Abstract
Collaboration among investigators, centers, countries, and disciplines is essential to advancing the care for traumatic brain injury (TBI). It is thus important that we “speak the same language.” Great variability, however, exists in data collection and coding of variables in TBI studies, confounding comparisons between and analysis across different studies. Randomized controlled trials can never address the many uncertainties concerning treatment approaches in TBI. Pooling data from different clinical studies and high-quality observational studies combined with comparative effectiveness research may provide excellent alternatives in a cost-efficient way. Standardization of data collection and coding is essential to this end. Common data elements (CDEs) are presented for demographics and clinical variables applicable across the broad spectrum of TBI. Most recommendations represent a consensus derived from clinical practice. Some recommendations concern novel approaches, for example assessment of the intensity of therapy in severely injured patients. Up to three levels of detail for coding data elements were developed: basic, intermediate, and advanced, with the greatest level of detail attained in the advanced version. More detailed codings can be collapsed into the basic version. Templates were produced to summarize coding formats, explanation of choices, and recommendations for procedures. Endorsement of the recommendations has been obtained from many authoritative organizations. The development of CDEs for TBI should be viewed as a continuing process; as more experience is gained, refinement and amendments will be required. This proposed process of standardization will facilitate comparative effectiveness research and encourage high-quality meta-analysis of individual patient data.
Key words: clinical studies, common data elements, data coding, data collection, standardization, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a field of medicine with one of the greatest unmet needs (Maas et al., 2008). Globally, its incidence is increasing, mainly due to increasing traffic in low- and middle-income countries (Maas et al., 2008). In high-income countries, traffic laws and improved auto safety have resulted in a decrease in the incidence of TBI caused by motor vehicle incidents. Nevertheless, TBI remains a leading cause of death and disability in Europe and the U.S., both in children and young adults. There is therefore a great need to advance clinical care. In practice, however, much uncertainty exists about the benefits and risks of many treatment modalities, and the evidence underpinning authoritative guideline recommendations is relatively weak. These problems are aggravated by the heterogeneity of TBI in terms of cause, pathology, severity, and prognosis. It seems unlikely that we will ever be able to mount adequately-powered trials to study all of the relevant treatment modalities.
Although randomized controlled trials (RCTs) are considered the preferred approach for investigating novel therapies, these are costly and logistically demanding. Moreover, it is doubtful whether results obtained in selected populations of subjects enrolled in clinical trials in research centers are generalizable to the broader settings in which most care for TBI patients is provided. Pooling data from multiple studies (individual patient data analysis), and comparative effectiveness research (CER) utilizing prospective observational data collection, can provide alternative sources of evidence that can be obtained in a more cost-efficient way. The direct relevance and potential of such approaches is illustrated by the results from the meta-analysis of individual patient data performed by the IMPACT study group, and by the fact that major advances in clinical care for TBI have resulted from previous observational studies, such as the U.S. Traumatic Coma Databank (Foulkes et al., 1991), the European Brain Injury Consortium (EBIC) Core Data Survey (Murray et al., 1999), The Vietnam Head Injury Study (Salazar et al., 1995), and the Trauma Audit And Research Network Registry (TARN; Patel et al., 2005). When undertaking high-quality observational data collection across multiple settings, or when analyzing individual patient data from various studies, standardization of data collection and coding is essential. In TBI, there is no lack of data, but we can never take full advantage of the potential resulting from the availability of these data if they have not been collected in a uniform way. A general consensus on choice and coding of variables (common data elements, CDEs) for TBI studies is not only highly desirable from a scientific point of view, but also from the perspective of cost-efficiency, because repeated development of case report forms for new studies will be obviated, and costs for funding agencies will consequently be reduced.
With these considerations in mind, an inter-agency workshop on standardization of data collection in TBI and psychological health was organized in March 2009 (Thurmond et al., 2010). General recommendations for collecting data on demographics and clinical assessment, neuroimaging studies, biomarkers, and outcomes for TBI, have previously been published (Duhaime et al., 2010; Maas et al., 2010; Manley et al., 2010; Wilde et al., 2010). Here we present the full scope of the recommendations for assessment and collection of clinical data in TBI trials and observational studies during the acute, subacute, and chronic phases. The global aim is to develop TBI common data elements for use across the broad spectrum of TBI. TBI was defined as: “An alteration in brain function, or other evidence of brain pathology, caused by an external cause” (Menon et al., 2010). The recommendations are presented in modular format to facilitate the production of common case report forms (CRFs).
Methods
The process for developing common data elements for TBI was consensus-driven. As a multidisciplinary working group (WG) with representation from many agencies and organizations, we prepared preliminary recommendations for presentation during the inter-agency workshop on “Standardization of Data Collection in TBI and Psychological Health,” which was held in March 2009 in Washington, D.C. The feedback obtained led to substantial refinements, which were discussed during three subsequent face-to-face meetings, and implemented in a beta version of the CDEs. This beta version was discussed during a 2-day meeting with international TBI experts from the fields of neurosurgery and intensive care medicine. Suggestions for further improvements were incorporated into subsequent releases. Templates were developed for each data element, providing information on definitions, coding formats, plausible values, recommendations for procedures, and explanation of choices. We sought to ensure compatibility with the National Institute of Neurological Disorders and Stroke (NINDS) broad common data elements project (www.commondataelements.ninds.nih.gov).
Structure of common data elements
The proposed CDEs contain all essential data elements for use across the broad spectrum of TBI. Related elements were combined in modules, which were grouped together in categories. For example, the data elements “age, gender, and race” are combined in the module “demographics” under the category “subject characteristics.”
In total, eight main categories were identified:
Participant/subject characteristics
Participant and family history
Injury/disease related events
Assessments and examinations
Treatments/interventions
Protocol experience
Adverse events and safety data
Outcome and function
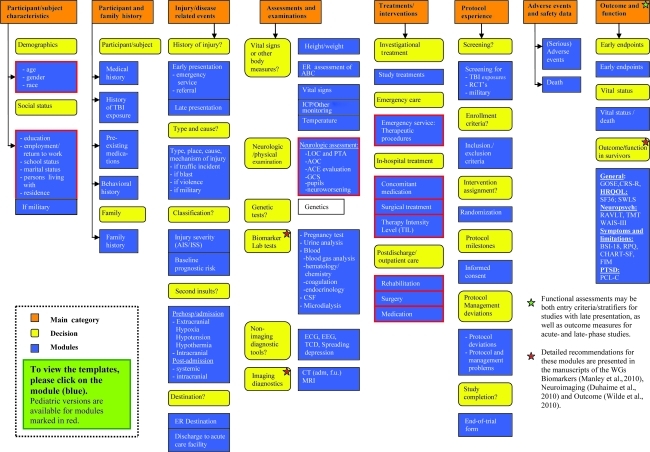
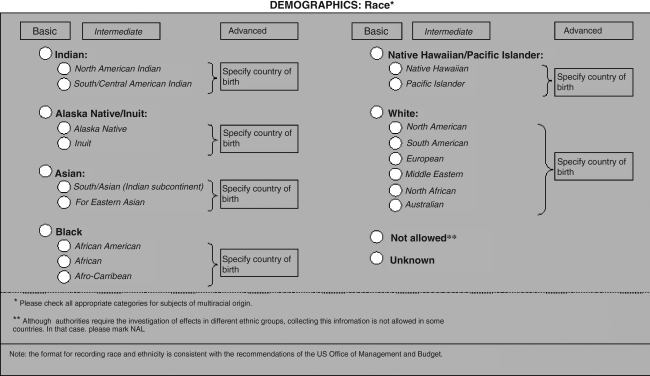
The overall structure of the CDEs is presented in Figure 1. We recognized that the required level of detail for coding elements may vary greatly according to the aim of a particular study. We therefore present up to three possible levels for coding each element: basic, intermediate, and advanced. The greatest level of detail is provided in the advanced version. In every case, the more detailed formats can be collapsed into the intermediate or basic versions, thus facilitating analysis of individual patient data across studies. Figure 2 provides an example of these three levels of coding. Many of the recommended elements represent “plug-in” elements and can be used multiple times in the development of a CRF. For example, assessments of the Glasgow Coma Scale score (GCS) and pupillary reactivity may be recorded pre-hospital, on admission, and repeatedly during the acute care phase.
FIG. 1.
Structure of common data elements. Related elements are combined in modules (blue), which are grouped together in categories (orange). A full listing of the common data elements, including codings and templates as well as explanations of the abbreviations can be found at www.tbi-impact.org.
FIG. 2.
Example of the three levels for coding a data element, with the greatest level of detail in the advanced format.
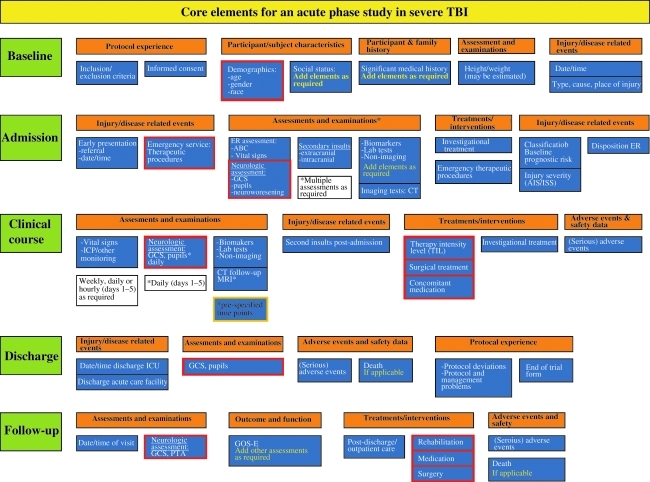
The selection of CDEs and the level of detail for their coding will depend on specific study requirements. The proposed CDEs offer sufficient flexibility for broad application, as basic, intermediate, and advanced levels can be mixed when designing a CRF. An example of how data elements can be compiled for an acute care study of severe TBI is presented in Figure 3.
FIG. 3.
Example of how data elements can be used to design a case report form for an acute care study on severe TBI (TBI, traumatic brain injury; GCS, Glasgow Coma Scale; ICP, intracranial pressure; CT, computed tomography; PTA, post-traumatic amnesia; GOSE-E, Glasgow Outcome Scale-Extended; ICU, intensive care unit; MRI, magnetic resonance imaging; ER, emergency room; AIS, Abbreviated Injury Scale; ISS, Injury Severity Scale.
Description of clinical common data elements
A complete overview of the recommended clinical CDEs and their templates has been posted on the IMPACT website (www.tbi-impact.org). More general information will be additionally incorporated on the NINDS website (www.commondataelements.ninds.nih.gov). Below, we summarize a selection of the main recommendations differentiated by category.
Subject characteristics
The category “subject characteristics” contains modules on demographics (age, gender, and race/ethnicity), and social status (including education, employment, marital status, and living arrangements).
Age
Age can be recorded in years (for infants in weeks/months), or derived from the date of birth. We extensively discussed the preferred choices. Concerns existed that date of birth might be considered a potential patient identifier, or “protected health information,” requiring adherence to Health Insurance Portability and Accountability Act (HIPAA) regulations in the U.S. Nevertheless, recording date of birth is recommended for intermediate and advanced versions, because it is source-verifiable.
Recording age is considered essential to all TBI studies, because causes of injury and consequences for patterns of damage vary by age. Age is also a strong predictor of outcome (Bullock et al., 2000; Mushkudiani et al., 2007).
Race and ethnicity
There are no international standards for classifying race and ethnicity. Recommendations for reporting race in at least five categories are mandated by the Office of Management and Budget (OMB) of the United States government (www.whitehouse.gov/omb/fedreg-1997standards). We therefore took a pragmatic approach and chose to further subdivide the broad categories prescribed by the OMB at several levels. In subjects of multiracial origin, multiple categories may be marked.
Many reasons exist for recording race and ethnicity in TBI studies:
To detect possible disparities in pre-injury health and access to health care in the acute and post-acute phases after TBI.
To identify racial variations in drug pharmacokinetics or Pharmacodynamics.
To clarify the demonstrated association between race and outcome, which is not related to differences in cause of injury or to injury severity (Mushkudiani et al., 2007).
Race and ethnicity are overlapping concepts, but given the constraints of the OMB recommendations, they should be documented separately. It should be recognized that race is perhaps more a social and cultural construct, and that classification is not always anthropologically- or scientifically-based. Importantly, race should not be seen as a surrogate for genetic variation, as only approximately 10% of genetic variation occurs between races (Jorde and Wooding, 2004).
Education
We recommend recording both the number of years of education completed and the highest level achieved. Achievement is considered more relevant than attendance (number of years). Educational level is an important component of socioeconomic status, and the level of educational achievement is related to outcome in TBI.
Employment
Although return to work is often considered a relevant outcome parameter for subjects in the paid workforce, we should recognize that other social roles such as homemaker or volunteer worker are equally relevant. We therefore prefer the more general term “productive activity,” and recommend collecting data on these role activities separately.
Participant and family history
The category “participant and family history” contains modules on medical history, history of TBI exposure, pre-existing medications, behavioral history, and family history. In contrast to many previous studies in which data on pre-existing conditions and pre-existing medications have been recorded in free text format, we strongly recommend the use of pre-specified formats. This is becoming increasingly relevant, as data from the European Union (https://webgate.ec.europa.eu/idb/documents/2009-IDB-Report_screen.pdf), and the Centers for Disease Control and Preventions (CDC) in the U.S. (http://www.cdc.gov/traumaticbraininjury/tbi_ed.html), suggest that injuries in general, and TBI in particular, are increasing in individuals over the age of 60 years, who may suffer from a broader range of pre-existing conditions and who may take a wide range of medications, including anticoagulant medication and platelet aggregation inhibitors.
Documentation of a history of previous TBI reflects the increasing understanding that repetitive injuries cause incremental damage and may be an important risk factor for neuropsychological sequelae, Alzheimer's disease, and encephalopathy. For documenting lifetime history of TBI, we recommend use of the Ohio State University TBI Identification Method Short Form (Corrigan et al., 2007).
Injury/disease related events
The category “injury/disease related events” contains modules on presentation, injury severity, second insults, and destination after initial evaluation.
Presentation
We recommend different formats for recording details on initial evaluation and referral for patients presenting early, versus those presenting late. For patients who present early, referral policy, mode of transport, and emergency medical care, as well as time of arrival, are relevant. For patients presenting late after injury, the main reason for presentation is perhaps the more relevant factor, and this will facilitate later characterization of the population captured. Late presentation is particularly common in individuals with mild TBI. Military service members and athletes may tend to avoid seeking immediate care or to minimize symptoms in order to fulfill their mission/game objectives, and to not let down their comrades/team. Moreover, these groups are prone to repetitive injuries. Once these patients are in more usual or less structured environments, symptoms that seemed manageable may not resolve, and may lead injured individuals to seek care long after injury, or after repetitive exposures. Late presentations based on self-report present problems for clinicians required to diagnose these injuries long after the event, and for systems that seek to provide fair compensation to injured individuals. In such situations it can sometimes be very difficult to establish a diagnosis of mild TBI definitively.
Definitions of mild TBI vary considerably across studies (Comper et al., 2005). The American Congress of Rehabilitation Medicine has presented criteria for mild TBI, defining a loss of consciousness (LOC) of less than 30 min, and post-traumatic amnesia (PTA) of less than 24 h (American Congress of Rehabilitation Medicine, 1993). Alteration of consciousness (AOC) not involving LOC or PTA (i.e., being dazed or confused, or patient reports that they saw “stars” at the time of injury) is also included as indicating a mild TBI. Documentation of the presence of LOC, duration of PTA, other AOC (including confusion), and careful clinical interview are considered the best means of consolidating clinical evaluations and self-report to provide a diagnosis of TBI, and to characterize its severity in these patients. For assessing complaints and symptoms in studies of conscious subjects captured immediately after injury, we advocate the use of the structured assessment, such as that contained in acute concussion evaluation forms.
Type of injury
Traditionally, TBI is divided into closed versus penetrating injuries. We recommend a broader documentation of the type of injury as one of four categories: closed, penetrating, blast, and crush. This reflects the changing epidemiology and increased recognition of blast injuries of the brain as a specific entity (Wolf et al., 2009; Ling et al., 2009). Crush injuries result from a slow mechanical force applied to the skull, and are therefore different from acceleration/deceleration or impact traumas.
Place and cause of injury
Although nearly every TBI study conducted in the past has attempted to capture essential information on place and (external) cause of injury, approaches to coding have been inconsistent and often confuse different aspects. For example, categories such as road traffic incident or fall may be lumped together with home, suicide attempt, or work. We recommend a clearer separation, in which place of injury captures information on the location (e.g., street, home, work, or sports field), and the element “cause of injury,” being more directed towards a causative factor (e.g., road traffic incident or fall). We further advocate recording whether injuries were intentional or not.
Accurate documentation of cause and mechanism of injury is important for two reasons. First, the type of brain damage that may be expected varies by injury mechanism (e.g., more contusions are seen in patients who have sustained a fall, and potentially unique injury patterns are associated with explosions/blasts). Second, it is important from a perspective of prevention. For more detailed recording of the mechanisms of injury caused by road traffic incidents, we strongly recommend documenting the function of the victim and that of the other party separately. This is relevant because vulnerable road users (pedestrians, cyclists, and motor cyclists) are particularly at risk, and account for almost half of all deaths due to road traffic incidents (WHO/OMS, 2009). In keeping with the addition of blast as a separate type of brain trauma, we also include explosions/blasts as a mechanism of injury that should be documented in detail.
Classification
We recommend a broad and multidimensional approach to classifying the severity of both brain injury and extracranial injuries. Few studies conducted in the past have explored the assessment and influence of extracranial injuries. Extracranial injuries, however, occur frequently in combination with TBI, and are associated with poorer outcome, increased pain, and increased medication use, perhaps more so in patients with mild to moderate injuries (McMahon et al., 1999; Van Leeuwen et al., in preparation). For assessment of the severity of extracranial injuries, we recommend the use of a simplified version of the Abbreviated Injury Scale (AIS; Association for the Advancement of Automotive Medicine, 1990), and calculation of the Injury Severity Score (ISS; Baker et al., 1974). For TBI patients, we further consider it important to document the coexistence and severity of spinal injuries separately.
Severity of brain damage is commonly assessed by measuring the depth and duration of LOC, and/or duration of PTA, and by quantifying the extent of structural damage through neuroimaging studies. Subjects with TBI are commonly grouped into three distinct categories according to GCS score: severe (GCS score 3–8), moderate (GCS score 9–12), or mild (GCS score 13–15). Although we strongly support the continued use of the GCS as an indication of the severity of brain damage, we should recognize that the degree of severity spans a spectrum, and that categorization into a limited number of categories leads to the loss of valuable information. Widely variable patterns of injury and pathology may be seen on structural imaging in patients with similar grades of clinical severity as assessed by GCS score (Saatman et al., 2008). A more comprehensive and multidimensional approach to the classification of TBI is advocated, but realization of this goal will require further research. An alternative approach to quantifying severity is by prognostic classification, in which the baseline prognostic risk for early mortality or functional outcome as assessed by the Glasgow Outcome Scale (GOS) is calculated. Validated models developed in large patient samples are now available (MRC CRASH Trial Collaborators, 2008; Steyerberg et al., 2008). We advocate increased use of these models. Consequently, documentation of the core predictors (Table 1) utilized in these models is considered essential for all TBI studies.
Table 1.
Elements Required for the IMPACT and CRASH Prognostic Models
|
IMPACT |
CRASH |
|||
|---|---|---|---|---|
| Core model | Extended model | Lab model | Core model | CT model |
| Age | Core model plus: | Extended model plus: | Age | Core model plus: |
| Motor score | Hypoxia | Glucose | GCS score | Petechial hemorrhages |
| Pupil reactivity | Hypotension CT classification Traumatic subarachnoid hemorrhage on CT Epidural mass on CT |
Hemoglobin | Pupil reactivity Major extracranial njury |
Obliteration of the third ventricle or basal cisterns Subarachnoid bleeding Midline shift Non-evacuated hematoma |
GCS, Glasgow Coma Scale; CT, computed tomography; IMPACT, International Mission for Prognosis and Analysis of Clinical Trials in TBI; CRASH, Corticosteroid Randomisation After Significant Head Injury.
Second insults
Second insults may be systemic or intracranial and can aggravate processes of secondary damage in a brain rendered vulnerable by the primary injury. Systemic insults (e.g., hypoxia, hypotension, and hypo/hyperthermia) may occur pre-hospital, during transport, and in-hospital. We recommend different formats for coding such insults in the pre-hospital situation and in-hospital. Because accurate measurements are not always possible in the pre-hospital situation, we recommended a broad categorization. In-hospital, however, more detailed assessments are available. In the advanced version we therefore recommend detailed recordings of depth and duration of lower values, for example by presenting the percentage of time during which pre-defined ranges of values occur over a given period.
Assessments and examinations
The category “assessments and examinations” contains modules on vital signs and other body measures (e.g., height and weight), neurological assessments (GCS score, LOC, PTA, and AOC), genetics, biomarkers, and lab tests, as well as imaging and non-imaging diagnostic tools. At minimum, we advocate recording of vital signs (e.g., blood pressure, heart rate, temperature, and oxygen saturation) on admission, and also on a daily basis during the acute phase of the study. In the ICU environment, recording blood pressure and intracranial pressure (ICP) on an hourly basis is recommended in order to permit determination of the cerebral perfusion pressure (CPP). In the analysis phase, we recommend that all hourly data are referenced to date and time of injury, since this represents the only fixed time event that is common to all patients. Consensus on procedures for zeroing the ICP monitor is required, and we suggest zero calibration to the level of the foramen of Monro. In instances in which a ventriculostomy is in place, ICP measurements will clearly depend on whether the ventriculostomy is left open, or kept closed, and only opened for elevations in ICP above a given threshold. There is no consensus on this topic, and for now, we recommend that clear information be provided about clinical practice in the context of the study. Indeed, provision of such information may allow us to undertake a CER analysis that addresses which of these approaches is better. It should be recognized, however, that ICP measurements obtained by ventricular fluid pressure monitoring during continuous cerebrospinal fluid drainage are likely to be inaccurate, underestimating the real ICP.
Treatments and interventions
The category “treatments and interventions” includes modules on study treatments (investigational treatments), emergency care, in-hospital treatment, and rehabilitation/post-acute care.
Emergency service therapeutic procedures
The contribution of early events and the importance of ultra-early management of acute TBI have only recently been acknowledged. Even small degrees of suboptimal early management may have profound effects on outcome by creating a ripple effect, exacerbating or accelerating underlying pathophysiological processes. Recommended elements for data collection include management of airway, breathing, and circulation, as well as the necessity for emergency intra- or extracranial surgery.
In-hospital treatment
The module on in-hospital treatment includes elements for concomitant medication, intra- or extracranial surgery, and therapy intensity level. Recording concomitant medication is often viewed as a nightmare by research personnel, but is essential, especially in the context of clinical trials of investigational medications, in order to capture drug interactions. In addition to documenting the generic names of the medications given, we recommend a broad categorization in order to facilitate analysis.
In previous trials, details of surgery have generally been entered in a free text format. This has in many cases precluded any meaningful analysis. We therefore recommend the use of pre-defined categories. A proposal is presented, but we realize that this may be controversial and will elicit debate. For example, we do not consider insertion of chest tubes or the implantation of a ventricular catheter for the sole purpose of monitoring as a surgical procedure. Practical experience and a process of validation will ultimately be needed to determine further refinements.
Interpretation of ICP is not possible without knowledge of the intensity of therapy directed at ICP/CPP control. Modern neurocritical care practices have substantially blunted our ability to use ICP as a surrogate marker for a range of pathophysiological processes. It is possible to control ICP by intensifying ICP/CPP therapies until the system terminally decompensates. In this context, the intensity of ICP/CPP targeted therapy may be a more sensitive measure of the severity of pathophysiology, and of the ability of a novel intervention to modify such pathophysiology. Therapy intensity level (TIL) has commonly been recorded on an hourly basis, but this is resource-intensive. Further, ICU practices have changed, with most high-grade interventions now being used in a continuous fashion. Given this context, we had doubts whether hourly recording of TIL justifies the investment in time. We therefore propose a novel approach, with the expectation that this will offer a transparent and useful approach, coupled with a lower burden of data capture than hourly recording. For the basic level, we propose a simple five-category scale, which permits a global approach to summarize the overall intensity of therapy over the entire treatment period or on a daily basis (Table 2). At the intermediate level, the use of specific treatment modalities are scored on a daily basis, or more frequently if required (Table 3). At the advanced level, details of fluid balance (volume loading), and administered doses of hyperosmolar fluids and vasopressors are also captured. The intermediate and advanced levels permit calculation of a numerical summary score, which is compatible with the TIL score proposed for use in pediatric TBI (Shore et al., 2006; Table 3). A great advantage of this method is that it allows a commonality of approach in pediatric and adult populations. While the approach is ready for pilot study, we recommend formal validation before widespread use.
Table 2.
Therapy Intensity Level (TIL): Basic
| ○ | TIL 0: |
| No specific ICP-directed therapy | |
| ○ | TIL 1: Basic ICU care: |
| Sedation for ventilator/endotracheal tube tolerance | |
| Volume/vasoactives for non-CNS cause (e.g., sepsis, myocardial injury) | |
| Head-up positioning (ventilator bundle) | |
| Normocapnia (Paco2 ≥ 40 mm Hg) | |
| ○ | TIL 2: Mild |
| Higher levels of sedation | |
| Vasopressors/volume for CPP support | |
| Low-dose osmotic therapy | |
| Mild hypocapnia (Paco2: 4.6–5.3 kPa; 35–40 mm Hg) | |
| CSF drainage < 120 mL/d (<5 mL/h) | |
| ○ | TIL 3: Moderate |
| Higher doses of osmotic therapy | |
| Moderate hypocapnia (Paco2: 4.0–4.5 kPa; 30–35 mm Hg) | |
| Mild hypothermia (>35°C) | |
| CSF drainage ≥ 120 mL/d (≥5 mL/h) | |
| ○ | TIL 4: Extreme |
| Profound hypocapnia (Paco2: < 30 mm Hg) | |
| Temperature < 35°C | |
| Metabolic suppression with intravenous anesthetics | |
| Surgery for refractory ICP (decompression/lobectomy) |
ICP, intracranial pressure; CSF, cerebrospinal fluid; Paco2, partial arterial carbon dioxide pressure; ICU, intensive care unit; CPP, cerebral perfusion pressure; CNS, central nervous system.
Table 3.
Therapy Intensity Level (TIL): Intermediate
| |
|
|
Assignment of scores |
|
|---|---|---|---|---|
| Score | Max score | |||
| ○No | ○Yes | Head elevation for ICP control | 1 | |
| ○No | ○Yes | Nursed flat (180°) for CPP management | 1 | 1 |
| ○No | ○Yes | Sedation (low-dose as required for mechanical ventilation) | 1 | |
| ○No | ○Yes | Higher-dose sedation for ICP control (not aiming for burst suppression) | 2 | |
| ○No | ○Yes | Metabolic suppression for ICP control with high-dose barbiturates or propofol | 5 | |
| ○No | ○Yes | Neuromuscular blockade (paralysis) | 3 | 8 |
| ○No | ○Yes | CSF drainage < 120 mL/d (<5 mL/h) | 2 | |
| ○No | ○Yes | CSF drainage ≥ 120 mL/d (≥5 mL/h) | 3 | 3 |
| ○No | ○Yes | Fluid loading for maintenance of cerebral perfusion | 1 | |
| ○No | ○Yes | Vasopressor therapy required for management of cerebral perfusion | 1 | 2 |
| ○No | ○Yes | Mild hypocapnia for ICP control (PaCO2 4.6–5.3 kPa [35–40 mm Hg]) | 1 | |
| ○No | ○Yes | Moderate hypocapnia for ICP control (PaCO2 ≥ 4 kPa [30 mm Hg]) | 2 | |
| ○No | ○Yes | Intensive hypocapnia for ICP control (PaCO2 < 4 kPa [30 mm Hg]) | 4 | 4 |
| ○No | ○Yes | Hyperosmolar therapy with mannitol up to 2 g/kg/24 h | 2 | |
| ○No | ○Yes | Hyperosmolar therapy with hypertonic saline up to 0.3 g/kg/24 h | 2 | |
| ○No | ○Yes | Hyperosmolar therapy with mannitol > 2 g/kg/24 h | 3 | |
| ○No | ○Yes | Hyperosmolar therapy with hypertonic saline > 0.3 g/kg/24 h | 3 | 6 |
| ○No | ○Yes | Treatment of fever (>38°C) or spontaneous temperature of 34.5°C | 1 | |
| ○No | ○Yes | Mild hypothermia for ICP control with a lower limit of 35°C | 2 | |
| ○No | ○Yes | Hypothermia below 35°C | 5 | 5 |
| ○No | ○Yes | Intracranial operation for progressive mass lesion, not scheduled on admission | 4 | |
| ○No | ○Yes | Decompressive craniectomy | 5 | 9 |
| Total maximal score: | 38a | |||
Maximum score corresponds to maximum score of pediatric version (Shore et al., 2006).
ICP, intracranial pressure; CSF, cerebrospinal fluid; Paco2, partial arterial carbon dioxide pressure; CPP, cerebral perfusion pressure.
Rehabilitation and post-acute care
Consistent methods for tracking service utilization following treatment or acute care discharge are lacking. Several major issues must be considered when developing CDEs for use in the rehabilitation setting. First, there can be multiple pathways of care prior to initiation of post-acute care. Second, disparities in access to post-acute care may influence the recovery process and confound outcome assessment in acute care studies. A major challenge in the post-acute care phase is posed by the highly variable intervals at which data are recorded, confounding comparability of studies and interpretation of their results. Thus it is the recommendation of this WG to develop a standard procedure for documenting post-acute service utilization after TBI at predetermined, fixed time periods.
Approximately 20% of those hospitalized acutely have sequelae serious enough to require and benefit from inpatient rehabilitation, which can occur in a variety of settings. Patients who are not expected to benefit from an active inpatient rehabilitation program are discharged home, to an outpatient rehabilitation program, nursing home, or other long-term care facility. Both in- and outpatient rehabilitation programs focus on a variety of therapies (e.g., physical therapy, occupational therapy, and speech therapy), to assist individuals in addressing a wide range of newly-acquired impairments and activity limitations that may be cognitive, behavioral, or physical in nature, with a goal of achieving the highest level of independence possible. Insurance coverage and patient financial resources may affect length of rehabilitation. The variability in access to and intensity of post-acute care currently provided indicates a need to explore preferred approaches with comparative effectiveness research. Standardization of data collection is fundamental to such studies.
Protocol experience
This category includes modules on screening, enrolment criteria, informed consent, randomization, protocol compliance, and study completion. Many of these are study-specific. The modules on screening and informed consent warrant special emphasis.
Screening
The importance and relevance of documenting the results of screening procedures have been severely under-recognized in TBI. We present formats to capture exposure to TBI in civilian and military settings, and formats for use in randomized clinical trials. In RCTs, as well as in prospective observational studies, documentation of patient eligibility screening and exclusion is essential to monitor for the possibility of inadvertent selection bias, as mandated by the CONSORT Statement (Schulz et al., 2010).
Informed consent
Accepted approaches to informed consent procedures in acutely mentally incapacitated patients such as TBI patients and those in an emergency situation vary considerably between and even within countries. The necessity for and validity of proxy consent in such emergency situations is subject to much debate (Kompanje et al., 2005; Stocchetti et al., 2004). It has been argued that proxy consent cannot be considered a substitute for the respect of the autonomy of individuals, and concerns have been raised about the validity of decision making by proxy in emergency situations. The approaches taken must comply with national regulations and be accepted by the local IRB. We recognize the following main types of informed consent procedures:
Informed consent: consent given on the basis of verbal or written information given by the patient.
Proxy consent: consent given by someone else other than the patient (e.g., a legal representative or relative of the patient).
Consent by an independent physician: consent by a physician not directly related to the researcher or the department of the researcher, with no conflict of interest with the research project.
Deferred consent: consent given after enrollment by the patient (deferred patient consent), or proxy (deferred proxy consent).
Waiver of consent/exception from informed consent (EFIC): partially waived consent, or waiver or alteration of all elements of consent (e.g., no verbal and no written consent).
EFIC was introduced in the U.S. to allow emergency research in the setting of a life-threatening disorder. EFIC is subject to very strict rules and regulations, as written in the Federal Register (21 CFR 50.24). These rules include that it must be reviewed by the FDA, and requires both community consultation and adequate public disclosure. Where possible, patient or proxy consent should be sought later, but is not considered mandatory (e.g., in case of death, or when no relatives can be found). In every case in which EFIC procedures are followed, concerted efforts for obtaining (deferred) consent should be documented.
Accurate documentation of informed consent procedures is mandatory for all clinical studies, and for those without study-related interventions. Accurate documentation of the informed consent procedures employed, the time of obtaining consent, and where appropriate the time of subsequent written confirmation, is highly relevant and is required from a legal and moral perspective, and is mandatory to comply with ethical regulations.
Adverse events and safety data
Adverse event and safety data reporting is already largely standardized under the guidelines of regulatory authorities and institutional review boards. However, templates are given on the above-referenced websites.
Outcome and function
Information on mortality is important to determine whether death was related to the injury or to other factors, to determine risk factors for death, and to identify causes of death that could possibly be prevented. We recommend recording the underlying cause of death. When extended data recording is undertaken, we further propose the listing of the three main causes leading to death using International Classification of Diseases, 10th Revision (ICD-10) codes. We further recommend investigation of the comparability between causes of death as captured in acute care studies by investigators, versus those captured by nosologists, the medical record coders assigning ICD-9 or ICD-10 codes.
Recommendations on the selection of instruments for assessing outcome have been proposed by Wilde and colleagues (2010). Considerable overlap exists with functional assessments to gauge progress during the post-acute care phase, and as such we recommend that wherever possible, the instruments recommended by Wilde and colleagues should be used. Relevant domains for the assessment of progress during rehabilitation include the resolution of symptoms, functional independence, and assessments of neuropsychological function. No single measure, however, exists that can capture the progress of a patient during all phases of recovery after TBI. We advocate further research of the development of a valid, global clinical assessment tool for use in TBI rehabilitation.
Much interest in the occurrence of symptoms suggestive of post-traumatic stress disorder (PTSD) has arisen from the recent military experience, but relatively little is known about this in civilian TBI (Kennedy et al., 2007). We therefore recommend the routine administration of the PTSD Checklist–Civilian Version (PCL-C) in all patients following TBI.
Discussion
We successfully developed general consensus on the coding of clinical data elements for use across the broad spectrum of TBI. The data elements are presented in a format for use as “building blocks” in the development of case report forms for TBI studies. We hope that these recommendations for common data elements will promote better comparisons between studies, and facilitate meta-analysis of individual patient data across studies. Standardization of data elements is essential in order to facilitate systematic reviews of evidence, and to implement prospective comparative effectiveness research in the field of TBI.
The recommendations presented here have resulted from a large interagency initiative to find “an integrated approach to research in psychological health and traumatic brain injury” (Thurmond et al., 2010). Within this initiative, four working groups on TBI addressed aspects of standardization concerning demographics and clinical assessment, biomarkers, neuroimaging, and outcome. The proceedings of the workshop have previously been published (Duhaime et al., 2010; Maas et al., 2010; Manley et al., 2010; Wilde et al., 2010). For detailed information on the recommendations for neuroimaging, biomarkers, and outcome, we refer the reader to these publications. The diversity and specific characteristics of the topics addressed by the working groups resulted in a different emphasis in these recommendations. For example, in the recommendations of the biomarkers and imaging groups, emphasis was placed on standardization of techniques and procedures, while in the outcomes group the main emphasis was on the selection of instruments. For demographics and clinical assessments, we considered standardization of coding of the variables the most important component. The selection of the variables to be recorded in a given study will be determined by the specific nature and focus of that study.
An element that might be considered crucial for an acute-phase study, for example, may be totally irrelevant for an epidemiological or rehabilitation study. We consequently concluded that at present, there is no rational foundation for either an evidence-based or consensus-based recommendation for the selection of clinical variables. We considered it more relevant to propose different levels of coding for consistent and compatible documentation of variables across the diversity of settings seen in TBI.
We consider the process of developing standardization of data collection in TBI studies to be of great importance, and is crucial to advance the care of TBI patients in the future. The recommendations have been well received in the field, and endorsements have been obtained from the American Association of Neurological Surgeons (AANS)/CNS section on Neurotrauma and Critical Care, the International and National Neurotrauma Societies, and the European Brain Injury Consortium. We emphasize, however, that the process of standardization is, and will remain, an ongoing process. The current proposals for common data elements represent a beta version, which will require further refinement and validation in clinical practice. An ongoing observational study coordinated by Dr. Geoff Manley at the University of California–San Francisco is a first approach towards such validation. Following subsequent refinements, further validation in broader settings will be required. We recognize that much additional work is needed. First, the modules require translation into a web-based entry format with pull-down menus and automated data checks. Second, we recognize that approaches to analysis of the parameters that are continuously monitored in an ICU setting, such as ICP, have not yet been addressed. Current approaches are often crude and widely divergent, using only momentary or summary measures. Here we see a great need for the use of advanced information technology, and further research into the best approaches to these analyses.
Standardizing data collection and coding formats for TBI constitutes one of the most important steps forward in the field of clinical TBI research, and paves the way for achieving more successful results in the future.
Acknowledgments
The meetings and activities of the working group “Demographics and Clinical Assessment” were supported by funding in the context of the interagency initiative towards “an integrated approach to Research in Psychological Health and Traumatic Brain Injury” (National Institutes of Health (NIH)-NINDS; the National Institute on Disability and Rehabilitation Research; the Department of Veterans Affairs; the Defense and Veterans Brain Injury Center; and the Defense Centers of Excellence). The development of CDEs was further supported by a supplemental grant from NIH-NINDS (NS 042691). The authors greatly acknowledge the input and advice obtained from these international experts: Giuseppe Citerio (Italy), Peter Hutchinson (U.K.), Bertil Romner (Denmark), Juan Sahuquillo (Spain), Franco Servadei (Italy), and Nino Stocchetti (Italy). We are grateful to Nele Kleisz and Anne-Claire van Harderwijk for administrative support in developing the common data elements and templates, and in preparing this manuscript.
The views expressed are those of the authors and do not necessarily reflect those of the agencies or institutions with which they are affiliated, including the U.S. Department of Veterans Affairs, the U.S. Department of Defense, the U.S. Department of Education, the U.S. Department of Health and Human Services, the National Institutes of Health, the National Institute of Mental Health, or the Uniformed Services University of the Health Sciences. This work is not an official document, guidance, or policy of the U.S. government, nor should any official endorsement be inferred.
Author Disclosure Statement
No competing financial interests exist.
References
- American Congress of Rehabilitation Medicine Mild Traumatic Brain Injury Committee. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- Association for the Advancement of Automotive Medicine. AAAM; Des Plaines, IL: 1990. 1990. The Abbreviated Injury Scale, 1990 Revision; pp. 15–24. [Google Scholar]
- Baker S.P. O'Neill B. Haddon W. Long W.B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- Bullock R.M. Chesnut R.M. Clifton G.L. Ghajar J. Marion D.W. Narayan R.K. Newell D.W. Pitts L.H. Rosner M.J. Walters B.C. Wilberger J.E. Management and prognosis of severe traumatic brain injury. Guidelines for the management of severe traumatic brain injury. J. Neurotrauma. 2000;17:451–627. [Google Scholar]
- Comper P. Bisschop S. Carnide N. Tricco A. A systematic review of treatments for mild traumatic brain injury. Brain Inj. 2005;19:863–880. doi: 10.1080/02699050400025042. [DOI] [PubMed] [Google Scholar]
- Corrigan J.D. Bogner J.A. Initial reliability and validity of the OSU TBI Identification Method. J. Head Trauma Rehabil. 2007;22:318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- Duhaime A. Gean A. Haacke E. Hicks R. Wintermark M. Mukherjee P. Brody D. Latour L. Riedy G. Common Data Elements neuroimaging advisory panel members. Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehab. 2010;91:1661–1666. doi: 10.1016/j.apmr.2010.07.238. [DOI] [PubMed] [Google Scholar]
- Foulkes M.A.Eisenberg H.M.Jane J.A.Marmarou A.Marshall L.F.the TCDB research group 1991The traumatic coma data bank: design, methods, and baseline characteristics J. Neurosurg 758–13. 2045924 [Google Scholar]
- Jorde L.B. Wooding S.P. Genetic variation, classification and “race”. Nat. Genetics Suppl. 2004;36:S28. doi: 10.1038/ng1435. [DOI] [PubMed] [Google Scholar]
- Kennedy J.E. Jaffee M.S. Leskin G.A. Stokes J.W. Leal F.O. Fitzpatrick P.J. Posttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injury. J. Rehabil. Res. Dev. 2007;44:895–920. doi: 10.1682/jrrd.2006.12.0166. [DOI] [PubMed] [Google Scholar]
- Kompanje E.J.O. Maas A.I.R. Hilhorst M.T. Slieker F.J.A. Teasdale G.M. Ethical considerations on consent procedures for emergency research in severe and moderate traumatic brain injury. Acta Neurochir. (Wien.) 2005;147:633–639. doi: 10.1007/s00701-005-0525-3. ; discussion 639–640. [DOI] [PubMed] [Google Scholar]
- Ling G. Bandak F. Armonda R. Grant G. Ecklund J. Explosive blast neurotrauma. J. Neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Harrison-Felix C.L. Menon D.K. Adelson D. Balkin T. Bullock R. Engel D.C. Gordon W. Langlois J. Lew H.L. Robertson C. Temkin N. Valadka A. Verfaellie M. Wainwright M. Wright D.W. Schwab K. Common data elements for traumatic brain injury: Recommendations from the Interagency Working Group on Demographics and Clinical Assessment. Arch. Phys. Rehab. Med. 2010;91:1641–1649. doi: 10.1016/j.apmr.2010.07.232. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R. Stocchetti N. Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Manley G.T. Ramon Diaz-Aristia R. Brophy M. Engel D. Goodman G. Gwinn-Hardy K. Hayes R. Ling G. Ottens A. Tortella F. Veenstra T. Common data elements for traumatic brain injury: Recommendations from the Biospecimens and Biomarkers Working Group. Arch. Phys. Rehab. Med. 2010;91:1667–1672. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- McMahon C.G. Yates D.W. Campbell F.M. Hollis S. Woodford M. Unexpected contribution of moderate traumatic brain injury to death after major trauma. J. Trauma. 1999;47:891–895. doi: 10.1097/00005373-199911000-00013. [DOI] [PubMed] [Google Scholar]
- Menon D.K.M. Schwab K. Wright D.W.W. Maas A.I.R. Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehab. 2010;91:1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- MRC CRASH Trial Collaborators. Perel P. Arango M. Clayton T. Edwards P. Komolafe E. Poccock S. Roberts I. Shakur H. Steyerberg E. Yutthakasemsunt S. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G. Teasdale G.M. Braakman R. Cohadon F. Dearden M. Iannotti F. Karimi A. Lapierre F. Maas A. Ohman J. Persson L. Servadei F. Stocchetti N. Trojanowski T. Unterberg A. The European Brain Injury Consortium survey of head injuries. Acta Neurochir. (Wien.) 1999;141:223–236. doi: 10.1007/s007010050292. [DOI] [PubMed] [Google Scholar]
- Mushkudiani N.A. Engel D.C. Steyerberg E.W. Butcher I. Lu J. Marmarou A. Slieker F. McHugh G.S. Murray G.D. Maas A.I. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24:259–269. doi: 10.1089/neu.2006.0028. [DOI] [PubMed] [Google Scholar]
- Patel H.C. Bouamra O. Woodford M. King A.T. Yates D.W. Lecky F.E. Trauma Audit and Research Network. Trends in head injury outcome from 1989 to 2003 and the effect of neurosurgical care: an observational study. Lancet. 2005;366:1538–1544. doi: 10.1016/S0140-6736(05)67626-X. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Workshop Scientific Team and Advisory Panel Members. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar A.M. Schwab K. Grafman J.H. Penetrating injuries in the Vietnam War. Traumatic unconsciousness, epilepsy, and psychosocial outcome. Neurosurg. Clin. North Am. 1995;6:715–726. [PubMed] [Google Scholar]
- Shore P. Adelson P.D. Kochanek P. Hand L. Roy L. Reliability and validity of the pediatric intensity level of therapy (pilot) scale: A measure of the use of intracranial pressure-directed therapies. Crit. Care Med. 2006;34:1981–1987. doi: 10.1097/01.CCM.0000220765.22184.ED. [DOI] [PubMed] [Google Scholar]
- Schulz K.F. Altman D.G. Moher D. the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;23(340):c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg E.W. Mushkudiani N. Perel P. Butcher I. Lu J. McHughm G.S. Murray G.D. Marmarou A. Roberts I. Habbema J.D. Maas A.I. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;8:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchetti N. Dearden M. Karimi A. Lapierre F. Maas A. Murray G. Ohman J. Persson L. Servadei F. Trojanowski T. Unterberg A. New European directive on clinical trials: implications for traumatic head injury research. Intensive Care Med. 2004;30:517–518. doi: 10.1007/s00134-003-2142-z. [DOI] [PubMed] [Google Scholar]
- Thurmond V.A. Hicks R.A. Gleason T. Miller A.C. Szuflita N. Orman J. Schwab K. Advancing integrated research in psychological health and Traumatic Brain Injury: Common Data Elements (CDE) Arch. Phys. Med. Rehab. 2010;91:1633–1636. doi: 10.1016/j.apmr.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen N. Lingsma H.F. Perel P. Lecky F. Roozenbeek B. Lu J. Shakur H. Weir J. Steyerberg E.W. Maas A.I. Prognostic value of major extracranial injury in traumatic brain injury: An individual patient data meta analysis in 39,274 patients (submitted for publication. [DOI] [PubMed]
- WHO/OMS. Geneva: World Health Organisation; 2009. Global status report on road safety: Time for action. [Google Scholar]
- Wilde E. Whiteneck G. Bogner J. Bushnik T. Cifu D. Dikmen S. French L. Giacino J. Hart T. Malec J. Millis S. Novack T. Sherer M. Tulsky D. Vanderploeg R. von Steinbuechel N. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehab. 2010;91:1650–1660. doi: 10.1016/j.apmr.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Wolf S.J. Bebarta V.S. Bonnett C.J. Pons P.T. Cantrill S.V. Blast injuries. Lancet. 2009;374:405–415. doi: 10.1016/S0140-6736(09)60257-9. [DOI] [PubMed] [Google Scholar]