Abstract
Objective
The aim is to evaluate the effectiveness of a manualized 12-week supportive–expressive group therapy program among primary breast cancer patients treated in community settings, to determine whether highly distressed patients were most likely to benefit and whether therapist’s training or experience was related to outcome.
Method
Three hundred and fifty-three women within one year of diagnosis with primary breast cancer were randomly assigned to receive supportive–expressive group therapy or to an education control condition. Participants were recruited from two academic centers and nine oncology practices, which were members of NCI’s Community Clinical Oncology Program (CCOP) and were followed over 2 years.
Results
A 2 × 2 × 19 analysis of variance was conducted with main effects of treatment condition, cohort, and baseline distress and their interactions. There was no main effect for treatment condition after removing one subject with an extreme score. Highly distressed women did not derive a greater benefit from treatment. Therapist training and psychotherapy experience were not associated with a treatment effect.
Conclusions
This study provides no evidence of reduction in distress as the result of a brief supportive–expressive intervention for women with primary breast cancer. Future studies might productively focus on women with higher initial levels of distress.
Keywords: support groups, primary breast cancer, breast cancer groups, group therapy, psychosocial interventions, cancer support groups, treatment outcome, therapist effectiveness
Introduction
Breast cancer patients often experience significant psychological distress after diagnosis and during initial treatment. Several recent studies of women newly diagnosed with early stage breast cancer have found high levels of psychiatric morbidity, particularly anxiety and affective disorders [1,2]. Although psychological distress among cancer patients often abates with time [1,3–5], as many as 22–43% have been shown to meet criteria for a psychiatric disorder six months later [1,6]. There is evidence that primary breast cancer patients continue to be vulnerable to psychological distress for many years [7].
There has been considerable research on group therapy for cancer patients over the last 20 years, and in recent years a number of studies have examined the benefits of group interventions for early stage breast cancer [8–22]. The interventions in these investigations have ranged from unstructured [11–14,17,20,21,23] to structured approaches [8–10,12,13,16,18,19], with results showing benefits for both structured and unstructured groups. By pitting a structured psychoeducation group against an unstructured peer support group, the psychoeducation group was found to be more effective [12,13]. However, it has been suggested and demonstrated that the quality of the facilitation of the unstructured groups may be a critical factor influencing outcome [14,24,25].
In this study, we were interested in demonstrating the benefits of a brief supportive–expressive group therapy as well as whether this intervention could be transferred to community oncology settings. To our knowledge, there are few group therapy studies for cancer survivors that have included multiple sites [16,26,27], and fewer still that considered the impact of site [16] or group composition [14,16].
The aim of the present study was to test the supportive–expressive group therapy model in community oncology practices utilizing personnel already working with these populations. To conduct this study of group therapy for primary breast cancer patients, we developed a brief version of the supportive–expressive model [28]. This intervention, which encourages building social support, emotional expression and the examination of existential concerns, has been shown to reduce mood disturbance and trauma symptoms and improve coping in metastatic breast cancer patients, [29,30] but, except for a feasibility study conducted in preparation for this randomized trial [20,21], it has not been tested in women with primary breast cancer. Indeed, some might question whether primary breast cancer patients would benefit from an intervention that emphasizes examining existential concerns as opposed to an emphasis on putting the cancer behind them. Our first aim was to test the efficacy of this intervention for reducing mood disturbance for women who received the intervention compared with a control group. While the prognosis for primary breast cancer patients is significantly better than it is for metastatic breast cancer patients, a diagnosis of primary breast cancer nevertheless activates existential concerns about isolation, death, one’s identity, and life’s meaning and examining these concerns in a supportive environment may be beneficial.
A second aim was to examine who was most likely to benefit from the intervention. To our knowledge there has been limited research addressing this question. In Goodwin’s recent study of supportive–expressive group therapy for metastatic patients, she and her colleagues found that group therapy benefited women who were more distressed at baseline but that it did not benefit women who were less distressed at that time [31]. Another study examined the benefit of receiving a supportive intervention in women undergoing radiotherapy and also found that women who were initially highly distressed experienced the greatest benefit from the program [32]. A third study sought to identify the type of cancer patient most likely to benefit from a psychosocial intervention and found that older patients were less likely to receive immediate benefit from a psychosocial intervention, although they derived comparable benefit over time [33]. For the present study, we hypothesized that women who were highly distressed would receive the greatest benefit from a brief supportive–expressive group therapy intervention.
Finally, we trained and utilized a range of professionals (nurses, social workers and psychologists) to conduct the intervention [34]. Although some research indicates that therapist degree, level of training and years of experience have no impact on effect sizes in psychotherapy outcome studies [35–38], others have found these variables to be positively associated with treatment efficacy [39–41]. One study of particular relevance to this report found that groups led by psychologists had the largest effect size in a brief cognitive-existential intervention study with early stage breast cancer patients [42]. In our current study, our aim was to test whether therapists’ education and experience were related to treatment efficacy.
We hypothesized that patients randomly assigned to the group therapy condition would show a greater reduction in mood disturbance over time compared with those randomized to the control condition. We also hypothesized that women who were highly distressed at baseline would show the greatest benefit from participating in a support group, and that therapists with more training and experience would be most effective in reducing distress.
Methods
Sample
Participants were women who met the following inclusion criteria: (1) diagnosis of primary, biopsy-proven breast cancer, stages I through IIIA; (2) diagnosis occurred no more than 12 months prior to recruitment; (3) completion of initial surgical treatment; and (4) no detectable disease present. Exclusion criteria included: (1) evidence of metastases beyond adjacent lymph nodes, including chest wall involvement, bone or viscera; (2) recurrence of the cancer prior to randomization; (3) diagnosis of other cancers (except for basal cell or squamous cell carcinoma of the skin or in situ cervical cancer) within the past 10 years; (4) any other major medical problems likely to limit life expectancy to less than 10 years; (5) a history of major psychiatric illness for which the patient was hospitalized or medicated, except for a diagnosis of depression or anxiety treated for a period of less than one year; and (6) attendance at a cancer support group for more than two months.
Participants were recruited at nine Community Clinical Oncology Program practice groups in the community and two academic sites, Stanford University and the University of Rochester. The Community Clinical Oncology Program is a nationwide research consortium funded by the Division of Cancer Control of the National Cancer Institute and comprises geographically diverse medical oncology groups in private practice across the United States. Informed consent was received from all participants and the study protocol was reviewed and approved by each institution’s institutional review board.
Study design
Recruitment
The study was designed so that each site would recruit patients in two waves. However, three sites completed only the first wave. The main reasons for refusing to participate in this study were that potential subjects were not interested, the distance to travel to the support group was too great, or they were already in a support group. Of those women who were invited to participate in this trial, the acceptance rate was approximately 25% at rural sites and 35–45% at urban sites.
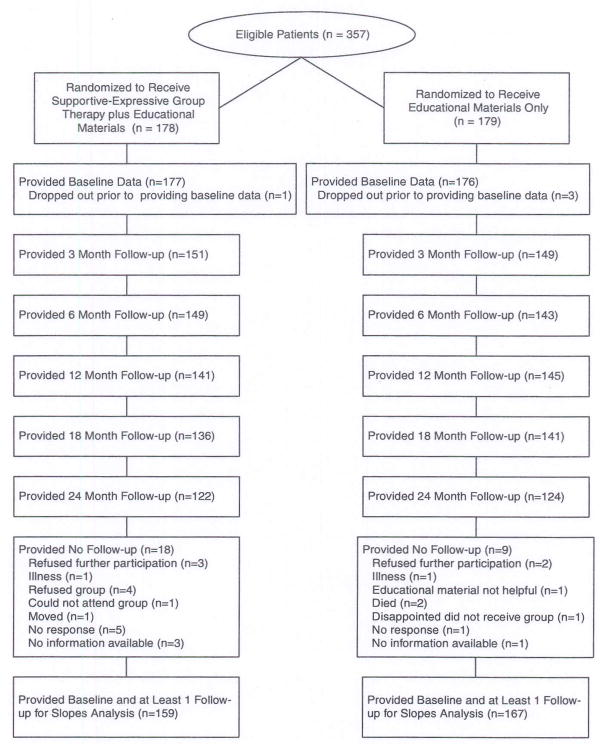
Three hundred and fifty-three participants were recruited into this study with a mean time of 8.7 months (±4.1) since diagnosis. Recruitment began in February 1994 and ended in May 1996. Follow-up assessments began in May 1994 and the final assessments were completed in June 1998. Among the women who were randomized to the support group condition (n = 177), a mean of 9.1 months (±4.1) elapsed between being diagnosed with cancer and entering a support group. See Figure 1 for a breakdown of the flow of participants through the study.
Figure 1.
Flow diagram of participants involvement in the multicenter randomized trial for primary breast cancer patients.
Randomization
For each wave, women were randomly assigned to the support group treatment or to an education control condition. Each site recruited potential participants and confirmed their eligibility with Stanford. Eligible participants completed the baseline assessment and when all baseline assessments for a particular site were completed, participants were randomized.
Random assignment followed a method combining elements of biased coin randomization with adaptive randomization [43]. The probability of randomization at each entry point was adjusted by taking sample sizes of the two groups at each site and crucial clinical characteristics into account in order to exert a constant pressure both to balance the size of the two groups and to decrease the possibility of bias on crucial entry variables prognostic of psychosocial and medical outcome. These stratification variables were: (1) type of surgical procedure: breast conservation vs modified radical mastectomy; (2) axillary node involvement: 0 vs 1–3 vs 4 or more positive; (3) age: < 50 vs 50 or older; and (4) estrogen receptor status: positive vs negative.
Education materials
Educational materials were mailed to all participants in both conditions and consisted of a brief videotape on breast self-examination as well as pamphlets published by the American Cancer Society. These pamphlets covered such topics as facts on cancer, breast self-exams, cooking, helping children understand, sexuality, radiation, chemotherapy and breast changes. None of these pamphlets discussed the importance of emotional expression or social support or provided advice on coping.
Intervention
The treatment group received supportive–expressive group therapy [30,44,45] as described in the treatment manual [28]. This is an unstructured intervention designed to build new bonds of social support, encourage expression of emotion, enhance communication with physicians and nurses, enhance symptom control, and deal directly with existential concerns such as fears of dying and death, changes in self and body image, making meaning out of the illness, feelings of isolation and reordering life priorities. Therapists are instructed to listen for the presence of these themes and to encourage their discussion. Special attention is paid to the expression of emotion with the therapists using emotion as a marker for what may be important to focus on in that session. Therapists are also instructed to listen for the absence of emotion as an indicator of the avoidance of important topics. Groups met weekly for 12 weeks. Each meeting lasted 90 min and was composed of up to 10 members and two co-therapists. On average, women attended 8 out of 12 sessions.
Group therapists and training
There were two therapists at each of the participating sites. Therapists included 10 nurses, 11 social workers and 3 psychologists. Six therapist pairs led both waves of groups at their site and seven therapist pairs ran one group only. Training consisted of a 2-day training workshop, studying the treatment manual and facilitating a pilot group. The pilot groups were intended to give the therapists an opportunity to practice implementing the model and to receive feedback. Proficiency in implementing the pilot groups was not formally assessed. Ten of the 24 study therapists did not conduct a pilot group or attend the training workshop. However, only two groups were led by therapists with no pilot group experience and one of those was a Stanford group in which the therapists received additional training. Therapists were given four tests throughout the course of the training program. This involved watching videotaped segments of actual groups and then writing the intervention they would make if they were the therapist facilitating the group situation depicted on the videotape. The results of these training tests and outcome of the pilot groups are reported elsewhere [34]. All therapists received supervision of the groups they conducted for this randomized trial. This involved receiving written feedback on two videotaped sessions and direct observation of another group session by an expert in the intervention.
Measures
Outcome measures
Participants completed a battery of questionnaires at six different time points: Baseline (before randomization), 3 (immediately post-intervention), 6, 12, 18 and 24 months. Questionnaires were completed via mail.
Primary outcome measure
The primary outcome measure for this trial was the Profile of Mood States Questionnaire (POMS) [46], which is a 65-item, adjective-rating scale designed to measure mood states. It has excellent psychometric properties, is sensitive to change and is frequently used in psychosocial studies of cancer [47]. Participants were asked to indicate how they felt during the past week by rating each word (e.g. ‘tense,’ ‘angry’ and ‘sad’) on a 5-point Likert-type scale ranging from ‘not at all’ to ‘extremely.’ It yields a score for total mood disturbance (TMD). TMD, based on the total score of the POMS, was the main outcome. The POMS was chosen as the primary outcome measure because it is widely used and because it was the main outcome measure in our previous research on supportive–expressive group therapy with metastatic breast cancer patients [29,30].
Secondary outcome measures
These included The Hospital Anxiety and Depression Scale (HADS) [48], Mini-Mental Adjustment to Cancer Scale (MAC) [49], Courtauld Emotional Control Scale [50], Impact of Event Scale [51], Stanford Self-Efficacy Scale for Serious Illness [52], CARES Medical Interaction Subscale [53], Family Relations Index [54,55], Sleep Measure adapted from the Stanford Sleep Questionnaire and Assessment of Wakefulness [56], Pain Measure [57] and Yale Social Support Index [58,59].
Statistical methods
Power analysis
The test of effectiveness of the treatment was based on a t-test with N = 24 degrees of freedom (main effect of treatment from ANOVA). For the POMS TMD score, from earlier studies, the within group standard deviation is approximately 8, and a clinically significant change on the POMS is taken as 5 units. Thus, the standardized mean difference is 5/8 = 0.625. In the critical effect size metric used in Kraemer [60], this translates to a critical effect size of 0.3. Using a 1% test (to protect against Type 1 error incurred in testing multiple primary outcomes) to obtain 99% power, the necessary number of subjects was computed as 227 + 24 = 251 [61]. However, it was noted that if site differences were found, the sample size in homogeneous subgroups of sites would be considerably less. At the extreme, the sample size per individual site would be only 40, which for the above effect size would yield only 50% power per site. Furthermore, subgroup analyses were proposed that would also subdivide the total sample. Thus, we calculated that with 480 subjects, there will be more than sufficient power to detect the differences of interest, including adequate representation at each site to well characterize that site, to detect any possible site differences and to do appropriate subgroup analyses.
Analytic strategy
Based on a general linear model, a three-way analysis of variance was conducted for all of the outcomes of interest. This model included main effects for treatment condition (group vs education), baseline severity (high and low), and cohort. To identify distress level at baseline, we chose a cut-off score of 37 on the baseline score of the POMS to divide the sample into high distressed and low distressed and keeping blind to the change scores on the POMS over time. This cut-off was determined a priori and based on the norms published by Cella and colleagues [47], in which a score of 37 was the mean score of cancer patients within 2 months of diagnosis and pretreatment. Given that participants in this study were a mean of 8.7 months post-diagnosis and were all post-surgery when they entered the study, we concluded that a score of 37 or higher would indicate high distress. Cohort represents the participants recruited at each site by wave (first or second group of 20 patients each in the intervention or control conditions). This was necessary because not all sites completed two waves of recruitment. Thus, eight sites consisted of two cohorts and three sites consisted of one cohort for a total of 19 cohorts.
Because participants were not selected based on level of distress, there was an uneven distribution of high and low distressed participants. Three cohorts had no highly distressed participants and thus three cells in the ANOVA had missing values on the POMS. Thus, a 2 × 2 × 19 ANOVA model that included imputed values for the missing cells was tested as well as a reduced model (2 × 2 × 16) that eliminated the three cohorts with missing cells.
The primary dependent variable was individual slopes on the POMS, operating as indicators of the trajectory over the 2-year study period. Slopes were derived from the regression of POMS scores obtained at baseline and each available follow-up, regressed on the time elapsed (in months) since the baseline assessment, for each participant. All tests were two tailed. We conducted a modified intention-to-treat analysis. Thus, we included participants regardless of whether they complied with randomization. However, we also required that they had at least one follow-up assessment in order to calculate a slope. Those participants with no follow-up data were dropped from the analysis. We chose not to carry the baseline score forward and thereby assume a flat slope. Additional analyses revealed that conducting a true intention-to-treat analysis would not have altered the outcomes as reported. There were 159 women from the treatment condition and 167 women from the control condition who provided at least one follow-up.
Effect sizes (Cohen’s d) based on treatment and control conditions were calculated. The direction of the effect sizes was reversed so that a positive effect size indicates improvement in the desired direction for the treatment group relative to the control group. To assess therapist effects on the outcome, the associations of educational training and therapy experience with the effect sizes were examined for each therapist using Spearman rank-order correlations. Therapist training was operationalized according to whether the therapist had a mental health degree (social work or psychology) versus a nursing degree. Therapist experience was based on the number of years of experience doing psychotherapy.
Results
Demographic and medical variables are described in Table 1. There were no differences between the randomized treatment and control groups (N = 177 and N = 176, respectively) at baseline on demographic or medical status variables except for stage of disease (Chi-square = 6.32, p = 0.04). Fifteen women had stage 3 disease in the control condition (8.5% from the control condition) compared with six women in the treatment condition (3.4% from the treatment condition)
Table 1.
Summary of demographic and medical characteristics of the sample of women with primary breast cancer
| Control (N = 176) | Treatment (N = 177) | |
|---|---|---|
| Age at study entry | 49.7 (10.6) | 49.8 (10.9) |
| Married | 136 (77.3%) | 134 (75.7%) |
| Number of children (biological) | 1.2 (1.1) | 1.2 (1.2) |
| Education | ||
| Less than high school | 3 (1.7%) | 3 (1.7%) |
| Graduated from high school | 42 (23.9%) | 36 (20.3%) |
| Completed trade school | 5 (2.8%) | 9 (5.1%) |
| Some college | 51 (29.0%) | 51 (28.8%) |
| Bachelor’s degree | 26 (14.7%) | 41 (23.2%) |
| Some graduate school | 10 (5.7%) | 14 (7.9%) |
| Master’s degree. | 33 (18.8%) | 18 (10.2%) |
| Ph.D., M.D., and/or J.D. | 6 (3.4%) | 5 (2.8%) |
| Employment | ||
| Not employed | 57 (32.4%) | 49 (27.7%) |
| Part time (< 30 hours/week) | 30 (17.0%) | 34 (19.2%) |
| Full time | 89 (50.6%) | 94 (53.1%) |
| Household income | ||
| Less than $20 000 | 21 (13.4%) | 16 (10.2%) |
| $20 000–$39 999 | 22 (14.0%) | 39 (24.9%) |
| $40 000–$59 999 | 42 (26.7%) | 35 (223%) |
| $60 000–$79 999 | 27 (17.2%) | 27 (17.2%) |
| $80 000–$99 999 | 18 (11.5%) | 20 (12.7%) |
| $100 000 or above | 27 (17.2%) | 20 (12.7%) |
| Ethnicity | ||
| Black | 6 (3.5%) | 4 (2.3%) |
| Asian American | 4 (2.3%) | 3 (1.7%) |
| Mexican American/Chicana | 2 (1.1%) | 0 (0.0%) |
| Other Hispanic/Latina | 1 (0.6%) | 0 (0.0%) |
| Native American | 11 (6.4%) | 5 (2.9%) |
| White/European American | 149 (86.1%) | 162 (93.1%) |
| Time from diagnosis to study entry (months) | 6.9 (3.7) | 7.5 (3.8) |
| Stage of disease | ||
| I | 70 (40.7%) | 87 (50.0%) |
| II | 87 (50.6%) | 81 (46.6%) |
| III | 15 (8.7%) | 6 (3.4%) |
| Estrogen receptor | ||
| Negative | 55 (31.2%) | 54 (30.5%) |
| Positive | 117 (66.5%) | 117 (66.1%) |
| Unknown | 4 (2.3%) | 6 (3.4%) |
| Auxiliary node involvement | ||
| None | 105 (59.7%) | 119 (67.2%) |
| Positive | 43 (24.4%) | 36 (20.4%) |
| Unknown | 28 (15.9%) | 22 (12.4%) |
| Surgery | ||
| Lumpectomy | 81 (46.0%) | 82 (46.3%) |
| Modified radical mastectomy | 95 (54.0%) | 95 (54.7%) |
| Hormone therapy | 73 (41.59%) | 73 (41.2%) |
| Chemotherapy | 121 (68.8%) | 115 (65.3%) |
| Radiation | 85 (48.3%) | 86 (48.6%) |
Three hundred and twenty-six women provided follow-up data and were included in the analysis. For women in the treatment condition, the mean slope on the POMS was −0.52 (SD = 1.57) and for women in the education control condition the mean slope was −0.13 (SD = 2.39). An exploratory analysis revealed an extreme outlier in the control condition with a slope of 25.04. This participant completed the baseline and 3-month assessment only, with TMD scores of 50 and 156, respectively. Owing to the high distress she was experiencing, she dropped out of the study. It was decided to exclude this observation from the analysis. Removing the outlier resulted in a mean slope of −0.28 (SD = 1.37) on the POMS for the control condition. Table 2 shows the number of women, by condition who completed questionnaires at each assessment point and the number who provided at least one follow-up assessment.
Table 2.
POMS scores for each assessment point by condition with outlier removed
| Condition | Baseline |
3 Months |
6 Months |
12 Months |
18 Months |
24 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | |
| Control | 21.67(29.07) | 175 | 17.07(27.61) | 148 | 16.36(32.18) | 142 | 14.75 (30.65) | 144 | 12.25(26.17) | 140 | 9.05(26.19) | 123 |
| Treatment | 27.59 (32.11) | 177 | 18.72 (30.45 | 151 | 19.54 (30.65 | 149 | 14.07(27.84) | 141 | 13.16 (26.08 | 136 | 13.69(30.67) | 122 |
We found that there was neither a main effect for treatment in either the model using imputed data (F(1,252) =0.85, p = 0.36) or in the reduced model (F(1,211) = 1.8, p = 0.18), nor an interaction effect for treatment by level of distress in either the model with imputed data (F (18,252) = 0.46, p = 0.50) or reduced model (F (15,211) = 0.52, p = 0.47). Similarly, no effects were found on any of the secondary outcome measures.
When we analyzed the data with the outlier included, we found that there was a statistically significant difference in the mean slope of change in TMD among women who received group therapy compared with those in the control condition for the model with imputed data and the reduced model (F (1,253) =4.7, p = 0.031 and F (1,212) = 6.5, p = 0.001, respectively). There were significant condition by baseline distress effects favoring the women who were highly distressed and in the treatment condition (F (18,253) = 3.9, p = 0.05 and F (15,212) = 4.3, p = 0.04). There were also significant differences across cohorts and condition by cohort interaction, as well as a significant three-way interaction of baseline distress by condition by cohort.
When including the outlier, beneficial effects of treatment were found on the secondary outcomes of HADS anxiety, HADS depression, MAC helpless/hopeless and Yale instrumental support using both the model with imputed data and the reduced model (F (1,253) = 4.5, p = 0.034 and F (1,212) = 3.9, p = 0.049, respectively, for anxiety; F (1,253) = 5.4, p = 0.021 and F (1,212) = 6.3, p = 0.013, respectively, for depression; F (1,253) = 5.2, p = 0.024 and F (1,212) = 5.3, p = 0.022, respectively, for MAC helpless/hopeless; F (1,253) = 5.5, p = 0.020 and F (1,212) = 6.4, p = 0.012, respectively, for instrumental support). Using the reduced model, there was a treatment effect for Yale negative support favoring the treatment condition (F (1,212) =4.1, p = 0.044). Condition by baseline distress interactions were found on HADS anxiety, HADS depression, Yale informational support for both the imputed data model and the reduced model (F (1,253) = 6.0, p= 0.015 and F (1,212) = 5.4, p = 0.021, respectively, for anxiety; F (1,253) = 6.9, p = 0.009 and F (1,212) = 6.3, p = 0.012, respectively, for depression; F (1,253) = 9.4, p = 0.002 and F (1,212) = 8.3, p = 0.004, respectively, for Yale informational support). For both anxiety and depression, the highly distressed women in the treatment condition showed the greatest improvement. The highly distressed in the education group improved the most on informational support.
Spearman’s rho correlation of effect size with number of years experience conducting psychotherapy and whether or not the therapist had a mental health degree were rho = 0.02 (p = 0.96) and rho = 0.15 (p = 0.62), respectively.
Discussion
We found no effect on distress for brief supportive–expressive group therapy for women with recently diagnosed primary breast cancer in this randomized multicenter trial. Neither our hypothesis that primary breast cancer patients would benefit from 12 weeks of supportive–expressive group therapy was supported nor was our hypothesis that those women who were highly distressed would benefit the most. However, we also found that when we included the extreme outlier there were significant treatment effects suggesting those effects were being carried by this one observation. The same pattern was noted for the secondary outcomes. We found that neither having a mental health degree nor experience in conducting psychotherapy was correlated with effect size.
While we cannot know with any certainty why we failed to show a benefit of brief supportive–expressive group therapy for primary breast cancer patients, there are a variety of possible explanations. The first reason we must consider is that a brief supportive–expressive group therapy intervention may not be helpful for newly diagnosed primary breast cancer patients. It is possible that this existentially oriented intervention is not appropriate for this population perhaps because their concerns are more pragmatic than existential. On the other hand, it is interesting to note that many of the women who participated in this intervention were sorry to see it end and in fact wanted the group to continue. This suggests that they found the group therapy to be helpful. It is also unclear to what extent focusing on existential concerns is beneficial for some cancer patients but not others. Further research is needed to see if there are characteristics that distinguish between who finds an existential approach helpful and who does not.
A second possibility is that the intervention was not long enough. Although there is evidence that interventions as brief as 6 weeks are helpful for cancer patients [62,63], perhaps an existentially oriented intervention requires more time in order to be effective. In a meta-analysis, Sheard and Maguire [24] found longer interventions to be more effective, although they included those interventions with 8 hours or more of therapy in the long intervention group and that is where the present study would also fall.
Third, most of the women who participated in this trial were not highly distressed. Only one-third of the sample met our cut-off for high distress. Thus, the failure to find an overall treatment effect may be because there was simply not enough room for improvement. It is possible that women were too far out from their initial diagnosis and many may have returned to their more typical level of mood disturbance, thereby obviating any possible effects the intervention might have had. However, we did not have an interaction effect for baseline distress by condition, suggesting that even the highly distressed did not benefit from this intervention.
The fourth possibility is that the intervention was not implemented as successfully as it might have been. One of the objectives of this trial was to see if this intervention could be readily disseminated. We provided a treatment manual, a training workshop, supervision for a pilot group and supervision for the groups in the randomized trial and have evidence that the therapists learned from the training program we provided [34]. However, we did not assess their actual performance in facilitating the groups. We used therapists with a range of training and experience and found that neither training nor experience was associated with effect size contrary to other reports in the literature [42].
Finally, our sample size may not have been adequate to test the hypothesis.
There are number of lessons that can be drawn from this trial. The most striking is that a single outlier can transform the findings even in a relatively large data set. A second lesson involves caution in the use of stratified adaptive randomization. In cancer clinical trials it is common practice to stratify based on prognostic medical variables. However, in the case of studies similar to this one it would have been better to stratify based only on baseline level of distress. Although the prognostic medical variables we chose may have had an impact on distress, the best predictor of change in distress over time is prior distress. Given that a primary aim of this trial was to determine whether highly distressed patients were more likely to benefit, it would have been prudent to stratify based on baseline distress and thereby have avoided the problem of empty cells. In addition, this would have ensured a larger sample of highly distressed patients and consequently increased the power to detect a difference.
Another lesson learned was the challenge of conducting research with community partners who are scattered around the country. These were busy oncology practices and it was challenging to accommodate the demands of this study. Some sites struggled with recruitment and dropped out after the first cohort was completed. Monitoring the data collection from a distance was cumbersome at times and not always effective. For instance, not all sites collected data on attendance, thereby making it impossible to test for a dose effect. Utilization of CCOP removed some but not all barriers.
There are a number of limitations to this study. One is that we cannot know for certain whether there was a problem in the quality of the group facilitation. A careful analysis of the videotaped groups is needed to address this important question. Another limitation is that the sample size may have been too small to detect a treatment benefit. Participants were also not randomized according to level of distress, resulting in some empty cells which may have compromised our analyses.
This study demonstrates a variety of statistical problems that can arise as a result of missing data and empty cells in a randomized clinical trial (RCT). Multicenter RCTs are expensive and a challenge to implement. It is important that we design our studies in a way that maximizes our ability to answer our research questions and that we be aware of the potential for misleading results if the data are not properly analyzed. In conclusion, this study failed to provide evidence for the effectiveness of a brief supportive–expressive group therapy for primary breast cancer patients.
Acknowledgments
The authors gratefully acknowledge the participation of JoAnn Briles-Klein and Narayan Mudaliar, Wichita CCOP; H. Irving Pierce, Northwest CCOP; Patrick J. Flynn, Metro-Minnesota CCOP; Tarit K. Banerjee, Marshfield Medical Research Foundation CCOP; Vincent P. Vinciguerra, North Shore University Hospital CCOP; Richard Rosenbluth, Northern New Jersey CCOP; Ronald Hart, Oncology of Wisconsin CCOP; Jeffrey Kirshner, Syracuse Hematology–Oncology CCOP. We also extend our appreciation to Robert Carlson for his medical consultation. Finally, we thank all the women who participated in this study. This research was conducted in the Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine and was supported by grant CA61309 from the National Cancer Institute awarded to David Spiegel and by a grant from the Cummings Foundation awarded to Gary R. Morrow.
References
- 1.Gallagher J, Parle M, Cairns D. Appraisal and psychological distress six months after diagnosis of breast cancer. Br J Health Psychol. 2002;7(3):365–376. doi: 10.1348/135910702760213733. [DOI] [PubMed] [Google Scholar]
- 2.Kissane D, Clarke D, Ikin J, et al. Psychological morbidity and quality of life in Australian women with early-stage breast cancer: a cross-sectional survey. Med J Aust. 1998;169(4):192–196. doi: 10.5694/j.1326-5377.1998.tb140220.x. [DOI] [PubMed] [Google Scholar]
- 3.Edgar L, Rosberger Z, Nowlis D. Coping with cancer during the first year after diagnosis. Cancer. 1992;69(3):817–828. doi: 10.1002/1097-0142(19920201)69:3<817::aid-cncr2820690334>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg JA, Scott RN, Davidson PM, et al. Psychological morbidity in the first year after breast surgery. Eur J Surg Oncol. 1992;18(4):327–331. [PubMed] [Google Scholar]
- 5.Stanton AL, Danoff-Burg S, Huggins ME. The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psycho-Oncology. 2002;11:93–102. doi: 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- 6.Ford S, Lewis S, Fallowfield L. Psychological morbidity in newly referred patients with cancer. J Psychosom Res. 1995;39(2):193–202. doi: 10.1016/0022-3999(94)00103-c. [DOI] [PubMed] [Google Scholar]
- 7.Weitzner MA, Meyers CA, Stuebing KK, Saleeba AK. Relationship between quality of life and mood in long-term survivors of breast cancer treated with mastectomy. Support Care Cancer. 1997;5:241–248. doi: 10.1007/s005200050067. [DOI] [PubMed] [Google Scholar]
- 8.Antoni MH, Lehman JM, Kilbourn KM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20(l):20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 9.Antoni MH, Wimberly SR, Lechner SC, et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatry. 2006;163(10):1791–1797. doi: 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukui S, Kamiya M, Koike M, et al. Applicability of a Western-developed psychosocial group intervention for Japanese patients with primary breast cancer. Psycho-Oncology. 2000;9(2):169–177. doi: 10.1002/(sici)1099-1611(200003/04)9:2<169::aid-pon441>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Heiney SP, McWayne J, Hurley TG, et al. Efficacy of therapeutic group by telephone for women with breast cancer. Cancer Nurs. 2003;26(6):439–147. doi: 10.1097/00002820-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Helgeson VS, Cohen S, Schulz R, Yasko J. Education and peer discussion group interventions and adjustment to breast cancer. Arch Gen Psychiatry. 1999;56(4):340–347. doi: 10.1001/archpsyc.56.4.340. [DOI] [PubMed] [Google Scholar]
- 13.Helgeson VS, Cohen S, Schulz R, Yasko J. Long-term effects of educational and peer discussion group interventions on adjustment to breast cancer. Health Psychol. 2001;20(5):387–392. doi: 10.1037//0278-6133.20.5.387. [DOI] [PubMed] [Google Scholar]
- 14.Kissane DW, Bloch S, Smith GC, et al. Cognitive-existential group psychotherapy for women with primary breast cancer: a randomised controlled trial. Psycho-Oncology. 2003;12(6):532–546. doi: 10.1002/pon.683. [DOI] [PubMed] [Google Scholar]
- 15.Kissane DW, Love A, Hatton A, et al. Effect of cognitive-existential group therapy on survival in early-stage breast cancer. J Clin Oncol. 2004;22(21):4255–4260. doi: 10.1200/JCO.2004.12.129. [DOI] [PubMed] [Google Scholar]
- 16.Manne SL, Ostroff JS, Winkel G, et al. Couple-focused group intervention for women with early stage breast cancer. J Consult Clin Psychol. 2005;73(4):634–646. doi: 10.1037/0022-006X.73.4.634. [DOI] [PubMed] [Google Scholar]
- 17.Owen JE, Klapow JC, Roth DL, et al. Randomized pilot of a self-guided internet coping group for women with early-stage breast cancer. Ann Behav Med. 2005;30(1):54–64. doi: 10.1207/s15324796abm3001_7. [DOI] [PubMed] [Google Scholar]
- 18.Samarel N, Fawcett J, Tulman L. Effect of support groups with coaching on adaption to early stage breast cancer. Res Nurs Health. 1997;20:15–26. doi: 10.1002/(sici)1098-240x(199702)20:1<15::aid-nur3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Samarel N, Tulman L, Fawcett J. Effects of two types of social support and education on adaptation to early-stage breast cancer. Res Nurs Health. 2002;25:459–470. doi: 10.1002/nur.10061. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel D, Morrow GR, Classen C, et al. Effects of group therapy on women with primary breast cancer. Breast J. 1996;2(1):104–106. [Google Scholar]
- 21.Spiegel D, Morrow GR, Classen C, et al. Group psychotherapy for recently diagnosed breast cancer patients: a multicenter feasibility study. Psycho-Oncology. 1999;8(6):482–493. doi: 10.1002/(sici)1099-1611(199911/12)8:6<482::aid-pon402>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Vos PJ, Visser AP, Garssen B, Duivenvoorden HJ, de Haes HC. Effects of delayed psychosocial interventions versus early psychosocial interventions for women with early stage breast cancer. Patient Educ Couns. 2006;60(2):212–219. doi: 10.1016/j.pec.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Kissane DW, Bloch S, Miach P, Smith GC, Seddon A, Keks N. Cognitive-existential group therapy for patients with primary breast cancer—techniques and themes. Psycho-Oncology. 1997;6:25–33. doi: 10.1002/(SICI)1099-1611(199703)6:1<25::AID-PON240>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Sheard T, Maguire P. The effect of psychological interventions on anxiety and depression in cancer patients: results of two meta-analyses. Br J Cancer. 1999;80(11):1770–1780. doi: 10.1038/sj.bjc.6690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman AC, Mosier J, Leszcz M, et al. Group interventions for patients with cancer and HIV disease: Part I: effects on psychosocial an functional outcomes at different phases of illness. Int J Group Psychother. 2004;54(1):29–82. doi: 10.1521/ijgp.54.1.29.40376. [DOI] [PubMed] [Google Scholar]
- 26.Bordeleau L, Szalai JP, Ennis M, et al. Quality of life in a randomized trial of group psychosocial support in metastatic breast cancer: overall effects of the intervention and an exploration of missing data. J Clin Oncol. 2003;21(10):1944–1951. doi: 10.1200/JCO.2003.04.080. [DOI] [PubMed] [Google Scholar]
- 27.Esplen MJ, Hunter J, Leszcz M, et al. A multicenter study of supportive–expressive group therapy for women with BRCA1/BRCA2 mutations. Cancer. 2004;101(10):2327–2340. doi: 10.1002/cncr.20661. [DOI] [PubMed] [Google Scholar]
- 28.Classen C, Diamond S, Soleman A, Fobair P, Spira J, Spiegel D. Brief Supportive–Expressive Group Therapy for Women with Primary Breast Cancer: A Treatment Manual. Stanford University School of Medicine; Stanford, CA: 1993. [Google Scholar]
- 29.Classen C, Butler LD, Koopman C, et al. Supportive–expressive group therapy and distress in patients with metastatic breast cancer: a randomized clinical intervention trial. Arch Gen Psychiatry. 2001;58:494–501. doi: 10.1001/archpsyc.58.5.494. [DOI] [PubMed] [Google Scholar]
- 30.Spiegel D, Bloom JR, Yalom I. Group support for patients with metastatic cancer. A randomized outcome study. Arch Gen Psychiatry. 1981;38(5):527–533. doi: 10.1001/archpsyc.1980.01780300039004. [DOI] [PubMed] [Google Scholar]
- 31.Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345(24):1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 32.Vachon MLS, Lyall WAL, Rogers J, Cochrane J, Freeman SJJ. The effectiveness of psychosocial support during post-surgical treatment of breast cancer. Int J Psychiatry Med. 1982;11:365–372. doi: 10.2190/cwy6-xd2d-5db0-bbjj. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham AJ, Lockwood GA, Edmonds CV. Which cancer patients benefit most from a brief, group, coping skills program? Int J Psychiatry Med. 1993;23(4):383–398. doi: 10.2190/EQ7N-2UFR-EBHJ-QW4P. [DOI] [PubMed] [Google Scholar]
- 34.Classen C, Abramson s, Angell K, et al. Effectiveness of a training program for enhancing therapists’ understanding of the supportive–expressive treatment model for breast cancer groups. J Psychother Pract Res. 1997;6(3):211–218. [PMC free article] [PubMed] [Google Scholar]
- 35.Propst A, Paris J, Rosberger Z. Do therapist experience, diagnosis and functional level predict outcome in short term psychotherapy? Can J Psychiatry. 1994;39(3):168–176. doi: 10.1177/070674379403900309. [DOI] [PubMed] [Google Scholar]
- 36.Siqueland L, Crits-Christoph P, Barber JP, et al. The role of therapist characteristics in training effects in cognitive, supportive–expressive, and drug counseling therapies for cocaine dependence. J Psychother Prac Res. 2000;9(3):123–130. [PMC free article] [PubMed] [Google Scholar]
- 37.Stein DM. On the relationship between therapist experience and psychotherapy outcome. Clin Psychol Rev. 1984;4:127–142. [Google Scholar]
- 38.Smith ML, Glass G. Meta-analysis of psychotherapy outcome studies. Am Psychol. 1977;32:752–760. doi: 10.1037//0003-066x.32.9.752. [DOI] [PubMed] [Google Scholar]
- 39.Stein DM, Lambert MJ. Graduate training in psychotherapy: are therapy outcomes enhanced? J Consult Clin Psychol. 1995;63(2):182–196. doi: 10.1037//0022-006x.63.2.182. [DOI] [PubMed] [Google Scholar]
- 40.Crits-Christoph P, Baranackie K, Kurcias JS, et al. Meta-analysis of therapist effects in psychotherapy outcome studies. Psychother Res. 1991;1(2):81–91. [Google Scholar]
- 41.Burlingame GM, Paul S, Fuhriman A, Ogles BM. Implementing a time-limited therapy program: differential effects of training and experience. Psychotherapy. 1989;26(3):303–313. [Google Scholar]
- 42.Kissane DW, Bloch S, Smith GC, et al. Cognitive-existential group psychotherapy for women with primary breast cancer: a randomised controlled trial. Psycho-Oncology. 2003;12(6):532–546. doi: 10.1002/pon.683. [DOI] [PubMed] [Google Scholar]
- 43.Hannigan JF, Brown BW. Adaptive Randomization Biased Coin-design: Experience in a Cooperative Group Clinical Trial Technical Report. Division of Biostatistics, Stanford University; Stanford, CA: 1982. [Google Scholar]
- 44.Spiegel D, Yalom I. A support group for dying patients. Int J Group Psychother. 1978;28:233–245. doi: 10.1080/00207284.1978.11491609. [DOI] [PubMed] [Google Scholar]
- 45.Spiegel D, Classen C. Group Therapy for Cancer Patients: A Research-based Handbook of Psychosocial Care. Basic Books; New York: 2000. [Google Scholar]
- 46.McNair DM, Lorr M, Droppleman LF. Edits Manual for the Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971/1981/1992. [Google Scholar]
- 47.Cella DF, Tross S, Orav EJ, Holland JC, Silberfarb PM, Rafla S. Mood states of patients after the diagnosis of cancer. J Psychosoc Oncol. 1989;7(1/2):45–54. [Google Scholar]
- 48.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 49.Watson M, Law M, dos Santos M, Greer S, Baruch J, Bliss J. The mini-MAC: further development of the mental adjustment to cancer scale. J Psychosoc Oncol. 1994;12(3):33–46. [Google Scholar]
- 50.Watson M, Greer S. Development of a questionnaire measure of emotional control. J Psychosom Res. 1983;27:299–305. doi: 10.1016/0022-3999(83)90052-1. [DOI] [PubMed] [Google Scholar]
- 51.Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Giese-Davis J, Koopman C, Butler LD, et al. The Stanford Emotional Self-Efficacy Scale—cancer: reliability, validity, and generalizability. In: Nyklicek I, Temoshok LR, Vingerhoets A, editors. Emotional Expression and Health: Advances in Theory, Assessment and Clinical Applications. Brunner-Routledge; Hove, UK, New York: 2004. pp. 204–222. [Google Scholar]
- 53.Schag CA, Heinrich RL. Development of a comprehensive quality of life measurement tool: CARES. Oncology. 1990;4(5):135–138. [PubMed] [Google Scholar]
- 54.Holahan CJ, Moos RH. The quality of social support: measures of family and work relationships. Brit J Clin Psychol. 1983;22(3):157–162. [Google Scholar]
- 55.Moos R, Moos B. Family Environment Scale Manual. 2. Consulting Psychologists Press; Palo Alto, CA: 1986. [Google Scholar]
- 56.Stanford Sleep Disorders Research Program: Sleep Questionnaire and Assessment of Wakefulness. Stanford University School of Medicine; Stanford, CA: 1979. [Google Scholar]
- 57.Spiegel D, Bloom JR. Pain in metastatic breast cancer. Cancer. 1983;52(2):341–345. doi: 10.1002/1097-0142(19830715)52:2<341::aid-cncr2820520227>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 58.Seeman TE, Berkman LF. Structural characteristics of social networks and their relationship with social support in the elderly: who provides support. Soc Sci Med. 1988;26(7):737–749. doi: 10.1016/0277-9536(88)90065-2. [DOI] [PubMed] [Google Scholar]
- 59.Berkman LF. Social networks, support, and health: taking the next step forward. Am J Epidemiol. 1986;123(4):559–562. doi: 10.1093/oxfordjournals.aje.a114276. [DOI] [PubMed] [Google Scholar]
- 60.Kraemer HC. The methodological and statistical evaluation of medical tests: the dexamethasone suppression test in psychiatry. Psychoneuroendocrinology. 1987;12(6):411–427. doi: 10.1016/0306-4530(87)90076-x. [DOI] [PubMed] [Google Scholar]
- 61.Kraemer H, Theimann S. How Many Subjects?. Statistical Power Analysis in Research. Sage Publications; Newbury Park, CA: 1987. [Google Scholar]
- 62.Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma: effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50(9):681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]
- 63.Fawzy FI, Canada AL, Fawzy NW. Malignant melanoma: effects of a brief, structured psychiatric intervention on survival and recurrence at 10-year follow-up. Arch Gen Psychiatry. 2003;60(1):100–103. doi: 10.1001/archpsyc.60.1.100. [DOI] [PubMed] [Google Scholar]