Abstract
Recognizing and managing the effects of cerebral concussion is very challenging, given the discrete symptomatology. Most individuals with sports-related concussion will not score below 15 on the Glasgow Coma Scale, but will present with rapid onset of short-lived neurological impairment, demonstrating no structural changes on traditional magnetic resonance imaging (MRI) and computed tomography (CT) scans. The return-to-play decision is one of the most difficult responsibilities facing the physician, and so far this decision has been primarily based on neurological examination, symptom checklists, and neuropsychological (NP) testing. Diffusion tensor imaging (DTI) may be a more objective tool to assess the severity and recovery of function after concussion. We assessed white matter (WM) fiber tract integrity in varsity level college athletes with sports-related concussion without loss of consciousness, who experienced protracted symptoms for at least 1 month after injury. Evaluation of fractional anisotropy (FA) and mean diffusivity (MD) of the WM skeleton using tract-based spatial statistics (TBSS) revealed a large cluster of significantly increased MD for concussed subjects in several WM fiber tracts in the left hemisphere, including parts of the inferior/superior longitudinal and fronto-occipital fasciculi, the retrolenticular part of the internal capsule, and posterior thalamic and acoustic radiations. Qualitative comparison of average FA and MD suggests that with increasing level of injury severity (ranging from sports-related concussion to severe traumatic brain injury), MD might be more sensitive at detecting mild injury, whereas FA captures more severe injuries. In conclusion, the TBSS analysis used to evaluate diffuse axonal injury of the WM skeleton seems sensitive enough to detect structural changes in sports-related concussion.
Key words: diffuse axonal injury, diffusion tensor imaging, mean diffusivity, mild traumatic brain injury, sports-related concussion
Introduction
Cerebral concussion is the most common type of traumatic brain injury (TBI). The terms mild traumatic brain injury (mTBI), minor head trauma, and concussion, are often used interchangeably, since the neuropathological and neurobehavioral consequences are comparable. The definition of concussion has been narrowed over the past decades and has been primarily used in sports medicine, whereas the term mTBI is used predominantly in general medical contexts. The leading causes of TBI are varied, and include falls, motor vehicle accidents, and violence. Sports activities are a major cause of mTBI, and a study by the Centers for Disease Control and Prevention estimated that 300,000 sports-related concussions occur annually in the United States (Thurman et al., 1998). However, this study only included concussions for which the person reported a loss of consciousness (LOC), which has been reported to occur in only 8% (Schulz et al., 2004) and 19.2% (Collins et al., 2003) of sports-related concussions. Sports-related concussions are often unreported, either due to an athlete wanting to minimize the importance of the injury and not be taken out of play, or because the athlete doesn't realize the symptoms are consistent with concussion (McCrea et al., 2004). Given the fact that athletes tend not to report their injury, a more accurate approximation may be that 1.6 to 3.8 million sports-related concussions occur each year, which would also include concussions for which no medical treatment is sought (Langlois et al., 2006).
In recent years, sports-related concussions have received increasing attention from the scientific community and mass media. At the same time, the medical knowledge and understanding of concussion has deepened, and its definition was modified during the first international conference on concussion held in Vienna (Aubry et al., 2002). This definition, which we used in the current study, states that “concussion is defined as a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces,” that may or may not involve loss of consciousness (Aubry et al., 2002). The hallmark of concussion is confusion. Other common symptoms include headache, dizziness, nausea, memory dysfunction, difficulties with balance, visual and auditory disturbances, and increased emotional instability.
The assessment and management of return-to-play decisions are among the most difficult responsibilities facing the team physician, and to date this decision has been primarily based on the evaluation of symptoms and the neurological examination, including both cognitive function and balance. Immediately after injury, abbreviated sideline tools have been used to evaluate sports-related concussion (e.g., Standardized Assessment of Concussion [SAC], Standardized Concussion Assessment Tool [SCAT and SCAT2]; McCrea et al., 1998; McCrory et al., 2005, 2009a). During both the acute and recovery phases of sports-related concussion, formal neuropsychological (NP) testing has proven to be a useful tool for the assessment of cognitive function. NP testing can include standard paper and pencil testing, and more recently, abbreviated computerized batteries (e.g., Headminder, CogSport, and Immediate Post-Concussion Assessment and Cognitive Testing [ImPACT]; Collie et al., 2003; Erlanger et al., 2002; Lovell et al., 2007b). The use of baseline NP testing has become more widespread for varsity-level athletes participating in contact sports at both the high school and university levels.
Several recent guidelines for the management of sports-related concussion (Aubry et al., 2002; Cantu, 2001; Guskiewicz et al., 2004; Herring et al., 2005; McCrory et al., 2005, 2009b) have established that athletes presenting with symptoms at rest or exertion should not be allowed to return to play, and that young athletes should be treated more conservatively. In addition, a step-wise gradual progression in activity level and risk of contact should be implemented when allowing an athlete to return to activity. However, there are no specific guidelines to determine whether an athlete with transient and minimal symptoms should be allowed back into play during the same game, or how quickly a return-to-play progression should occur. The appropriate timing for return to play is also of particular importance with regard to the risk of recurrent concussions. Several studies have demonstrated that athletes who have sustained a first concussion are at higher risk of suffering future concussions (Guskiewicz et al., 2003; Zemper, 2003). Cumulative cognitive effects and slowed neurological function have also been reported by Gronwall and Wrightson (1975), and Guskiewicz and associates (2003). Moreover, there is evidence of increased risk for developing mild cognitive impairment, Alzheimer's disease, depression, and suicidality, in football players who sustained repetitive head injuries earlier in life (Guskiewicz et al., 2005, 2007a). Given the current lack of clarity concerning the long-term effects of concussion on cognitive function, and the complexities surrounding the decision for return to play, it seems imperative to gain a deeper understanding of the structural and functional neural mechanisms involved during concussion.
It is widely recognized that regular magnetic resonance imaging (MRI) and computed tomography (CT) scans are not sensitive enough to capture changes in the microstructure of the white matter of the brain that are a consequence of diffuse axonal injury (DAI; McCrory et al, 2009b). Diffusion tensor imaging (DTI), however, offers other advanced MR measures, which are more sensitive to focal ischemic lesions and to diffuse axonal damage (Horsfield et al., 1998). DTI provides information about the white matter (WM) microstructure and fiber tract integrity by measuring the brownian motion of water molecules in the brain (Basser and Jones, 2002; Johansen-Berg and Rushworth, 2009; Le Bihan et al., 2001). Directional information of the neural tracts is derived from the imaging data using at least six diffusion gradient directions, which are sufficient to compute the diffusion tensor. WM fiber tract integrity is assessed using the calculation of fractional anisotropy (FA; Pierpaoli et al., 1996), which quantifies the degree of a preferred diffusion direction in each voxel, and hence informs about fiber directionality (Pierpaoli et al., 1996). Overall diffusion in a tissue is characterized by mean diffusivity (MD), which is calculated as the mean of the three eigenvalues of the diffusion tensor (Mori, 2007). MD measures the overall, non-directional mobility of water molecules in the brain tissue.
Previous DTI studies of individuals with mTBI and persistent cognitive or neurobehavioral impairments demonstrate decreases in WM fiber tract integrity through measurements of FA (Niogi et al., 2008a), and/or MD (Lipton et al., 2008; Messé et al., 2010). Another study assessing microstructural integrity of WM (measured through FA) in normal control subjects and mTBI patients revealed significant correlations between attentional control and FA within the left anterior corona radiate, as well as memory performance and FA within the uncinate fasciculus (Niogi et al., 2008b). It should, however, be emphasized that so far all DTI studies using FA and MD measurements involved individuals with mTBI scoring in the 13–15 range on the Glasgow Coma Scale (GCS). Individuals with sports-related concussion will typically not score below 15 on the GCS, but will present with a rapid onset of short-lived neurological impairment involving a diverse set of symptoms, which resolve in many cases spontaneously.
Significant differences in brain activation patterns in individuals with mTBI and persistent symptoms have also been reported in functional MRI studies (Chen et al., 2004, 2008; McAllister et al., 2001; Ptito et al., 2007). Recent evidence suggests that abnormal brain activation patterns persist for months after injury, despite testing within the normal range during neurocognitive task performance (Chen et al., 2004; Lovell et al., 2007a; McAllister et al., 1999). This discrepancy may be due to a phase of early functional recovery driven by compensatory mechanisms in the brain (re-allocation of neurocognitive resources), followed by a prolonged neuronal recovery, which currently available clinical assessments cannot capture. Based on these findings, DTI may be a unique imaging marker to assess the severity of a concussion, and to predict recovery in individuals with mTBI. Furthermore, DTI may be a critical tool in refining the diagnosis, prognosis, and management of mTBI.
In order to assess WM fiber tract integrity with DTI during a later phase of neuronal recovery, we investigated a group of varsity level athletes with protracted symptoms. These athletes tested neuropsychologically in the abnormal range immediately after injury, and continued to present with symptoms for at least 1 month. In this study we test the hypothesis that DTI is sensitive enough to identify disruptions of WM fiber tract integrity in this group of individuals who sustained a sports-related concussion without LOC.
We believe the results of this study and others based on its findings will provide an imaging method capable of offering a more objective diagnostic tool for the assessment of sports-related concussion.
Methods
Subjects
Participants in this study included 10 college students who sustained a concussion (5 male, 5 female; mean age 19.7 years, SD 1.6 years), of which 9 were sports-related concussions (8 contact and 1 non-contact sports). All athletes were diagnosed with mTBI in the form of a concussion presenting with serious and prolonged symptoms. Since grading systems for concussion are infrequently used based on the publication of the International Consensus Agreements in 2001, severity of injury is often determined through the evaluation of nature, burden, and duration of symptoms, with symptoms such as amnesia and prolonged confusion being more severe. For the one subject who sustained a non-sports-related concussion, the injury occurred during a fall on the sidewalk when the subject's head hit the ground. Severity, symptomatology, and medical diagnosis of concussion were all consistent with those of the sports-related concussion subjects, thus warranting inclusion in this study. The majority of the subjects had a prior history of concussion (mean 3, SD 1.4); however, they had no prior history of any other diagnosed medical, genetic, or psychiatric disorder. Subjects with sports-related concussions were all participating in intercollegiate athletics, and were initially enrolled in the Princeton University Concussion Program as part of a “high-risk sport,” with the exception of two athletes who were either non-varsity level or in a lower-risk sport. The Princeton University Concussion Program entails baseline testing of high-risk sports for concussion using computerized neuropsychological tests (ImPACT). In addition, post-injury testing includes sideline assessment (SCAT and SCAT2), and hybrid neuropsychological tests (ImPACT and paper/pencil tests), that are interpreted by an outside consulting neuropsychologist. After their most recent sports-related concussion, subjects included in this study were evaluated by a certified athletic trainer and team physician. None of the subjects demonstrated symptomatology warranting further assessment by GCS (Teasdale and Jennett, 1974), or the use of any other diagnostic tool assessing LOC. Upon further evaluation by the team physician, several participants had a CT or MRI scan to rule out more severe injury (e.g., skull fracture or intracranial bleed), based on the presence of persisting symptoms, but results of these tests were within normal limits. However, all subjects tested abnormally on paper/pencil and computerized ImPACT tests (Lovell et al., 2007b) administered immediately after injury (within 24–48 h). Following concussion, individualized management and return-to-play decisions are made by the team physician. Typically, athletes are kept out of activity until their symptoms resolve and their balance and NP testing return to pre-injury levels, at which time they are allowed to slowly resume an exertional program that gradually introduces contact activities. The athletes in this study, however, were recruited at least 1 month post-injury due to report of persistent symptoms, despite the fact that performance on NP paper/pencil and ImPACT tests returned to normal for 3 subjects, and remained abnormal for 6 subjects, at the time of the MRI scan (at least 1 month post-injury; mean 115 days, SD 104 days, for the sports-related concussion subjects). Healthy control subjects included 10 sex- and age-matched athletes (5 male, 5 female; mean age 20.4 years, SD 1.8 years; 6 contact and 4 non-contact sports), who were in good physical condition with no prior history of concussion or head trauma, and no history of any psychiatric, neurological, or developmental disorder. Detailed concussion and control subject demographics are presented in Table 1.
Table 1.
Concussion and Control Subject Demographics, including Sex, Age, Sport, Number of Previous Concussions, and Symptoms Reported Immediately after Injury, and at Diffusion Tensor Imaging (DTI) Scan
|
Concussion subjects |
Control subjects |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Age | Sport | No. of concussions | Initial symptoms At injury | Persistent symptoms At DTI scan | Age | Sport |
| 1 | M | 21 | Football | 5 confirmed | Headache | Headache | 23 | Crew team |
| 2 | F | 18 | Hockey | 5 confirmed | Headache, dizziness, phonophobia, feeling slowed down, balance problems, difficulty concentrating and remembering | Headache, phonophobia, dizziness, balance problems, memory deficits, difficulty concentrating, drowsiness | 20 | Softball |
| 3 | M | 21 | Football | 2 confirmed 2 unconfirmed |
Headache, sleeping more than usual, drowsiness, irritability, nervousness, feeling more emotional, feeling mentally foggy, difficulty concentrating | Headache, dizziness, mentally foggy, difficulty concentrating | 21 | Football |
| 4 | F | 19 | Pole vaulter | 3 confirmed | Headache, balance problems, dizziness, fatigue, trouble falling asleep, sleeping less than usual, drowsiness, photophobia, feeling slowed down, mentally foggy, difficulty concentrating, visual problems | Headache, phonophobia, absentmindedness, difficulty concentrating and remembering, irritability | 19 | Cross country,* track,* figure skating* |
| 5 | M | 20 | Lacrosse | 3 confirmed | Headache, dizziness, fatigue, trouble falling asleep, sleeping more than usual, drowsiness, photophobia, mentally foggy, difficulty concentrating and remembering | Headache, verbal memory slowness | 22 | Sprint football |
| 6 | F | 19 | Rugby | 3 confirmed | Visual deficits, minimal confusion, headache, dizziness, nausea, “not feeling right” | Headache, photophobia, difficulty concentrating, working memory deficits, dizziness, phonophobia | 22 | Field hockey,* lacrosse,* squash* |
| 7 | M | 21 | Wrestling | 1 confirmed 1 unconfirmed | Headache, nausea, dizziness, fatigue, sleeping more than usual, feeling slowed down, mentally foggy, difficulty concentrating and remembering | Exertion-related symptoms (headache, dizziness, mentally foggy, difficulty concentrating, short-term memory problems, disturbed sleeping patterns) | 18 | Wrestling |
| 8 | F | 17 | Soccer | 3 confirmed | Headache, nausea, dizziness, fatigue, sleeping more than usual, drowsiness, photophobia, phonophobia, feeling slowed down, feeling mentally foggy, difficulty concentrating, visual problems | Pressure headaches, dizziness, nausea, dizziness, feeling slowed down, sleeping more than usual, trouble falling asleep, drowsiness, fatigue, more emotional, photophobia, phonophobia, mentally foggy, difficulty concentrating and remembering | 18 | Soccer |
| 9 | F | 19 | Soccer | 1 confirmed | Headache, dizziness, fatigue, trouble falling asleep, sleeping less than usual, drowsiness, photophobia, phonophobia, feeling slowed down, mentally foggy, difficulty concentrating and remembering | Headache | 19 | Soccer |
| 10 | M | 22 | N/A | 1 confirmed | Hypersensitivity to taste and smell, headache, balance problems, feeling slowed down | Headache | 22 | Cross country, track |
| Mean | 19.7 | 3 | 20.4 | |||||
Sports designated with an asterisk were played in high school. All other sports listed are collegiate sports.
In addition to these subjects, several moderate-to-severe TBI subjects were included in this study for comparison of DTI data with increasing injury severity. These participants included 2 moderate TBI subjects with sex- and age-matched (±2 years) controls, and 3 severe TBI subjects with sex- and age-matched (±3 years) controls. At the time of the MRI scan, all moderate and severe TBI subjects were at least 1 year post-injury. Moderate TBI was defined as a score between 9 and 12 on the first available GCS (Teasdale and Jennett, 1974), whereas severe TBI corresponded to a score of 8 or less. Similarly to the concussed/control groups, the moderate TBI/control subjects were of college age (moderate TBI ages: 21 and 19 years old; control ages: 23 and 21 years). Clinical MRI scans for the two moderate TBI subjects demonstrated the following injury characteristics: subject 1 was subjected to a motor vehicle accident (MVA), and showed hemorrhagic foci in right frontal and temporal lobe and small foci within the grey-white matter junction of the parietal and occipital lobes and basal ganglia, suggestive of axonal injury; subject 2 (MVA accident) demonstrated a cerebral contusion in the right frontal lobe.
The severe TBI/control subject pairs were on average about 27 years older (severe TBI ages: 45, 46, and 49 years; control ages: 42, 45, and 51 years). Clinical MRI scans for the 3 severe TBI subjects demonstrated the following injury characteristics: subject 1 (tree-logging accident), bifrontal infarction (right > left); subject 2 (MVA), bilateral frontal and temporal contusions, mild enlargement of the ventricles; subject 3 (MVA), left frontal and right temporal contusions.
All participants (and legal guardians where applicable) gave written consent/assent to participate in the study, which was approved by the Princeton University Institutional Review Panel for Human Subjects Research.
Data acquisition and imaging parameters
MR images were acquired on a Siemens Allegra 3.0 T head-only MRI machine with a circularly polarized head volume coil. High-resolution T1-weighted structural images were obtained using a gradient echo pulse sequence (MP-RAGE parameters: TR/TE/TI = 2.5 sec/4.38 msec/1.1 sec, matrix size 160 × 256 × 256 interpolated to 160 × 512 × 512, giving a final voxel size of 1 × 0.5 × 0.5 mm3). Diffusion-weighted images were acquired using an eddy-current compensated double spin-echo, echo-planar pulse sequence adapted from Reese and associates (2003). Diffusion encoding vectors were uniformly distributed through space using an electrostatic model (Jones et al., 1999). Vectors were rotated as a group to optimally use the existing gradient performance, which enables acquisition with uniformly distributed diffusion encoding directions that are greater than the performance of any individual gradient encoding axis. Diffusion scanning parameters were as follows: TR/TE = 10 sec/95 msec, matrix size 96 × 128 in plane (6/8 ppf zero-filled to 128 × 128), 66 axial slices, voxel size 1.71875 × 1.71875 mm in plane, slice thickness 2.5 mm. Diffusion images were acquired using 60 distinct gradient directions and a b-value of 1000 sec/mm2, in addition to two volumes with no diffusion weighting (b = 0; Jones, 2004). All diffusion scans were run three times and averaged.
Image processing
Initial diffusion image post-processing steps included eddy current correction, motion correction, and averaging of the three sets of 60 diffusion directions using software from FMRIB's Diffusion Toolbox, FDT (Smith et al., 2004). The brain mask automated output of the Brain Extraction Tool (BET; Smith, 2002) was manually touched-up to fix incorrectly excluded brain matter. This mask, along with the diffusion data, was used by DTIFit (Smith et al., 2004) to calculate the 3 × 3 diffusion tensor for each brain voxel, and subsequently compute FA and MD from the tensor's three eigenvalues (Basser et al., 1994).
TBSS analysis of the white matter skeleton for the concussion/control groups
Diffusion imaging analysis was carried out using Tract-Based Spatial Statistics (TBSS), part of the FSL software library (Smith et al., 2004, 2006). All subject FA data were transformed to a common space (MNI), using the nonlinear registration tool FNIRT (Andersson et al., 2007a, 2007b), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). MD data were subsequently aligned to MNI space with the resulting transform. Next, the mean FA image of the concussion/control subjects was created and thinned to construct a mean WM tract skeleton, which represents the centers of all tracts common to the concussion/control group. The mean WM skeleton was then thresholded to include only those voxels with FA > 0.25, which excludes regions of high between-subject variability in the minor tracts. Each subject's aligned FA data were subsequently projected onto the mean WM skeleton using the highest FA value on a line perpendicular to each point on the skeleton (Smith et al., 2006). Using the same projection, each subject's aligned MD data were also projected onto the WM skeleton. The resulting skeletonized data were fed into voxel-wise between-group statistics using a two-group comparison between concussed and control subjects in FSL's randomize program, which performs permutation testing (using 10,000 permutations) with threshold-free cluster enhancement and multiple comparison correction (Smith and Nichols, 2009). Statistical voxel-wise results for the following contrasts were derived: concussed MD > control MD and concussed FA < control FA were thresholded at corrected p ≤ 0.05. Voxels surviving threshold were clustered based on spatial extent. In addition to the minimum p value and total volume, the anatomical location of each significant cluster was determined based on reference to atlases of human WM anatomy (Bürgel et al., 2006; Mori et al., 2005; Wakana et al., 2004).
Comparison of concussion data to moderate and severe TBI
All moderate-to-severe TBI subjects' diffusion data and their respective control subjects' diffusion data were similarly processed through the TBSS processing pipeline to create skeletonized FA and MD images, using the WM skeleton previously created based on mean FA data of the concussion/control group. Lesion masks for the severe TBI subjects were used during the nonlinear registration of FA data to MNI space. Average skeleton voxel values for the most significant cluster found in the concussion/control group TBSS analysis were plotted to qualitatively assess structural changes with different levels of injury severity.
Results
TBSS statistical results of the white matter skeleton for the concussion/control groups
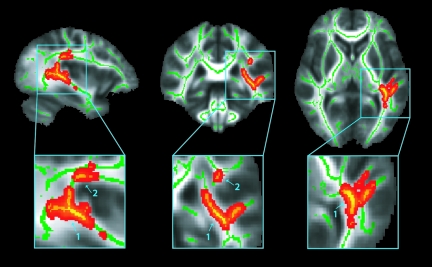
Results of TBSS analysis between the concussed and control groups yielded several clusters of significant (corrected p ≤ 0.05) voxels on the WM skeleton. All clusters, which were located in the left hemisphere, demonstrated increased MD for concussed subjects compared to their age- and sex-matched control subjects. Figure 1 shows the significant (corrected p ≤ 0.05) MD voxels on the WM skeleton with the two largest cluster divisions out of four identified by an arrow with the cluster number. For each cluster, the total number of voxels, the minimum p value, and the anatomic location are listed in Table 2. The largest cluster (961 voxels) containing the most significant voxel (p = 0.027) was located in the left hemisphere, spanning the sagittal stratum (including the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus), retrolenticular part of the internal capsule, the posterior thalamic radiation (including the optic radiation), the acoustic radiation, and the superior longitudinal fasciculus (SLF). The second largest cluster (58 voxels), also in the left hemisphere, was located along the superior longitudinal fasciculus, anterior and superior to the part of the SLF in the largest cluster.
FIG. 1.
Results of the Tract-Based Spatial Statistics (TBSS) analysis of the white matter (WM) skeleton. Voxels demonstrating significantly (corrected p ≤ 0.05) increased mean diffusivity (MD) values for the concussion subjects compared to their age- and sex-matched control subjects are shown in red-yellow. Voxels are thickened into local tracts and overlaid on the WM skeleton (green), and the group mean fractional anisotropy (FA) image (grayscale). The two largest cluster divisions out of four are identified by an arrow with the cluster number. Further cluster details are given in Table 2. Images are shown in radiological convention (right = subject's left) for slice coordinates: x = −36 mm, y = −35 mm, z = 4 mm.
Table 2.
Minimum p Value, Total Volume, and Anatomic Location of Each Cluster Containing Significant Mean Diffusivity (MD) Voxel-wise Results on the White Matter Skeleton
| |
MD significant clusters (corrected p ≤ 0.05) |
||
|---|---|---|---|
| Cluster number | Volume (mm3) | Minimum p value | Anatomical location in the left hemisphere |
| 1 | 961 | 0.027 | Sagittal stratum (inferior longitudinal fasciculus, inferior fronto-occipital fasciculus), retrolenticular part of the internal capsule, posterior thalamic radiation (including optic radiation), acoustic radiation, superior longitudinal fasciculus |
| 2 | 58 | 0.046 | Superior longitudinal fasciculus |
| 3 | 7 | 0.049 | Superior longitudinal fasciculus |
| 4 | 4 | 0.050 | Superior longitudinal fasciculus |
Interestingly, permutation testing of the two-group comparison between concussed and control subject FA data on the WM skeleton yielded no significant (corrected p ≤ 0.05) results.
Comparison of concussion results to moderate and severe TBI data
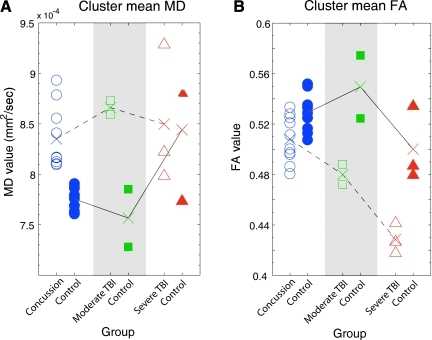
Using the largest and most significant cluster identified in the TBSS analysis, which demonstrated increased MD in the concussion group compared to their controls, average MD and FA values were determined for each subject (including all concussed, moderate TBI, severe TBI, and control subjects). Figure 2A shows average MD values for each individual subject separated by group, and reveals that brain-injured subjects tend to have increased MD compared to controls. Furthermore, concussion and control subject MD values are completely separable, and thus validate the significant findings for MD in these voxels between the two groups in the TBSS analysis (Fig. 1 and Table 2). Moderate TBI subjects show an even larger separation of MD from their controls. In contrast, the severe TBI subjects' MD values are similar to those of their controls. However, this finding may be confounded by typical age-related increases of MD in the normal population (Giorgio et al., 2010). Figure 2B shows average FA values for each individual subject separated by group, and reveals that brain-injured subjects tend to have decreased FA compared to controls. This difference was also manifest between the moderate-to-severe TBI subjects and their controls, despite age-related decreases in FA for the severe TBI group and their controls (Giorgio et al., 2010). On the contrary, substantial overlap of concussion and control subject FA values further validates the insignificant findings for FA in these voxels between the two groups in the TBSS analysis. These results demonstrate that MD might be a more sensitive measure than FA, particularly for detecting minor DAI such as that typically seen in individuals with sports-related concussions. The results also suggest that with increasing injury severity, FA may be a better marker for structural abnormalities.
FIG. 2.
Comparison of concussion results to moderate and severe TBI data. Group averages are denoted by “X.” Differences in group averages of the diffusion values with increasing injury severity are highlighted with a dotted black line. Differences across the control groups are highlighted with a solid black line. (A) Average mean diffusivity (MD) for each individual subject, separated by group. Note that brain-injured subjects tend to have increased MD compared to controls. (B) Average fractional anisotropy (FA) values for each individual subject, separated by group. Note that brain-injured subjects tend to have decreased FA compared to controls. MD may be more sensitive at detecting mild injury, whereas FA captures more severe injuries (TBI, traumatic brain injury).
Discussion
Results of the current study provide promising insights into the use of DTI as an imaging method sensitive enough to capture mild WM injury in individuals with sports-related concussion who experience persistent symptoms with no LOC. The TBSS image analysis technique utilized here reduces the alignment issues of traditional voxel-wise analyses, and reduces the limitations of a priori defined region-of-interest analyses that may dilute or eliminate detection of small structural lesions post-concussion. Furthermore, this is the first report of increased MD in individuals with sports-related concussion who experience persistent symptoms from an injury that did not warrant assessment by the GCS. Previous DTI studies of concussion or mild TBI have focused on subjects with GCS scores ranging from 13–15, and the assessment of FA (Niogi et al., 2008a,b; Rutgers et al., 2008), with the exception of Arfanakis and colleagues (2002), Inglese and co-workers (2005), Lipton and associates (2008), Wilde and colleagues (2008), Mayer and associates (2010), and Messé and co-workers (2010), all of whom assessed both FA and MD. Studies assessing WM fiber tract integrity in the early phase of recovery (days post-injury) in individuals with mTBI (GCS scores 13–15) describe conflicting results, with reports of decreased FA and/or increased MD (Arfanakis et al., 2002; Inglese et al., 2005; Miles et al., 2008), and reports of increased FA and/or decreased MD (Bazarian et al., 2007; Mayer et al., 2010; Wilde et al., 2008). Additionally, during later phases of recovery (months post-injury), studies assessing WM fiber tract integrity in individuals with persistent cognitive impairment reported decreased FA (Niogi et al., 2008a), and increased MD (Lipton et al., 2008). Collectively, these studies have reported abnormalities in a variety of brain regions, including the corona radiata, uncinate fasciculus, corpus callosum, inferior longitudinal fasciculus, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, and capsula interna, many of which were also identified in the current study.
Results of the current analysis suggest a lack of structural integrity located in the left temporal lobe, where association and projection fibers running anterior-posterior and superior-inferior merge or cross. The most significant cluster spans the retrolenticular part of the internal capsule and the posterior thalamic radiation, which contain reciprocal thalamo-cortical pathways, including the optic radiation that connects the lateral geniculate nucleus to the occipital lobe. In this region, anterior-posterior-oriented long association fibers from the inferior fronto-occipital fasciculus (connecting the frontal and occipital lobes), and the inferior longitudinal fasciculus (connecting the temporal and occipital lobes), merge with the thalamic fibers. Part of the identified cluster also follows the C-shaped trajectory of the SLF as it bends superior-inferiorly from the frontal lobe towards the temporal lobe. In the superior temporal lobe, several voxels of the cluster with increased MD lie along the acoustic radiation, which contains projection fibers that connect the medial geniculate body of the thalamus to the primary auditory cortex of Heschl's gyrus. This centrally-localized junction of several WM tracts might be particularly vulnerable in concussion, and hence may be more sensitive than other areas of the brain to injury from translational (linear) acceleration/deceleration forces, or rotational forces that disrupt the structural integrity of the tracts (Broglio et al., 2009; Viano et al., 2007). These findings are in close agreement with the results of a recent TBSS study by Messé and colleagues, that revealed increased MD in long association tracts of the inferior fronto-occipital fasciculus and inferior longitudinal fasciculus bilaterally, as well as in the corpus callosum in a group of mTBI subjects with persistent neurobehavioral impairment during the subacute phase.
The results of the current study are also surprisingly similar to those of a recent TBSS pilot study of veterans who sustained combat-related closed head TBI following active duty in Operation Iraqi Freedom and Operation Enduring Freedom (Kim and Jorge, 2010). Although the effects of blast-related TBI in veterans remain controversial (Levin et al., 2010), Kim and Jorge (2010) reported significantly higher MD and lower FA in veterans with TBI than controls in the following WM fiber tracts: left inferior longitudinal fasciculus, left superior longitudinal fasciculus, left retrolenticular internal capsule, and left sagittal stratum. These are the same regions identified in the current study, suggesting a similarity in the manifestation of military blast injuries and repetitive sports-related concussions with persistent symptoms, or identifying a region of the brain that is particularly susceptible to mild head trauma. Although the mechanisms of injury may differ, these results, when taken in combination, may open the door to future translational studies aimed at better understanding and treating the effects of mild brain injury in both civilian (specifically sports-related concussion) and military populations.
Evidence from histological, volumetric, and DTI studies supports enhanced structural integrity of the WM in these left hemispheric regions in the normal population. Larger left volumes than right volumes of the lateral geniculate body and the optic radiation, including the part of the optic radiation with increased MD in the current study, were observed in a histological voxel-based morphometry study by Bürgel and associates (1999), although no asymmetries in the acoustic radiation were identified by Rademacher and colleagues (2002), who used the same histological approach. A DTI tractography study by Barrick and co-workers (2007) showed a leftward asymmetry of the WM fiber tracts in the posterior segment of the arcuate fasciculus in the left hemisphere, similar to the region identified in the current study. Furthermore, a voxel-based DTI analysis by Büchel and associates (2004) revealed an asymmetry in the C-shaped structure of the posterior part of the SLF belonging to the arcuate fasciculus, with higher FA in the left hemisphere regardless of subject handedness. The observed increases in FA extended far into the temporal lobe in a similar manner as the current study's most significant cluster. In addition, Anderson and colleagues (1999) demonstrated in a post-mortem study that WM increases in the left posterior superior temporal lobe (near the planum temporale region) were due to more thickly myelinated axons. Collectively, these studies of normal controls lend support to a leftward lateralization of increased WM structure, possibly due to thicker myelin, in the centrally localized junction of merging and/or crossing fiber tracts identified by the most significant cluster in the current study. As a result, this brain region may be more susceptible to injury from the translational and rotational forces acting in sports-related concussion. Further research should address this issue, as a larger sample size may reveal other areas of DAI.
Mechanisms of injury were reported to the attending physician for each participant in the current study, although it was frequently difficult for the athlete to remember precisely how the injury occurred, and reported injuries often conflicted with film reviews when available. To date, the relationship between the mechanism of injury and the site of injury remains unclear, and no theoretical thresholds for injury have been established (Mihalik et al., 2007). As reported by Broglio and associates (2009), the type of forces (linear and rotational) acting on the brain depends on both the site of the impact and the role of the player. Additionally, a player's physical condition, such as stronger neck muscles, may reduce the likelihood of a concussion (Viano et al., 2007). Guskiewicz and colleagues (2007b) and Mihalik and associates (2007) suggest that it may be difficult to establish a threshold for concussive injury for football players (let alone across other sports disciplines), given the varying magnitudes of impact and frequency of previous concussion (reported and unreported). Complete information regarding the laterality, location, and the mechanics of impact may provide the necessary link between injury mechanism and the location of DAI in future studies of sports-related concussion.
The current study's results also suggest that MD is a more sensitive measure than FA at detecting small structural WM abnormalities, which is in agreement with a recent TBSS study by Messé and associates (2010), who reported increased MD and no FA changes in individuals with mTBI (GCS score 13–15) and persistent neurobehavioral impairment in the subacute phase. Furthermore, a TBSS study by Acosta-Cabronero and colleagues (2010), investigating early-stage Alzheimer's disease, revealed that diffusivity measures (axial, radial, and mean) were highly significant and far more sensitive than FA. Collectively, these studies suggest that MD may be a better measure for detecting subtle structural damage such as that caused by concussion, regardless of whether it is due to fiber reorganization, increased membrane permeability, destruction of intracellular compartments, or glial alterations (Acosta-Cabronero et al., 2010; Beaulieu, 2002). Enhanced sensitivity of MD to abnormalities in WM structure in the current study is further substantiated through qualitative assessment of average MD and FA values in the most significant cluster with increasing injury severity. For the concussed group, MD was more indicative of structural damage than FA. However, as injury severity increased, FA emerged as the diffusion parameter that is more indicative of structural damage, although age was confounded in the severe TBI group. This finding is substantiated by a 2009 TBSS study by Perlbarg and associates, that examined WM integrity in individuals with severe TBI during the subacute phase (Perlbarg et al., 2009). These investigators also reported decreased FA and no diffusivity differences between severe TBI subjects with unfavorable 1-year outcomes and severe TBI subjects with favorable 1-year outcomes. Additionally, subjects with favorable outcomes exhibited FA values similar to those of normal healthy control subjects. In another TBI study by Huisman and associates (2004), of individuals with a mean GCS score4 of 8.7 (SD 3.7), they found decreased FA and no diffusivity differences in the posterior limb of the internal capsule, an area in close proximity to the region identified in the current study. Furthermore, FA correlated with GCS and Rankin scores in the posterior limb of the internal capsule, whereas diffusivity values showed no correlation (Huisman et al., 2004). The results of the moderate and severe TBI subjects in the current study are only qualitative in nature, due to the very small sample size, and consequently there are limitations to the conclusions that can be inferred. Nonetheless, it is of interest to present these findings to demonstrate that the current method is capable of detecting FA differences in long fiber tracts outside of brain lesions in severe TBI. FA has typically been the DTI measure of choice in previous TBI studies, although the current findings on the continuum of the levels of injury severity suggest that future research in mild TBI should utilize MD as an additional measure. A study by Heiervang and associates (2006) revealed that FA is more variable among normal subjects than MD. Therefore, if the changes in FA and MD are small, as would be expected in the current study's subject population of concussed individuals, only MD group differences will be detectable with a small sample size. A larger sample size may be needed to overcome the higher between-subject FA variability, and to detect subtle group differences in FA. In summary, minor injuries in a small sample size in whom the lesions are not sizeable enough to provoke large directional (orientation) changes in diffusion, but are substantial enough to provoke small changes in overall diffusion, will be detected with greater sensitivity by MD than by FA.
The primary focus of the current study was to assess whether DTI offers measures sensitive enough to capture DAI in sports-related concussion not warranting assessment with the GCS, and to additionally identify the locations of DAI in the brain. NP testing and concussion history, done as part of Princeton University's Concussion Program, was administered according to their protocol for use in medical evaluation. As such, it inherently provided some between-subject variability in the testing time intervals post-injury, and in the testing time intervals from date of DTI scan. This may explain the insignificant correlations of MD with neurocognitive (NP testing) measures. Similarly, there was no relationship between MD and concussion history, although self-report of concussions is inherently troublesome, as athletes often minimize symptoms and do not report their injuries (McCrea et al., 2004). Additionally, the current study was not designed to target a particular brain area with specialized NP tests. Although ImPACT targets many brain areas, it may not be specific enough to cognitively target the brain region identified with MD differences in the current study. Future research should address this issue by utilizing an NP testing protocol with rigorous time intervals and specific tests targeted to the brain region under investigation.
The NP data acquired closest to the DTI scan (at least 1 month post-injury) revealed that this study's concussion subject population was a mixture of subjects testing neuropsychologically normal (n = 3), and neuropsychologically abnormal (n = 6). Interestingly, all subjects continued to experience persistent symptoms and showed a lack of WM fiber tract integrity despite their NP testing results. Additionally, all subjects with the exception of one were able to continue their normal college coursework following concussion. This raises important questions: Can the brain compensate for potential cognitive deficits resulting from structural abnormalities in sports-related concussion subjects who test NP normal by drawing upon other neurocognitive resources that are individual-specific, such as intelligence quotient (IQ) and level of general cognitive ability, or are the results of NP testing in subjects who performed within the normal range confounded by a learning or motivational effect? If the brain can compensate, this opens the door to future research aimed at identifying the brain regions that are compensating, and in a sense are “working overtime,” to make up for any cognitive deficiencies based on the structural deficit. It also opens the door to future strategies aimed at improving evaluation, management, and treatment of concussion. On the other hand, athletes repeat the same battery of NP tests numerous times after injury to evaluate cognition. Athletes are also aware that the NP test results will be used to determine whether they are allowed to return to play. Therefore athletes may be more motivated to perform well on the tests post-injury than when initially tested at baseline. If the results of NP testing are confounded by learning or motivational effects, this supports the need for a more objective diagnostic tool for the assessment of sports-related concussion, which may involve the use of DTI to measure MD.
Collectively, these results suggest that in addition to current NP tests, DTI may be another useful tool for clinicians when making return-to-play decisions to help reduce vulnerability to recurrent injuries. Whether the structural changes demonstrated in this pilot study of athletes having suffered more than one concussion and demonstrating symptoms that persist for at least 1 month post-injury also occur in athletes with uncomplicated concussions (concussion with symptoms, cognitive function, and balance testing returning to normal within 7–14 days) remains to be evaluated. Ultimately, identifying and tracking the effects of concussion demands a longitudinal assessment of the relationship between structural integrity as assessed by DTI scans, functional ability as assessed by fMRI scans, and performance/cognitive ability on NP tests in the acute, subacute, and long-term phases of recovery after concussion. For these reasons, this pilot study is being followed with a longitudinal combined DTI/fMRI study of a larger sample size to refine, clarify, and provide a more detailed understanding of mild TBI, specifically concussion.
Currently, there are no routine imaging protocols used to evaluate sports-related concussion besides traditional clinical MR imaging (T1-weighted), or CT scans, neither of which can detect subtle structural changes, and thus are inadequate for use in the assessment and management of concussion. This pilot study provides evidence of structural changes in the WM of the brain, creating a link between concussive injury with persistent symptoms and changes on DTI. These findings emphasize the importance of athletes to report any possible concussion, and thus to reduce the chance of re-injury. Furthermore, the current study's results suggest DTI may serve as a biomarker for concussion, and thus may provide an objective diagnostic tool to help determine the severity of injury, and to manage concussions, particularly with regard to return-to-play decisions.
Acknowledgments
This work was funded by National Institutes of Health grant no. HD049546-01, the Peter R. and Cynthia K. Kellogg Foundation, and the SIBIL Foundation for Self-determination and International Relations in Liechtenstein.
Author Disclosure Statement
No competing financial interests exist.
References
- Acosta-Cabronero J. Williams G.B. Pengas G. Nestor P.J. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain. 2010;133:529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- Anderson B. Southern B.D. Powers R.E. Anatomic asymmetries of the posterior superior temporal lobes: A post-mortem study. Neuropsychiatry Neuropsychol. Behav. Neurol. 1999;12:247–254. [PubMed] [Google Scholar]
- Andersson J.L.R. Jenkinson M. Smith S. Non-linear optimization. FMRIB technical report TR07JA1. 2007a. www.fmrib.ox.ac.uk/analyis/techrep. www.fmrib.ox.ac.uk/analyis/techrep from.
- Andersson J.L.R. Jenkinson M. Smith S. Non-linear registration, aka Spatial normalization. FMRIB technical report TR07JA2. 2007b. www.fmrib.ox.ac.uk/analysis/techrep. www.fmrib.ox.ac.uk/analysis/techrep from.
- Arfanakis K. Haughton V.M. Carew J.D. Rogers B.P. Dempsey R.J. Meyerand M.E. Diffusion tensor MR imaging in diffuse axonal injury. Am. J. Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Aubry M. Cantu R. Dvorak J. Graf-Baumann T. Johnston K. Kelly J. Lovell M. McCrory P. Meeuwisse W. Schamasch P. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001. Br. J. Sports Med. 2002;36:6–10. doi: 10.1136/bjsm.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick T.R. Lawes I.N. Mackay C.E. Clark C.A. White matter pathway asymmetry underlies functional lateralization. Cereb. Cortex. 2007;17:591–598. doi: 10.1093/cercor/bhk004. [DOI] [PubMed] [Google Scholar]
- Basser P.J. Jones D.K. Diffusion tensor imaging: Theory, experimental design and data analysis—A technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basser P.J. Mattiello J. Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin-echo. J. Magn. Reson. Ser. B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J. Zhong J. Blyth B. Zhu T. Kavcic V. Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: A pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Broglio S.P. Sosnoff J.J. Shin S. He X. Alcaraz C. Zimmerman J. Head impacts during high school football: A biomechanical assessment. J. Athletic Training. 2009;44:342–349. doi: 10.4085/1062-6050-44.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C. Raedler T. Sommer M. Sach M. Weiller C. Koch M.A. White matter asymmetry in the human brain: A diffusion tensor MRI study. Cereb. Cortex. 2004;14:945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Bürgel U. Amunts K. Hoemke L. Mohlberg H. Gilsbach J.M. Zilles K. White matter fiber tracts of the human brain: Three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29:1092–1105. doi: 10.1016/j.neuroimage.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Bürgel U. Schormann T. Schleicher A. Zilles K. Mapping of histologically identified long fiber tracts in human cerebral hemispheres to the MRI volume of a reference brain: Position and spatial variability of the optic radiation. Neuroimage. 1999;10:489–499. doi: 10.1006/nimg.1999.0497. [DOI] [PubMed] [Google Scholar]
- Cantu R.C. Post traumatic (retrograde/anterograde) amnesia: pathophysiology and implications in grading and safe return to play. J. Athl. Train. 2001;36:244–248. [PMC free article] [PubMed] [Google Scholar]
- Chen J.K. Johnston K.M. Frey S. Petrides M. Worsley K. Ptito A. Functional abnormalities in symptomatic concussed athletes: An fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Chen J.K. Johnston K.M. Petrides M. Ptito A. Recovery from mild head injury in sports: evidence from serial functional magnetic resonance imaging studies in male athletes. Clin. J. Sport Meds. 2008;18:241–247. doi: 10.1097/JSM.0b013e318170b59d. [DOI] [PubMed] [Google Scholar]
- Collie A. Maruff P. Makdissi M. McCrory P. McStephen M. Darby D. CogSport: reliability and correlation with conventional cognitive tests used in postconcussion medical evaluations. Clin. J. Sport Med. 2003;13:28–32. doi: 10.1097/00042752-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Collins M.W. Iverson G.L. Lovell M.R. McKeag D.B. Norwig J. Maroon J. On field predictors of neuropsychological and symptom deficit following sports-related concussion. Clin. J. Sport Med. 2003;13:222–229. doi: 10.1097/00042752-200307000-00005. [DOI] [PubMed] [Google Scholar]
- Erlanger D.M. Kaushik T. Broshek D. Freeman J. Feldman D. Festa J. Development and validation of a web-based screening tool for monitoring cognitive status. J. Head Trauma Rehabil. 2002;17:458–476. doi: 10.1097/00001199-200210000-00007. [DOI] [PubMed] [Google Scholar]
- Giorgio A. Santelli L. Tomassini V. Bosnell R. Smith S. De Stefano N. Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall D. Wrightson P. Cumulative effect of concussion. Lancet. 1975;306:995–997. doi: 10.1016/s0140-6736(75)90288-3. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K.M. Bruce S.L. Cantu R.C. Ferrara M.S. Kelly J.P. McCrea M. Putukian M. Valovich McLeod T.C. National Athletic Trainers' Association Position Statement: Management of Sport-Related Concussion. J. Athl. Train. 2004;39:280–297. [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K.M. Marshall S.W. Bailes J. McCrea M. Cantu R.C. Randolph C. Jordan B.D. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–724. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K.M. Marshall S.W. Bailes J. McCrea M. Harding H.P., Jr. Matthews A. Mihalik J.R. Cantu R.C. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 2007a;39:903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K.M. McCrea M. Marshall S.W. Cantu R.C. Randolph C. Barr W. Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA concussion study. JAMA. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K.M. Mihalik J.P. Shankar V. Marshall S.W. Crowell D.H. Oliaro S.M. Ciocca M.F. Hooker D.N. Measurement of head impacts in collegiate football players: relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurgery. 2007b;61:1244–1252. doi: 10.1227/01.neu.0000306103.68635.1a. [DOI] [PubMed] [Google Scholar]
- Heiervang E. Behrens T.E.J. Mackay C.E. Robson M.D. Johansen-Berg H. Between session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage. 2006;33:867–877. doi: 10.1016/j.neuroimage.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Herring S. Bergfeld J. Boland A. Boyajian-O'Neill L.A. Cantu R.C. Hershman E. Indelicato P. Jaffe R. Kibler W.B. McKeag D.B. Pallay R. Putukian M. Concussion (mild traumatic brain injury) and the team physician: A consensus statement. Med. Sci. Sports Exerc. 2005;37:2012–2016. doi: 10.1249/MSS.0b013e3182342e64. [DOI] [PubMed] [Google Scholar]
- Horsfield M.A. Larsson H.B. Jones D.K. Gass A. Diffusion magnetic resonance imaging in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1998;64:S80–S84. [PubMed] [Google Scholar]
- Huisman T.A. Schwamm L.H. Schaefer P.W. Koroshetz W.J. Shetty-Alva N. Ozsunar Y. Wu O. Sorensen A.G. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. Am. J. Neuroradiol. 2004;25:370–376. [PMC free article] [PubMed] [Google Scholar]
- Inglese M. Makani S. Johnson G. Cohen B.A. Silver J.A. Gonen O. Grossman R.I. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H. Rushworth M.F. Using diffusion imaging to study human connectional anatomy. Annu. Rev. Neurosci. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- Jones D.K. Horsfield M.A. Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 1999;42:515–525. [PubMed] [Google Scholar]
- Jones D.K. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study. Magn. Reson. Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Kim J. Jorge R.E. Diffusion tensor MRI in combat-related traumatic brain injury. Clin. Transl. Sci. 3, 2010:S45. [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K.E. Centers for Disease Control, National Center for Injury Prevention and Control; Atlanta, GA: 2006. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths. [Google Scholar]
- Le Bihan D. Mangin J. Poupon C. Clark C.A. Pappata S. Molko N. Chabriat H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Wilde E. Troyanskaya M. Petersen N. Scheibel R. Newsome M. Radaidah M. Wu T. Yallampalli R. Chu Z. Li X. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Lipton M.L. Gellella E. Lo C. Gold T. Ardekani B.A. Shifteh K. Bello J.A. Branch C.A. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma. 2008;25:1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- Lovell M.R. Collins M.W. Podell K. Powell J. Maroon J. NeuroHealth Systems, LLC; Pittsburgh, PA: 2007b. Immediate post-concussion assessment and cognitive testing (Version 2.0) [Google Scholar]
- Lovell M.R. Pardini J.E. Welling J. Collins M.W. Bakal J. Lazar N. Roush R. Eddy W.F. Becker J.T. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007a;61:352–359. doi: 10.1227/01.NEU.0000279985.94168.7F. [DOI] [PubMed] [Google Scholar]
- Mayer A.R. Ling J. Mannell M.V. Gasparovic C. Phillips J.P. Doezema D. Reichard R. Yeo R.A. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W. Saykin A.J. Flashman L.A. Sparling M.B. Johnson S.C. Guerin S.J. Mamourian A.C. Weaver J.B. Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McAllister T.W. Sparling M.B. Flashman L.A. Guerin S.J. Mamourian A.C. Saykin A.J. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McCrea M. Hammeke T. Olson G. Leo P. Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin. J. Sports Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- McCrea M. Kelly J.P. Randolph C. Kluge J. Bartolic E. Finn G. Baxter B. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J. Head Trauma Rehabil. 1998;13:27–35. doi: 10.1097/00001199-199804000-00005. [DOI] [PubMed] [Google Scholar]
- McCrory P. Johnston K. Meeuwisse W. Aubry M. Cantu R. Dvorak J. Graf-Baumann T. Kelly J. Lovell M. Schamasch P. Summary and agreement statement of the Second International Conference on Concussion in Sport, Prague 2004. Br. J. Sports Med. 2005;39:196–204. doi: 10.1136/bjsm.2005.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P. Meeuwisse W. Johnston K. Dvorak J. Aubry M. Molloy M. Cantu R. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. J. Clin. Neurosci. 2009b;16:755–763. doi: 10.1016/j.jocn.2009.02.002. [DOI] [PubMed] [Google Scholar]
- McCrory P. Meeuwisse W. Johnston K. Dvorak J. Aubry M. Molloy M. Cantu R. SCAT2. Br. J. Sports Med. 2009a;43:i85–i88. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- Messé A. Caplain S. Paradot G. Garrigue D. Mineo J.-F. Ares G.S. Ducreux D. Vignaud F. Rozec G. Desal H. Pélégrini-Issac M. Montreuil M. Benali H. Lehéricy S. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum. Brain Mapp. n/a. 2010 doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalik J.P. Bell D.R. Marshall S.W. Guskiewicz K.M. Measurement of head impacts in collegiate football players: an investigation of positional and event-type differences. Neurosurgery. 2007;61:1229–1235. doi: 10.1227/01.neu.0000306101.83882.c8. [DOI] [PubMed] [Google Scholar]
- Miles L. Grossman R. Johnson G. Babb J. Diller L. Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22:115–122. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- Mori S. Introduction to Diffusion Tensor Imaging. Elsevier; Oxford, U.K.: 2007. [Google Scholar]
- Mori S. Wakana S. Nagae-Poetscher L.M. van Zijl P.C.M. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Lee H. Suh M. Zimmerman R.D. Manley G.T. McCandliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008b;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Sarkar R. Lee H. Meeker M. Zimmerman R.D. Manley G.T. McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008a;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlbarg V. Puybasset L. Tollard E. Lehéricy S. Benali H. Galanaud D. Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: A diffusion tensor imaging study using voxel-based approaches. Hum. Brain Mapp. 2009;30:3924–3933. doi: 10.1002/hbm.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C. Jezzard P. Basser P.J. Barnett A. Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Ptito A. Chen J.K. Johnston K.M. Contributions of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. NeuroRehabilitation. 2007;22:217–227. [PubMed] [Google Scholar]
- Rademacher J. Bürgel U. Zilles K. Stereotaxic localization, intersubject variability, and interhemispheric differences of the human auditory thalamocortical system. Neuroimage. 2002;17:142–160. doi: 10.1006/nimg.2002.1178. [DOI] [PubMed] [Google Scholar]
- Reese T.G. Heid O. Weisskoff R.M. Wedeen V.J. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn. Reson. Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rueckert D. Sonoda L.I. Hayes C. Hill D.L.G. Leach M.O. Hawkes D.J. Non-rigid registration using free-form deformations: Application to breast MR images. IEEE Trans. Med. Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Rutgers D.R. Toulgoat F. Cazejust J. Fillard P. Lasjaunias P. Ducreaux D. White matter abnormalities in mild traumatic brain injury: A diffusion tensor imaging study. Am. J. Neuroradiol. 2008;29:514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M.R. Marshall S.W. Mueller F.O. Yang J. Weaver N.L. Kalsbeek W.D. Bowling J.M. Incidence and risk factors for concussion in high school athletes, North Carolina, 1996–1999. Am. J. Epidemiol. 2004;160:937–944. doi: 10.1093/aje/kwh304. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Nichols T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localization in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Jenkinson M. Johansen-Berg H. Rueckert D. Nichols T.E. Mackay C.E. Watkins K.E. Ciccarelli O. Cader M.Z. Matthews P.M. Behrens T.E.J. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Jenkinson M. Woolrich M.W. Beckmann C.F. Behrens T.E.J. Johansen-Berg H. Bannister P.R. De Luca M. Drobnjak I. Flitney D.E. Niazy R.K. Saunders J. Vickers J. Zhang Y.Y. De Stefano N. Brady J.M. Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Teasdale G. Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;304:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Branche C.M. Sniezek J.E. The epidemiology of sport-related traumatic brain injuries in the United States: Recent developments. J. Head Trauma Rehabil. 1998;13:1–8. doi: 10.1097/00001199-199804000-00003. [DOI] [PubMed] [Google Scholar]
- Viano D.C. Casson I.R. Pellman E.J. Concussion in professional football: biomechanics of the struck player—part 14. Neurosurgery. 2007;61:313–327. doi: 10.1227/01.NEU.0000279969.02685.D0. [DOI] [PubMed] [Google Scholar]
- Wakana S. Jiang H. Nagae-Poetscher L.M. van Zijl P.C.M. Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. McCauley S.R. Hunter J.V. Bigler E.D. Chu Z. Wang Z.J. Hanten G.R. Troyanskaya M. Yallampalli R. Li X. Chia J. Levin H.S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Zemper E.D. Two year prospective study of relative risk of a second cerebral concussion. Am. J. Phys. Med. Rehabil. 2003;82:653–659. doi: 10.1097/01.PHM.0000083666.74494.BA. [DOI] [PubMed] [Google Scholar]