outcomes in patients with reperfused myocardial infarction. Basic Res Cardiol. 2006;101:400–407. doi: 10.1007/s00395-006-0619-y. [DOI] [PubMed] [Google Scholar]
- 30.Jacoby C, Molojavyi A, Flogel U, Merx MW, Ding Z, Schrader J. Direct comparison of magnetic resonance imaging and conductance microcatheter in the evaluation of left ventricular function in mice. Basic Res Cardiol. 2006;101:87–95. doi: 10.1007/s00395-005-0542-7. [DOI] [PubMed] [Google Scholar]
- 31.Janssens S, Dubois C, Bogaert J, The-unissen K, Deroose C, Desmet W, Kal-antzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymar-kowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 32.Kedziorek DA, Gilson WD, Stuber M, Huang G, Blush E, Cosby K, Korosoglou G, Soto AV, Bulte JWM, Hofmann LV, Kraitchman DL. Mesenchymal stem cell therapy in a rabbit hindlimb ischemia model. J Am Coll Cardiol. 2007;49:362A. [Google Scholar]
- 33.Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17:513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 34.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 35.Kustermann E, Roell W, Breitbach M, Wecker S, Wiedermann D, Buehrle C, Welz A, Hescheler J, Fleischmann BK, Hoehn M. Stem cell implantation in ischemic mouse heart: a high-resolution magnetic resonance imaging investigation. NMR Biomed. 2005;18:362–370. doi: 10.1002/nbm.967. [DOI] [PubMed] [Google Scholar]
- 36.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 37.Mani V, Saebo KC, Itskovich V, Samber DD, Fayad ZA. GRadient echo Acquisition for Superparamagnetic particles with Positive contrast (GRASP): sequence characterization in membrane and glass superparamagnetic iron oxide phantoms at 1.5T and 3T. Magn Reson Med. 2006;55:126–135. doi: 10.1002/mrm.20739. [DOI] [PubMed] [Google Scholar]
- 38.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 39.Modo M, Cash D, Mellodew K, Williams SC, Fraser SE, Meade TJ, Price J, Hodges H. Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. Neuroimage. 2002;17:803–811. [PubMed] [Google Scholar]
- 40.Partlow KC, Chen J, Brant JA, Neubauer AM, Meyerrose TE, Creer MH, Nolta JA, Caruthers SD, Lanza GM, Wickline SA. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. Faseb J. 2007;21(8):1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 41.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Hol-schermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soto AV, Gilson WD, Kedziorek D, Fritzges D, Izbudak I, Young RG, Pittenger MF, Bulte JW, Kraitchman DL. MRI tracking of regional persistence of feridex-labeled mesenchymal stem cells in a canine myocardial infarction model. J Cardiovasc Magn Reson. 2006;8:89–90. [Google Scholar]
- 44.Strauer BE, Brehm M, Zeus T, Koster-ing M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 45.Stuber M, Gilson WD, Kedziorek D, Bulte JW, Kraitchman DL. Positive contrast visualization of iron oxide-labeled stem cells using inversion recovery with ON-resonant water suppression (IRON) Magn Reson Med. 2007;58:1072–1077. doi: 10.1002/mrm.21399. [DOI] [PubMed] [Google Scholar]
- 46.Stuckey DJ, Carr CA, Martin-Rendon E, Tyler DJ, Willmott C, Cassidy PJ, Hale SJ, Schneider JE, Tatton L, Harding SE, Radda GK, Watt S, Clarke K. Iron particles for noninvasive monitoring of bone marrow stromal cell engraftment into, and isolation of viable engrafted donor cells from, the heart. Stem Cells. 2006;24:1968–1975. doi: 10.1634/stemcells.2006-0074. [DOI] [PubMed] [Google Scholar]
- 47.Tai JH, Foster P, Rosales A, Feng B, Hasilo C, Martinez V, Ramadan S, Snir J, Melling CW, Dhanvantari S, Rutt B, White DJ. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes. 2006;55:2931–2938. doi: 10.2337/db06-0393. [DOI] [PubMed] [Google Scholar]
- 48.Tannous BA, Grimm J, Perry KF, Chen JW, Weissleder R, Breakefield XO. Metabolic biotinylation of cell surface receptors for in vivo imaging. Nat Methods. 2006;3:391–396. doi: 10.1038/nmeth875. [DOI] [PubMed] [Google Scholar]
- 49.van den Bos EJ, Baks T, Moelker AD, Kerver W, van Geuns RJ, van der Giessen WJ, Duncker DJ, Wielopolski PA. Magnetic resonance imaging of haemorrhage within reperfused myocardial infarcts: possible interference with iron oxide-labelled cell tracking? Eur Heart J. 2006;27:1620–1626. doi: 10.1093/eurheartj/ehl059. [DOI] [PubMed] [Google Scholar]
- 50.Walczak P, Kedziorek D, Gilad AA, Lin S, Bulte JW. Instant MR labeling of stem cells using magnetoelectroporation. Magn Reson Med. 2005;54(4):769–774. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- 51.Walter G, Barton ER, Sweeney HL. Noninvasive measurement of gene expression in skeletal muscle. Proc Natl Acad Sci USA. 2000;97:5151–5155. doi: 10.1073/pnas.97.10.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 53.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 54.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]