Abstract
Activity-based therapies such as passive bicycling and step-training on a treadmill contribute to motor recovery after spinal cord injury (SCI), leading to a greater number of steps performed, improved gait kinematics, recovery of phase-dependent modulation of spinal reflexes, and prevention of decrease in muscle mass. Both tasks consist of alternating movements that rhythmically stretch and shorten hindlimb muscles. However, the paralyzed hindlimbs are passively moved by a motorized apparatus during bike-training, whereas locomotor movements during step-training are generated by spinal networks triggered by afferent feedback. Our objective was to compare the task-dependent effect of bike- and step-training after SCI on physiological measures of spinal cord plasticity in relation to changes in levels of neurotrophic factors. Thirty adult female Sprague-Dawley rats underwent complete spinal transection at a low thoracic level (T12). The rats were assigned to one of three groups: bike-training, step-training, or no training. The exercise regimen consisted of 15 min/d, 5 days/week, for 4 weeks, beginning 5 days after SCI. During a terminal experiment, H-reflexes were recorded from interosseus foot muscles following stimulation of the tibial nerve at 0.3, 5, or 10 Hz. The animals were sacrificed and the spinal cords were harvested for Western blot analysis of the expression of neurotrophic factors in the lumbar spinal cord. We provide evidence that bike- and step-training significantly increase the levels of brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and NT-4 in the lumbar enlargement of SCI rats, whereas only step-training increased glial cell-derived neurotrophic factor (GDNF) levels. An increase in neurotrophic factor protein levels that positively correlated with the recovery of H-reflex frequency-dependent depression suggests a role for neurotrophic factors in reflex normalization.
Key words: complete transection, cycling, exercise, locomotor training, spinal cord injury
Introduction
Spinal cord injury (SCI) unequivocally reduces the amount of activity performed by an individual (van den Berg-Emons et al., 2008). Rehabilitative strategies routinely integrate activity-based therapies to induce repetitive activation of the neuromuscular system, to limit further structural/anatomical loss and facilitate functional recovery. Activity-dependent processes triggered by exercise affect multiple neurotrophic factors by increasing the level of brain-derived neurotrophic factor (BDNF; Dupont-Versteegden et al., 2004; Gomez-Pinilla et al., 2004; Hutchinson et al., 2004), glial cell-derived neurotrophic factor (GDNF; Dupont-Versteegden et al., 2004), neurotrophin-3 (NT-3; Gomez-Pinilla et al., 2004; Hutchinson et al., 2004), and neurotrophin-4 (NT-4; Skup et al., 2002) in the spinal cord and hindlimb muscles. Neurotrophic factors are powerful regulators of neuronal survival, maintenance, and synaptic strength. After SCI, exogenous neurotrophic factors can rescue neurons (Novikova et al., 2000, 2002), promote regeneration (Bregman et al., 2002; Dolbeare and Houlé, 2003; Ma et al., 2010; Ye and Houlé, 1997), and facilitate functional recovery (Boyce et al., 2007; Fortun et al., 2009; Jakeman et al., 1998). Neurotrophic factors also have significant effects on the development of motor pathways (Seebach et al., 1999), and synaptic transmission (Mendell and Arvanian, 2002; Mendell et al., 2001).
Studies in which BDNF action was blocked using TrkB-IgG chimera showed that BDNF is required for the success of instrumental spinal learning (Gomez-Pinilla et al., 2007) and motor recovery (Ying et al., 2008). High levels of BDNF within the lumbar spinal cord are associated with an increased effect of descending drive on the tibial motoneuronal pool, normalization of motoneuronal properties, resolution of allodynia, and improved locomotor recovery (Beaumont et al., 2008; Hutchinson et al., 2004; Ying et al., 2008). Recent findings indicate that BDNF serum levels are increased as well in SCI athletes (Rojas Vega et al., 2008).
After SCI, the loss of supraspinal control and the subsequent increase in the gain of afferent feedback causes the excitability of spinal reflexes to be exacerbated. Therefore, the ability to modulate spinal reflexes is decreased, resulting in long-term undesirable effects such as inappropriate timing of activation of muscles during movement (Knikou, 2010; Nielsen et al., 2007). Hyperreflexia is also manifest by the incapacity to decrease the transmission of the H-reflex, the electrical analog of the stretch reflex, when successive volleys are transmitted from group Ia afferents to motoneurons (Field-Fote et al., 2006; Grey et al., 2008; Lee et al., 2005, 2007; Lloyd, 1957; Thompson et al., 1992). Impaired depression of the spinal reflexes with repetitive stimulation has been correlated with spasticity, and passive exercise helps recover H-reflex depression and decrease spasticity in animals (Reese et al., 2006; Yates et al., 2008b), and humans (Kiser et al., 2005), after SCI. Spastic patients also responded better to bike-training than did non-spastic individuals (Phadke et al., 2009).
Task-specific training has emerged as a crucial concept when designing rehabilitation strategies (de Leon et al., 1998; Edgerton et al., 1997). Although motor skills achieved by practicing one task are not transferable to another skill, other functional gains such as prevention of muscle mass loss and decreased spasticity result from several types of exercise. Both bike- and step-training consist of alternating movements that rhythmically stretch and shorten hindlimb muscles; however, step-training entails an active loading component that does not appear to be achieved with passive bicycling.
The objectives of this study were threefold: (1) characterize spinal plasticity evoked by bike- and step-training after a complete SCI at the thoracic level as measured by changes in neurotrophic factor levels and H-reflex excitability; (2) compare spinal plasticity with different training paradigms, passive bicycling versus step-training on a treadmill; and (3) investigate the relationship between neurotrophic factor protein levels and recovery of H-reflex frequency-dependent depression.
Methods
All procedures were performed in accordance with protocols approved by the Drexel University College of Medicine Institutional Animal Care and Use Committee, and followed National Institutes of Health guidelines for the care and use of laboratory animals.
Surgical procedures and postoperative care
Thirty adult female Sprague-Dawley rats underwent a complete spinal transection at a low thoracic level (T12) under aseptic conditions. Briefly, the rats were anesthetized with isoflurane (1–2%) in oxygen. A laminectomy was performed at the T10–T11 vertebral level and the dura was carefully opened. A 2-mm cavity was created by gentle aspiration, and saline-soaked surgical foam was used to achieve hemostasis. The complete removal of spinal cord tissue was confirmed by the retraction of the rostral and caudal portions of the cord, by examining the ventral floor of the spinal canal, and by subsequent histology. The dura was closed with 10-0 sutures. Overlying muscles were sutured and the skin incision was closed with wound clips. The animals were given dextrose in saline (5 mL SC), buprenorphine (0.05 mg/kg IM) for 3 days as an analgesic, and ampicillin (100 mg/kg SC) for 7 days to prevent infection. The bladders were expressed manually twice a day until the voiding reflex returned (∼14 days).
Bicycle and locomotor training
The rats were randomly assigned to one of three groups: passive cycling (n = 8, bike-trained group), step-training on a treadmill with perineal stimulation and manual assistance (n = 14, step-trained group), or no training (n = 8, untrained group). Beginning on day 5, the exercised groups received 15 min of daily exercise, 5 days a week, for 4 weeks. Briefly, animals undergoing passive bicycling were secured in a support harness with the flaccid hindlimbs hanging. The feet were secured to the pedals with surgical tape. This custom motor-driven apparatus moved the hindlimbs through a complete range of motion at a rate of 45 rpm, as described previously (Houlé et al., 1999; Ollivier-Lanvin et al., 2010; Skinner et al., 1996).
Animals receiving step-training were placed over a motorized treadmill belt (∼9–11 cm/sec) with the forelimbs resting on an acrylic glass platform. Perineal stimulation was continuously provided to induce and maintain bipedal locomotor movements (Etlin et al., 2010). Weight support of the hindlimbs was provided by the experimenter and adjusted to prevent collapsing of the hindlimbs; the animals typically performed weight-bearing stepping with plantar placement of the foot 5–10 days after the onset of the training regimen (unpublished observations). Weight support was then solely provided in between stepping episodes when no perineal stimulation was provided. Step-trained animals provided with perineal stimulation could perform >20 consecutive steps (see Supplementary Video 1; see online supplementary material at http://www.liebertonline.com).
H-reflex recordings and analysis
In a terminal experiment, the rats were anaesthetized with an IP injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). The tibial nerve was dissected free, mounted on a bipolar hook electrode for stimulation, and skin flaps were used to form a pool of mineral oil to prevent desiccation. Bipolar wire electrodes (Cooner Wire, Chatsworth, CA) were inserted into the interosseus muscles for EMG recordings and the ground electrode was inserted into the skin of the leg.
H-reflexes were evoked via an isolated pulse stimulator (A-M Systems, Carlsborg, WA), delivering single bipolar pulses (100 μsec) to the tibial nerve. H- and M-waves in the interosseus muscle were recorded in response to a range of increasing stimulus intensities to determine the threshold and the maximal response amplitude for both the M- and H-wave (Mmax and Hmax). The stimulation intensity that elicited Hmax response was then used for three series of 17 consecutive stimulation pulses delivered at different frequencies (0.3, 5, and 10 Hz). Before termination, the 0.3-Hz series was repeated to verify that the M-wave was still within 95% of the initial trial, otherwise the trial was discarded. The EMG recordings were amplified (1000 × ; A-M Systems), and bandpass filtered (10–5 kHz). The signal was digitized (10 kHz) and fed to custom software.
Response latency (onset of response), peak latency (delay to reach peak), and peak-to-peak amplitude were measured for the H and M responses evoked by single pulses. The recruitment curve was plotted by expressing the amplitude of the response as a function of stimulus intensity. Mmax, the response of all motor units with supramaximal stimulation of axons of the tibial nerve, and Hmax, the maximal H-reflex amplitude, were determined. The Hmax:Mmax ratio was calculated to assess the relative proportion of motoneurons recruited through the monosynaptic reflex loop as compared to the activation of the entire motor pool. This measure is thought to give an estimate of motoneuronal excitability. The Hmax:M ratio was also calculated to estimate the relative activation of the motor pool required to reach maximal reflex amplitude. The slope of the recruitment curve was evaluated from a linear regression line fitted to the recruitment curve, as reported by others (Thompson et al., 1992).
For the analysis of frequency-dependent depression of the H-reflex, the first five responses to a train of stimulation were discarded to allow reflex stabilization. The 12 remaining responses were then averaged for every train frequency (0.3, 5, and 10 Hz). Peak-to-peak amplitude of the M and H responses were then measured. H-reflex amplitude was normalized to Mmax, and the change in H-reflex response at 5 Hz and 10 Hz was calculated as a percentage of the response measured at 0.3 Hz.
Western blots
Thirty days after spinal cord transection, the rats were overdosed with Euthasol® (390 mg/kg sodium pentobarbital and 50 mg/kg phenytoin IP). The T10–L6 spinal cord was rapidly harvested, the spinal roots and meninges were stripped off, and four separate spinal segments (T10–T11, T12–L1, L2–L3, and L4–L6) were prepared and placed in cold RIPA buffer (50 mM Tris buffer [pH 7.6], 0.1% SDS, 0.25% deoxycholate, and 150 mM NaCl), in the presence of protease and phosphatase inhibitors (Roche Diagnostics, Indianapolis, IN), 2 mM phenyl-methyl sulfonyl-fluoride (PMSF), and 1 mM sodium fluoride.
Tissue blocks were sonicated and centrifuged at 14,000g for 40 min at 4°C. The supernatants were collected and aliquots were stored at −80°C. For Western blot analysis, the samples were boiled in Laemmli sample buffer for 5 min, and equal amounts of total protein were separated on 10% SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF) membranes (BioRad, Hercules, CA). Each nitrocellulose replica was blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20 (TBS-T), probed with primary rabbit polyclonal antibodies against BDNF (1:400; Abcam, Cambridge, MA), GDNF (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), NT-3 (1:200; Abcam), or NT-4 (1:200; Santa Cruz Biotechnology), followed by incubation with the horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (IgG; Jackson ImmunoResearch Laboratories, West Grove, PA). Blots for each sample were run two or three times for each primary antibody to ensure replication of the results.
To confirm equal loading of protein in each lane, the blots were stripped using buffer containing 65 mM Tris buffer (pH 6.8), 2% SDS, and 1% β-mercaptoethanol for 30 min, and re-probed with mouse monoclonal anti-actin antibody (1:8000; Sigma-Aldrich, St. Louis, MO). Immunoreactivity was detected using an enhanced chemiluminescence kit (ECL; Amersham Biosciences, Piscataway, NJ), and optical densities of the protein bands were assessed using GeneTool Analysis software (Syngene, Frederick, MD). Values for each sample were normalized to actin and combined for each group. Final data (mean ± standard error of the mean [SEM]) are presented as a ratio of the untrained group, which was assigned a value of 1.0.
Statistical analysis
A one-way analysis of variance (ANOVA) followed by the Holm-Sidak post-hoc test were used to determine significant differences across groups for all data unless stated otherwise. If the sample variables did not fit a normal distribution or were not equally variant, a one-way ANOVA on ranks followed by Dunn's post-hoc test was performed.
A two-way ANOVA followed by the Holm-Sidak post-hoc test was used to assess whether stimulation frequency and treatment group had a significant effect on the amplitude of the H-reflex, and to evaluate if the interaction of these factors affected the variable. All data are reported as mean ± SEM. The Fisher's exact test evaluating the frequency distribution was used to identify differences between groups for the occurrence of H-reflexes with a threshold below the motor threshold. Linear regression analysis was used to correlate neurotrophic factor levels to the rate of H-reflex depression. Statistical analysis was performed using Sigma Plot software 11.0 and PASW Statistics 18. For all statistical tests, the significance level was set to p < 0.05.
Results
Bike-training and step-training increase neurotrophic factor levels in the spinal cord
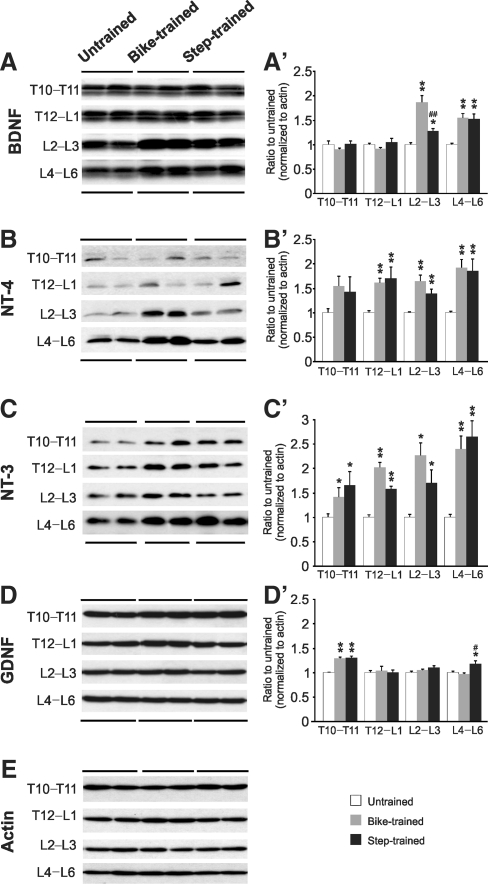
Figure 1 illustrates representative Western blots with positive bands for BDNF (∼18 kDa), NT-4 (∼27 kDa), NT-3 (20 kDa), and GDNF (∼37 kDa) protein, for two untrained, two bike-trained, and two step-trained animals. Protein expression levels were averaged and compared across groups for separate spinal levels rostral (T10–T11) and caudal (T12–L1, L2–L3, and L4–L6) to the lesion site. Bike- and step-training increased the expression of BDNF in the L2–L6 lumbar cord compared to untrained animals (Fig. 1A and A’). This increase was significantly larger in the bike-trained groups than in the step-trained group in the upper lumbar segments (L1–L3), but not in the lumbar enlargement (L4–L6). NT-4 (Fig. 1B and B’) and NT-3 protein levels (Fig. 1C and C’) were amplified in all caudal segments of the lumbar spinal cord after bike-training or step-training. The GDNF protein level was increased in the L4–L6 region after step-training, but not after bike-training (Fig. 1D and D’). Only NT-3 and GDNF levels were increased in spinal segments rostral to the transection injury (T10–T11) following either exercise regimen. We observed that bike-training tended to have a larger effect than step-training at L1–L3 for BDNF, NT-3, and NT-4 (significant only for BDNF), but in the L4–L6 region they were very close in expression level.
FIG. 1.
Western blots showing the expression of brain-derived neurotrophic factor (BDNF) (A), neurotrophin-4 (NT-4) (B), NT-3 (C), and glial cell-derived neurotrophic factor (GDNF) (D) rostral to the injury site (T10–T11), at the lesion site (T12–L1), and caudal to the injury site (L2–L3 and L4–L6), in untrained, bike-trained, and step-trained animals (n = 8 per group). Overall, bike- and step-training increased the expression of BDNF (A’), NT-3 (B’), NT-4 (C’), and GDNF (D’). Also, step-training increased GDNF protein levels in the lumbar enlargement more than bike-training (D’). The blots were reprobed with anti-actin to provide an indication of total protein loaded per lane (E). BDNF, NT-3, NT-4, and GDNF proteins were detected and quantified by Western blots using actin as a standard control (*p < 0.05 versus untrained; **p < 0.001 versus untrained; #p < 0.05 versus bike-trained; ##p < 0.001 versus bike-trained).
Step-training modifies spinal excitability
The H-reflex is frequently used to assess motoneuronal excitability after an injury to the spinal cord with or without treatment. It provides an estimation of neurotransmission from Ia afferents to target motoneurons. A single stimulus evokes two subsequent EMG responses: the initial M-wave, which results from the direct activation of motor axons, and the delayed H-wave, evoked by the synaptic activation of motoneurons by group Ia muscle afferents. Therefore, the H-reflex yields information about the properties of the monosynaptic reflex. Table 1 describes the features of the H-reflex in the different groups of animals, indicating that there were no significant differences between untrained and trained groups with regard to latency, amplitude, and threshold of M- and H-waves. For example, the H-reflex response latency and peak latency were 8.49 ± 0.20 msec and 9.31 ± 0.23 msec, respectively, in untrained animals, and this was not modified by bike-training or step-training. Exercise did not further modify M-wave response latency and peak latency compared to untrained animals (2.30 ± 015 msec and 3.24 ± 0.12 msec, respectively).
Table 1.
Properties of the M-Wave and H-Reflex: Latency, Amplitude, and Threshold
| Motor threshold (mA) | M-wave latency (msec) | M-wave peak latency (msec) | H-reflex latency (msec) | H-reflex peak latency (msec) | H-reflex threshold (xMT) | Hmax(xMT) | |
|---|---|---|---|---|---|---|---|
| Untrained (n = 8) | 0.028 ± 0.007 | 2.30 ± 0.15 | 3.24 ± 0.12 | 8.49 ± 0.20 | 9.31 ± 0.23 | 1.02 ± 0.09 | 1.5 ± 0.2 |
| Bike-trained (n = 8) | 0.040 ± 0.005 | 2.19 ± 0.11 | 3.00 ± 0.16 | 8.06 ± 0.20 | 8.93 ± 0.20 | 0.99 ± 0.06 | 1.4 ± 0.2 |
| Step-trained (n = 12) | 0.037 ± 0.008 | 2.33 ± 0.11 | 3.07 ± 0.11 | 8.07 ± 0.18 | 8.94 ± 0.19 | 0.96 ± 0.02 | 1.2 ± 0.1 |
Values are mean ± SEM.
MT, motor threshold.
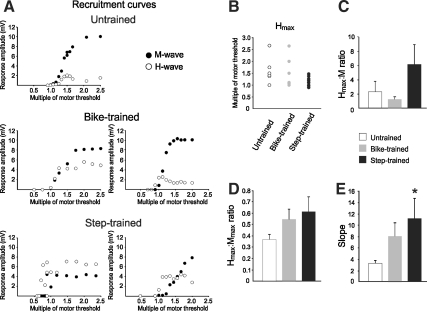
Motor threshold (MT), which is the lowest stimulation intensity evoking an M-wave, was similar across groups (Table 1). On average, the H-reflex appeared slightly above MT in untrained animals (1.02 ± 0.09 MT), whereas it required a weaker stimulation intensity in exercised animals (bike-trained: 0.99 ± 0.06 MT; step-trained: 0.96 ± 0.02 MT), but this difference was not significant. Further analysis showed that the H-reflex appeared at stimulation intensities lower than MT in 14% of untrained, 50% of bike-trained, and 79% of step-trained animals. Fisher's exact test revealed an increased occurrence of an H-response below MT in step-trained animals versus the other groups (p < 0.001).
Recruitment curves were constructed by plotting the H-reflex amplitude against a series of stimulus intensities, represented here as a multiple of the motor threshold. Figure 2A illustrates a recruitment curve with an initial linear component (∼1–1.3 MT), followed by an asymptotic portion (∼1.5 T), with an M-wave reaching maximal amplitude around 2 MT in untrained animals, as has been reported previously (Thompson et al., 1992). We observed differences in the shape of the recruitment curves after bike-training and step-training. The Hmax tended to occur closer to MT than in untrained animals (Fig. 2A and B), and consequently occurred when the M-wave was of much smaller amplitude, close to MT, in the ascending part of the recruitment curve. This is expressed as an increase in the Hmax:M ratio (Fig. 2C), which represents the fraction of motoneurons activated to reach a maximal H-reflex.
FIG. 2.
(A) Representative recruitment curves for individual animals in the untrained, bike-trained, and step-trained groups. The untrained animals display Hmax ∼1.5 MT, and Mmax ∼2 MT. Bike- and step-training shifted the Hmax value toward 1 MT. (B) Hmax occurrence expressed as a multiple of MT. (C) The Hmax:M ratio tends to increase with step-training, suggesting that Hmax occurs when M is of smaller amplitude than in untrained animals. (D) The Hmax:Mmax ratio tends to increase with exercise. (E) The slope of the recruitment curve is significantly steeper in step-trained animals than in untrained animals (MT, motor threshold; Mmax, maximal response amplitude for the M-wave; Hmax, maximal response amplitude for the H-wave).
The Hmax:Mmax ratio estimates the fraction of motoneurons recruited via the H-reflex relative to the activation of the entire motor pool. There was a tendency toward an increased ratio in exercised animals, with values of 0.546 for bike-trained animals, and 0.614 for step-trained animals, compared to 0.367 in the untrained group (Fig. 2D). Statistical tests were below the desired power because of the wide range of values displayed in the exercised groups. As illustrated in Figure 2A, exercised animals displayed inconsistent recruitment curves, with some of the step-trained animals even showing H-reflex amplitude larger than the M-wave amplitude (i.e., Hmax:Mmax > 1).
The slope of the recruitment curve is one of the determinants of the gain of the reflex pathway. It has been shown to be markedly reduced at 28 days after SCI, with values around 3.35, versus 6.79 for intact animals (Thompson et al., 1992). We observed an increase in slope after exercise, from 3.217 in untrained animals to 7.990 in bike-trained and 11.064 in step-trained animals. Only step-training significantly increased slope compared to untrained animals (p < 0.046). Together, the decrease in H-reflex initiation threshold and steeper slope of the recruitment curve in the step-trained group suggest an increase in reflex gain.
The modulation of the H-reflex is recovered after exercise
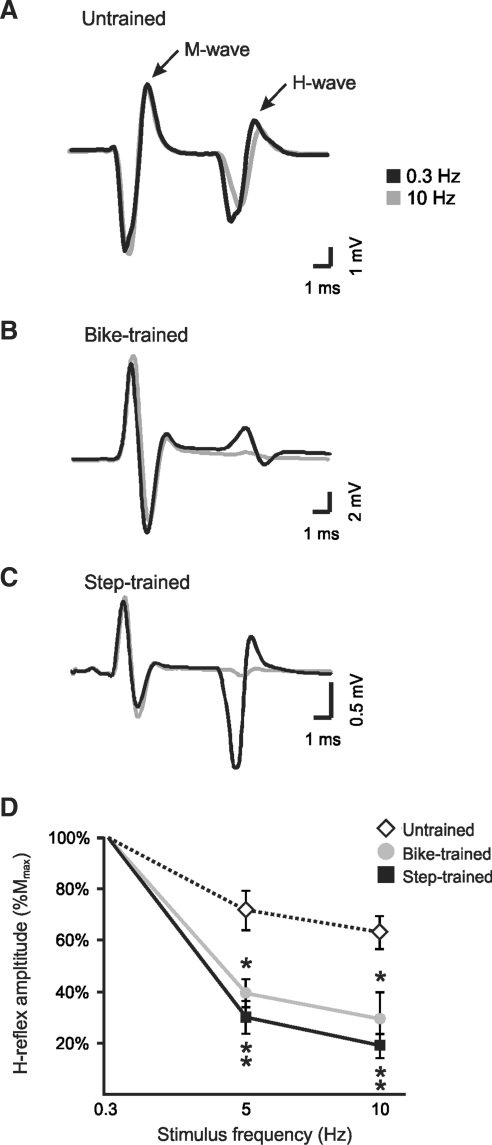
Figure 3 shows representative recordings of the H-reflex at 0.3 Hz and 10 Hz from individual untrained (A), bike-trained (B), and step-trained (C) animals. These recordings demonstrate that increasing the frequency of stimulation leads to a smaller decrease in H-reflex amplitude in untrained animals (78%), compared to bike-trained (18%) or step-trained (4%) animals. Figure 3D illustrates the H-reflex amplitude (normalized to Mmax) expressed as a percentage of the baseline value obtained at a frequency of 0.3 Hz. Therefore, the lower the value on the y axis, the greater the reflex depression, whereas higher y values indicate less depression of the H-reflex. As a group, exercised animals displayed a better modulation of the H-reflex both at 5 Hz and 10 Hz compared to the untrained group (Fig. 3D), going from 71% (untrained) at 5 Hz, to 39% in bike-trained and 29% in step-trained animals, and from 62% (untrained) at 10 Hz, to 29% in bike-trained and 18% in step-trained animals. A two-way ANOVA revealed statistically significant differences across stimulation frequency (p < 0.001), across experimental groups (p < 0.001), and for the interaction between frequency and group (p < 0.001). Post-hoc comparisons showed that 5-Hz and 10-Hz values were different than 0.3-Hz values in all groups, and that bike-trained and step-trained values were different from untrained animals, both at 5 Hz and 10 Hz, suggesting that exercise leads to restoration of H-reflex depression.
FIG. 3.
H-reflex frequency-dependent depression is recovered after 30 days of step-training and bike-training in spinal cord injury (SCI) animals. H-reflexes were evoked by the stimulation of the tibial nerve and recorded from the interosseus muscles. Representative averages of H-reflex recordings following a train of stimulation at 0.3 Hz (black), and 10 Hz (gray), in untrained (A), bike-trained (B), and step-trained (C) animals. There was a modest decrease in the average amplitude of the H-reflex at 10 Hz compared to 0.3 Hz in untrained animals, whereas this decrease was considerable in bike-trained and step-trained animals. Overall, the animals in the exercised groups showed a marked reduction of the H-reflex amplitude as the stimulation frequency increased (i.e., increased depression of the reflex), compared to the untrained group (D). There was a significant decrease in H-reflex amplitude in bike-trained and step-trained animals compared to untrained animals at both 5 Hz and 10 Hz (D; *p < 0.05; **p < 0.001).
Relationship between neurotrophic factor levels and H-reflex depression
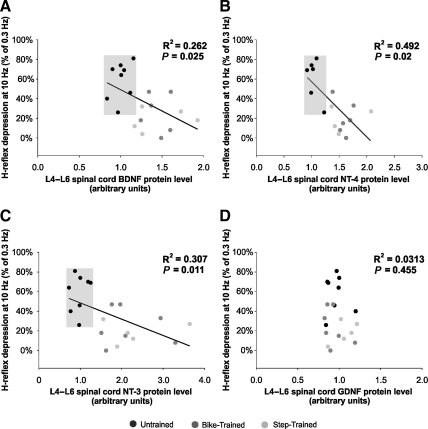
To determine if there was a relationship between neurotrophic factor levels and recovery of the H-reflex, BDNF, NT-3, NT-4, and GDNF protein levels for each animal were plotted against the individual amplitude of its H-reflex at 10 Hz (expressed as a percentage of the amplitude at 0.3 Hz). Linear regression analysis yielded a significant relationship between H-reflex frequency-dependent depression recovery and BDNF (p < 0.025, R2 = 0.262), NT-3 (p < 0.011, R2 = 0.307), and NT-4 protein levels (p < 0.002, R2 = 0.492), in the lumbar enlargement (Fig. 4). In addition, the untrained animals (gray boxes in Fig. 4) were linearly separable from the exercised groups, being segregated in the upper left corner (i.e., untrained animals showed low neurotrophic factor levels and little depression of the H-reflex). GDNF protein levels were not correlated with the depth of modulation of the H-reflex (Fig. 4D; p = 0.455, R2 = 0.0313). Frequency-dependent depression was low in untrained animals (high on the y axis), compared to exercised animals (low on the y axis), but the GDNF protein level was similar across all animals, (i.e., the values were clustered around 1 on the x axis).
FIG. 4.
Linear regression analysis between H-reflex depression and brain-derived neurotrophic factor (BDNF) (A), neurotrophin-4 (NT-4) (B), NT-3 (C), and glial cell-derived neurotrophic factor (GDNF) (D) protein levels in the L4–L6 spinal segments. There was a significant correlation between BDNF, NT-3, and NT-4 levels, and the depth of the H-reflex depression at 10 Hz (expressed as a percentage of 0.3 Hz). This correlation was not observed with GDNF. Untrained animals are segregated in the upper left quadrant, and are linearly separable from the exercised groups (gray boxes), for BDNF, NT-3, and NT-4, expressed as a function of H-reflex depression, but GDNF is not.
Discussion
Local reflexes are considered to play an important role after SCI (Pearson, 2001). The results of the present study demonstrate significant changes in a simple monosynaptic reflex pathway of the lumbar spinal cord induced by step- or bike-training after SCI. Different measures of H-reflex excitability were analyzed in animals with complete spinal cord transection that were either sedentary (untrained), step-trained, or bike-trained: reflex threshold, slope of the recruitment curve, maximal H-reflex:maximal M-response (Hmax:Mmax ratio), and frequency-dependent depression. We showed that step-training decreased the H-reflex threshold and facilitated the recruitment of the motoneuronal pool in response to afferent input. Also, both step- and bike-training restored the frequency-dependent depression of the H-reflex, and increased neurotrophic factor levels in the spinal cord. This indicates both a facilitation of the transmission in this reflex pathway, and a better modulation when repetitive stimuli were applied. More importantly, the increased level of neurotrophic factors in the lumbar enlargement was predictive of the recovery of spinal reflex modulation. Effects of exercise were not limited to regions of the spinal cord directly associated with hindlimb activity (i.e., a significant increase of neurotrophic factors was observed in the lower thoracic cord, and even rostral to the complete transection lesion).
A primary objective of activity-based rehabilitation is to provide optimal sensorimotor cues to activate spinal networks. Sensory information is critical to motor learning, as the pattern and timing of information that is provided plays a critical role in functional recovery (de Leon et al., 1998, 1999). Stepping and biking are repetitive and rhythmical movements that involve alternation between flexion and extension of the hip, knee, and ankle. Passive biking provides a steady and timed afferent flow of information to the spinal cord, and does not necessitate high levels of postural control, whereas stepping is incontestably a more complex task, that involves weight-bearing, foot placement, and constant control of posture and position of limb joints. Our passive biking model does not involve cycle-to-cycle variation in the kinematics and sensorimotor feedback as is the case for stepping. Interestingly, some variation in the pattern has been shown to be of crucial importance for the control and recovery of repetitive movements (Cai et al., 2006; Hausdorff, 2005; Ziegler et al., 2010).
Step-training facilitates transmission in the H-reflex pathway
The H-reflex is an objective measure of the physiological changes occurring after SCI. The threshold for H-reflex initiation has been shown to occur below MT in some animals and above MT in others (Hosoido et al., 2009). We showed that the proportion of animals in which an H-reflex was elicited below the motor threshold was increased in the step-trained group. In addition, step-training increased the initial slope of the recruitment curve. The relatively steep slope of the recruitment curve indicated that once the minimum reflex excitability level was reached, additional units were readily recruited with only a minimal increase in the relative amplitude of the afferent volley. The excitatory drive to a motor pool required to initiate a reflex discharge and the subsequent progression of recruitment depends on the distribution of excitability among the motoneurons of a given pool (Hunt, 1955; Kernell and Hultborn, 1990).
Because the amplitude of the H-reflex is proportional to the number of motoneurons activated, an increase in Hmax:Mmax ratio after exercise would likely reflect an amplified transmission from Ia afferents to motoneurons. We observed a tendency toward an increase in Hmax:Mmax ratio after bike-training or step-training, but it did not reach statistical significance, mainly because some of the animals displayed an Hmax of higher amplitude than Mmax, yielding great variability in the ratio. Variability in electrophysiological features in step-trained animals has been reported previously, and has been shown to correlate with success of recovery (Petruska et al., 2007). Together, these results suggest that transmission in this reflex pathway was facilitated, at least in a subset of animals, and that exercise impacts individuals to varying degrees.
Our observations indicate that step-training increased the lumbar cord reflex excitability, and this suggests an increase in the gain of this reflex pathway. An enhancement of group Ia afferent-evoked monosynaptic excitatory postsynaptic potentials (EPSPs) has been reported previously in step-trained transected rats. An EPSP of large amplitude was associated with successful step-training, whereas poor steppers displayed EPSPs of smaller amplitude (Petruska et al., 2007). Evidence suggests that the recovery of locomotor function is facilitated by an increase in hindlimb reflex activity, produced in part by enhanced segmental synaptic input via collateral sprouting of primary afferent terminals (Murray and Goldberger, 1974).
Restoration of frequency-dependent depression is not load-dependent
When the spinal cord is intact, the amplitude of the H-reflex decreases considerably with increasing stimulus frequency (Lee et al., 2005; Ollivier-Lanvin et al., 2010; Thompson et al., 1992). Since biological stimuli are usually transmitted as repetitive signals, the mechanism that modulates motor output relative to repetitive input is thought to shape reflex excitability (Koerber and Mendell, 1991). This ability to attenuate the response is impaired after complete or incomplete SCI in animals and humans (Boulenguez et al., 2010; Grey et al., 2008; Ollivier-Lanvin et al., 2010; Thompson et al., 1992). Previous studies have shown that passive exercise (i.e., cycling) helps to recover the frequency-dependent depression of the H-reflex in animals (Reese et al., 2006; Skinner et al., 1996) and humans (Kiser et al., 2005; Phadke et al., 2009) after SCI. Our results suggest that both passive bicycling and step-training induce robust effects on the modulation of reflex pathways independently of the loading component, timing of muscle activation, and cycle-to-cycle variation. The rhythmic motion elicited by exercise provides recurrent signaling that modifies hyper-reflexive circuitry to restore frequency-dependent depression of the H-reflex.
The load experienced by the hindlimbs during cycling and stepping was not quantified in this study. It is unknown how much load was experienced during passive cycling in our animals; however, the animal was suspended in a harness to prevent weight-bearing on the hindlimb muscles. Previous reports have shown that the soleus muscle is activated during flexion of the foot (Houlé et al., 1999). In humans, the soleus muscle, which is also activated during flexion when pedaling, has been shown to be much smaller during passive than during active cycling, which requires force generation (Boorman et al., 1992). In these conditions, it is unlikely that much loading occurs during our bicycle training paradigm.
We cannot rule out a contribution of perineal stimulation in causing changes on our two key outcome measures. However, we believe it is unlikely that it was responsible for the improvement in H-reflex depression and increase in neurotrophin levels, because similar results were observed in bike-trained animals, which did not receive perineal stimulation. This suggests that exercise triggers these changes and not afferent feedback induced by perineal stimulation.
Mechanisms underlying frequency-dependent depression
Frequency-dependent depression provides an estimation of the inhibitory influence that contributes to changes in reflex excitability during movement. Hence, under dynamic conditions, the H-reflex undergoes cyclic changes with a variety of rhythmic behaviors, such as stepping (Crenna and Frigo, 1987), running (Capaday and Stein, 1987), and pedaling (Brooke et al., 1992). With reflex modulation being phase-dependent and task-dependent (reviewed in Rossignol et al., 2006), future studies need to address and compare the modulation of spinal reflexes during the actual motor task (i.e., bicycling or stepping).
The similarity between the H-reflex recorded from the interosseous muscle, and the monosynaptic reflex depression recorded from the tibial nerve, indicates that hyperreflexia after SCI is likely due to mechanisms of central origin rather than an impairment of the neuromuscular junction (Thompson et al., 1992). Several explanations were investigated, including increased electrical coupling mediated by gap junctions (Yates et al., 2008a), reciprocal facilitation of antagonistic muscles (Xia and Rymer, 2005), decreased presynaptic inhibition (Hultborn et al., 1996; Reese et al., 2006; Schindler-Ivens and Shields, 2000; Thompson et al., 1992), and changes in the persistent inward currents (PICs; Bennett et al., 2001; Hornby et al., 2006). PICs are thought to be involved in the development of spasticity after SCI. Interestingly, the recovery of the H-reflex depression is associated with a decrease in spasticity (Kiser et al., 2005; Phadke et al., 2009).
Neurotrophin level is predictive of the depth of modulation of the H-reflex
Postsynaptic factors can also regulate the frequency of transmission in reflex pathways (Koerber and Mendell, 1991). SCI involves changes in cellular properties and synaptic relationships in spinal neurons (Mendell, 1984), which are modulated by exercise (Beaumont et al., 2004, 2008; Petruska et al., 2007). Individually, animals that displayed higher levels of BDNF, NT-3, and NT-4, also exhibited better modulation of spinal reflexes.
In an elegant series of experiments, Boulenguez and colleagues proposed a new mechanism that could explain the disinhibition caused by the lack of descending influence after SCI, in spite of the increased levels of inhibitory transmitters in the spinal cord (Boulenguez et al., 2010). Their results suggest a role for KCC2, a potassium chloride co-transporter, in the depth of modulation of the frequency-dependent depression of the H-reflex (Boulenguez et al., 2010). Acute exogenous administration of BDNF increases KCC2 expression and restores frequency-dependent depression (Boulenguez et al., 2010). Hence, signaling pathways relating BDNF, TrkB receptors, and KCC2, have been described (Rivera et al., 2002, 2004). It is well established that exercise increases BDNF protein and mRNA levels in the spinal cord after SCI (Ying et al., 2005, 2008), and that blocking BDNF prevents favorable training effects (Gomez-Pinilla et al., 2007). Activity-dependent processes triggered by exercise include an increase in the expression of neurotrophic factors in the spinal cord that are associated with spinal learning and motor recovery after SCI. The correlation between BDNF, NT-3, and NT-4 protein levels, and the amount of recovery of the H-reflex depression evoked by repetitive stimuli, suggests a possible common pathway in support of the Vinay hypothesis.
Unlike BDNF, NT-3, and NT-4, which showed a general upregulation in the lumbar cord with exercise, GDNF was upregulated primarily in spinal segments rostral to the injury. This increase could influence trunk stability, and also stimulate regeneration above the injury level. In addition, only step-training upregulated GDNF protein levels in the lumbar enlargement. After SCI, the GDNF protein level is downregulated in the segment caudal to the lesion, suggesting a potential role in physiological and pathological conditions (Zhou et al., 2008). Delivery of exogenous GDNF can promote locomotor recovery (Cao et al., 2004; Hashimoto et al., 2005). A transient increase in GDNF expression at the lumbar level was previously described following passive exercise, but returned to untrained values within 30 days (Keeler et al., 2009), suggesting that the time course of GDNF modulation may be different for bike-training and step-training.
Conclusions
Our results suggest that both step-training and bike-training induce remarkable spinal cord plasticity that includes an increase in neurotrophic factor levels and a concomitant improvement in H-reflex depression. Neurotrophic factors induce spinal plasticity through synaptic rearrangement, but also act as chemo-attractants for elongating axons. Further studies are needed to test whether this local increase in neurotrophic factors can promote axonal outgrowth across a spinal lesion, thereby increasing the formation of new synaptic contacts with host neurons to further improve modulation of spinal reflexes. Future studies should also address the long-term effect of combined activity-based therapies and their association with functional recovery.
Supplementary Material
Acknowledgments
We thank Kassi Miller for performing bike-training, Drs. Jed Shumsky and Corey Hart for help with statistical analysis, and Dr. Jeffrey Petruska for suggestions made during the data analysis process. This study was supported by National Institutes of Health grant NS 055976 (to J.D.H.).
Author Disclosure Statement
No competing financial interests exist.
References
- Beaumont E. Houlé J.D. Peterson C.A. Gardiner P.F. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- Beaumont E. Kaloustian S. Rousseau G. Cormery B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci. Res. 2008;62:147–154. doi: 10.1016/j.neures.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Bennett D.J. Li Y. Harvey P.J. Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J. Neurophysiol. 2001;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Boorman G. Becker W.J. Morrice B.L. Lee R.G. Modulation of the soleus H-reflex during pedalling in normal humans and in patients with spinal spasticity. J. Neurol. Neurosurg. Psychiatry. 1992;55:1150–1156. doi: 10.1136/jnnp.55.12.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P. Liabeuf S. Bos R. Bras H. Jean-Xavier C. Brocard C. Stil A. Darbon P. Cattaert D. Delpire E. Marsala M. Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Boyce V.S. Tumolo M. Fischer I. Murray M. Lemay M.A. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J. Neurophysiol. 2007;98:1988–1996. doi: 10.1152/jn.00391.2007. [DOI] [PubMed] [Google Scholar]
- Bregman B.S. Coumans J.-V. Dai H.N. Kuhn P.L. Lynskey J. McAtee M. Sandhu F. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. In: L. McKerracher., editor; G. Doucet., editor; S. Rossignol., editor. Progress in Brain Research. Elsevier; Montreal: 2002. pp. 258–273. [DOI] [PubMed] [Google Scholar]
- Brooke J.D. McIlroy W.E. Collins D.F. Movement features and H-reflex modulation. I. Pedalling versus matched controls. Brain Res. 1992;582:78–84. doi: 10.1016/0006-8993(92)90319-5. [DOI] [PubMed] [Google Scholar]
- Cai L.L. Courtine G. Fong A.J. Burdick J.W. Roy R.R. Edgerton V.R. Plasticity of functional connectivity in the adult spinal cord. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1635–1646. doi: 10.1098/rstb.2006.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L. Liu L. Chen Z.Y. Wang L.M. Ye J.L. Qiu H.Y. Lu C.L. He C. Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain. 2004;127:535–549. doi: 10.1093/brain/awh072. [DOI] [PubMed] [Google Scholar]
- Capaday C. Stein R.B. Difference in the amplitude of the human soleus H reflex during walking and running. J. Physiol. 1987;392:513–522. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenna P. Frigo C. Excitability of the soleus H-reflex arc during walking and stepping in man. Exp. Brain Res. 1987;66:49–60. doi: 10.1007/BF00236201. [DOI] [PubMed] [Google Scholar]
- de Leon R.D. Hodgson J.A. Roy R.R. Edgerton V.R. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- de Leon R.D. Tamaki H. Hodgson J.A. Roy R.R. Edgerton V.R. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J. Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- Dolbeare D. Houlé J.D. Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF) J. Neurotrauma. 2003;20:1251–1261. doi: 10.1089/089771503770802916. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden E.E. Houlé J.D. Dennis R.A. Zhang J. Knox M. Wagoner G. Peterson C.A. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- Edgerton V.R. de Leon R.D. Tillakaratne N. Recktenwald M.R. Hodgson J.A. Roy R.R. Use-dependent plasticity in spinal stepping and standing. Adv. Neurol. 1997;72:233–247. [PubMed] [Google Scholar]
- Etlin A. Blivis D. Ben-Zwi M. Lev-Tov A. Long and short multifunicular projections of sacral neurons are activated by sensory input to produce locomotor activity in the absence of supraspinal control. J. Neurosci. 2010;30:10324–10336. doi: 10.1523/JNEUROSCI.1208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote E.C. Brown K.M. Lindley S.D. Influence of posture and stimulus parameters on post-activation depression of the soleus H-reflex in individuals with chronic spinal cord injury. Neurosci. Lett. 2006;410:37–41. doi: 10.1016/j.neulet.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortun J. Puzis R. Pearse D.D. Gage F.H. Bunge M.B. Muscle injection of AAV-NT3 promotes anatomical reorganization of CST axons and improves behavioral outcome following SCI. J. Neurotrauma. 2009;26:941–953. doi: 10.1089/neu.2008.0807. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Huie J.R. Ying Z. Ferguson A.R. Crown E.D. Baumbauer K.M. Edgerton V.R. Grau J.W. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148:893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Ying Z. Roy R.R. Hodgson J. Edgerton V.R. Afferent input modulates neurotrophins and synaptic plasticity in the spinal cord. J. Neurophysiol. 2004;92:3423–3432. doi: 10.1152/jn.00432.2004. [DOI] [PubMed] [Google Scholar]
- Grey M.J. Klinge K. Crone C. Lorentzen J. Biering-Sorensen F. Ravnborg M. Nielsen J.B. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp. Brain Res. 2008;185:189–197. doi: 10.1007/s00221-007-1142-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto M. Nitta A. Fukumitsu H. Nomoto H. Shen L. Furukawa S. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport. 2005;16:99–102. doi: 10.1097/00001756-200502080-00004. [DOI] [PubMed] [Google Scholar]
- Hausdorff J.M. Gait variability: methods, modeling and meaning. J. Neuroeng. Rehabil. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby T.G. Kahn J.H. Wu M. Schmit B.D. Temporal facilitation of spastic stretch reflexes following human spinal cord injury. J. Physiol. 2006;571:593–604. doi: 10.1113/jphysiol.2005.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoido T. Motoyama S. Goto M. Mori F. Tajima T. Hirata H. Wada N. Characteristics of H- and M-waves recorded from rat forelimbs. Neurosci. Lett. 2009;450:239–241. doi: 10.1016/j.neulet.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Houlé J.D. Morris K. Skinner R.D. Garcia-Rill E. Peterson C.A. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve. 1999;22:846–856. doi: 10.1002/(sici)1097-4598(199907)22:7<846::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Illert M. Nielsen J. Paul A. Ballegaard M. Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp. Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hunt C.C. Monosynaptic reflex response of spinal motoneurons to graded afferent stimulation. J. Gen. Physiol. 1955;38:813–852. doi: 10.1085/jgp.38.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson K.J. Gomez-Pinilla F. Crowe M.J. Ying Z. Basso D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Ichiyama R.M. Courtine G. Gerasimenko Y.P. Yang G.J. van den Brand R. Lavrov I.A. Zhong H. Roy R.R. Edgerton V.R. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J. Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakeman L.B. Wei P. Guan Z. Stokes B.T. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp. Neurol. 1998;154:170–184. doi: 10.1006/exnr.1998.6924. [DOI] [PubMed] [Google Scholar]
- Keeler B.E. Siegfried R.N. Liu G. Miller K.N. Santi L. Houlé J.D. 563.5 Neuroscience Meeting Planner. Chicago: Society for Neuroscience; 2009. Changes in gene expression are maintained over short and long periods of cycling exercise (Ex) after spinal cord injury (SCI) Program No. Online. [Google Scholar]
- Kernell D. Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Kiser T.S. Reese N.B. Maresh T. Hearn S. Yates C. Skinner R.D. Pait T.G. Garcia-Rill E. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J. Spinal Cord Med. 2005;28:241–245. doi: 10.1080/10790268.2005.11753818. [DOI] [PubMed] [Google Scholar]
- Knikou M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin. Neurophysiol. 2010;121:1655–1668. doi: 10.1016/j.clinph.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Koerber H.R. Mendell L.M. Modulation of synaptic transmission at Ia-afferent fiber connections on motoneurons during high-frequency stimulation: role of postsynaptic target. J. Neurophysiol. 1991;65:590–597. doi: 10.1152/jn.1991.65.3.590. [DOI] [PubMed] [Google Scholar]
- Lee J.K. Emch G.S. Johnson C.S. Wrathall J.R. Effect of spinal cord injury severity on alterations of the H-reflex. Exp. Neurol. 2005;196:430–440. doi: 10.1016/j.expneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Lee J.K. Johnson C.S. Wrathall J.R. Up-regulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp. Neurol. 2007;203:502–511. doi: 10.1016/j.expneurol.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D.P. Monosynaptic reflex response of individual motoneurons as a function of frequency. J. Gen. Physiol. 1957;40:435–450. doi: 10.1085/jgp.40.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckman S.M. Dyball R.E. Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J. Neurosci. 1994;14:4825–4830. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.H. Zhang Y. Cao L. Su J.C. Wang Z.W. Xu A.B. Zhang S.C. Effect of neurotrophin-3 genetically modified olfactory ensheathing cells transplantation on spinal cord injury. Cell Transplant. 2010;19:167–177. doi: 10.3727/096368910X492634. [DOI] [PubMed] [Google Scholar]
- Mendell L.M. Arvanian V.L. Diversity of neurotrophin action in the postnatal spinal cord. Brain Res. Brain Res. Rev. 2002;40:230–239. doi: 10.1016/s0165-0173(02)00205-9. [DOI] [PubMed] [Google Scholar]
- Mendell L.M. Modifiability of spinal synapses. Physiol. Rev. 1984;64:260–324. doi: 10.1152/physrev.1984.64.1.260. [DOI] [PubMed] [Google Scholar]
- Mendell L.M. Munson J.B. Arvanian V.L. Neurotrophins and synaptic plasticity in the mammalian spinal cord. J. Physiol. 2001;533:91–97. doi: 10.1111/j.1469-7793.2001.0091b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. Goldberger M.E. Restitution of function and collateral sprouting in the cat spinal cord: the partially hemisected animal. J. Comp. Neurol. 1974;158:19–36. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- Nielsen J.B. Crone C. Hultborn H. The spinal pathophysiology of spasticity—from a basic science point of view. Acta Physiol. (Oxf.) 2007;189:171–180. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Novikova L.N. Novikov L.N. Kellerth J.O. Differential effects of neurotrophins on neuronal survival and axonal regeneration after spinal cord injury in adult rats. J. Comp. Neurol. 2002;452:255–263. doi: 10.1002/cne.10381. [DOI] [PubMed] [Google Scholar]
- Novikova L.N. Novikov L.N. Kellerth J.O. Survival effects of BDNF and NT-3 on axotomized rubrospinal neurons depend on the temporal pattern of neurotrophin administration. Eur. J. Neurosci. 2000;12:776–780. doi: 10.1046/j.1460-9568.2000.00978.x. [DOI] [PubMed] [Google Scholar]
- Ollivier-Lanvin K. Keeler B.E. Siegfried R. Houlé J.D. Lemay M.A. Proprioceptive neuropathy affects normalization of the H-reflex by exercise after spinal cord injury. Exp. Neurol. 2010;221:198–205. doi: 10.1016/j.expneurol.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K.G. Could enhanced reflex function contribute to improving locomotion after spinal cord repair? J. Physiol. 2001;533:75–81. doi: 10.1111/j.1469-7793.2001.0075b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska J.C. Ichiyama R.M. Jindrich D.L. Crown E.D. Tansey K.E. Roy R.R. Edgerton V.R. Mendell L.M. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J. Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke C.P. Flynn S.M. Thompson F.J. Behrman A.L. Trimble M.H. Kukulka C.G. Comparison of single bout effects of bicycle training versus locomotor training on paired reflex depression of the soleus H-reflex after motor incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 2009;90:1218–1228. doi: 10.1016/j.apmr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Reese N.B. Skinner R.D. Mitchell D. Yates C. Barnes C.N. Kiser T.S. Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- Rivera C. Li H. Thomas-Crusells J. Lahtinen H. Viitanen T. Nanobashvili A. Kokaia Z. Airaksinen M.S. Voipio J. Kaila K. Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C. Voipio J. Thomas-Crusells J. Li H. Emri Z. Sipila S. Payne J.A. Minichiello L. Saarma M. Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas Vega S. Abel T. Lindschulten R. Hollmann W. Bloch W. Strüder H.K. Impact of exercise on neuroplasticity-related proteins in spinal cord injured humans. Neuroscience. 2008;153:1064–1070. doi: 10.1016/j.neuroscience.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Dubuc R. Gossard J.-P. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S. Shields R.K. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp. Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebach B.S. Arvanov V. Mendell L.M. Effects of BDNF and NT-3 on development of Ia/motoneuron functional connectivity in neonatal rats. J. Neurophysiol. 1999;81:2398–2405. doi: 10.1152/jn.1999.81.5.2398. [DOI] [PubMed] [Google Scholar]
- Skinner R.D. Houlé J.D. Reese N.B. Berry C.L. Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- Skup M. Dwornik A. Macias M. Sulejczak D. Wiater M. Czarkowska-Bauch J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp. Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- Thompson F.J. Reier P.J. Lucas C.C. Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J. Neurophysiol. 1992;68:1473–1486. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- van den Berg-Emons R. Bussmann J.B. Haisma J.A. Sluis T.A. van der Woude L.H. Bergen M.P. Stam H.J. A prospective study on physical activity levels after spinal cord injury during inpatient rehabilitation and the year after discharge. Arch. Phys. Med. Rehabil. 2008;89:2094–2101. doi: 10.1016/j.apmr.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Xia R. Rymer W.Z. Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord. 2005;43:14–21. doi: 10.1038/sj.sc.3101656. [DOI] [PubMed] [Google Scholar]
- Yates C.C. Charlesworth A. Reese N.B. Skinner R.D. Garcia-Rill E. The effects of passive exercise therapy initiated prior to or after the development of hyperreflexia following spinal transection. Exp. Neurol. 2008b;213:405–409. doi: 10.1016/j.expneurol.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates C. Charlesworth A. Allen S.R. Reese N.B. Skinner R.D. Garcia-Rill E. The onset of hyperreflexia in the rat following complete spinal cord transection. Spinal Cord. 2008a;46:798–803. doi: 10.1038/sc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.H. Houlé J.D. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp. Neurol. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- Ying Z. Roy R.R. Edgerton V.R. Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Ying Z. Roy R.R. Zhong H. Zdunowski S. Edgerton V.R. Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.L. Yang H.J. Li Y.M. Wang Y. Yan L. Guo X.L. Ba Y.C. Liu S. Wang T.H. Changes in Glial cell line-derived neurotrophic factor expression in the rostral and caudal stumps of the transected adult rat spinal cord. Neurochem. Res. 2008;33:927–937. doi: 10.1007/s11064-007-9536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M.D. Zhong H. Roy R.R. Edgerton V.R. Why variability facilitates spinal learning. J. Neurosci. 2010;30:10720–10726. doi: 10.1523/JNEUROSCI.1938-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.