Abstract
Background
The research question guiding this study was, “Does receiving individualized feedback about the findings of a research study that the hospice participated in affect clinical practice?” Three issues were examined: (1) Did anyone at the hospice recall receiving the research results? (2) Were the findings shared with the hospice staff? and (3) Did the findings influence clinical practice in the hospice?
Methods
The sample was 170 hospices that participated in a previous study examining the written materials used by hospices to prepare families for death. Participating hospices were sent individualized feedback concerning the signs of impending death and types of information that were present in their hospice's materials. Approximately 1 year later, participating hospices received a follow-up survey.
Results
Sixty-five hospices (40.1%) completed the survey, 33 of these (50.8%) said they received the results of the previous research, 9 (13.8%) said they did not, and 23 (35.4%) said they did not know. All hospices that said that they received the data shared it with the others in the agency. Twenty-six (78.8% of those who recalled receiving the data) said that they made some change to how they prepare families for the patient's death and 11 said the changes were related to the research results they received.
Conclusion
The findings of this study suggest that providing feedback to agencies or individuals who participate in some descriptive studies may be used to promote improvements in clinical care.
Introduction
To provide the best care, we need to act on the best evidence available. We know from previous research examining evidence-based medicine that many physicians will use research evidence when it is made available to them,1,2 but access to research continues to be a barrier to evidence-based medicine.3,4 In nursing as well, access and knowledge of research findings was a primary barrier to integrating research into practice.5,6 Accessibility to research is cited as a barrier in other professions as well.7
One way to penetrate this barrier is to provide information directly back to the clinicians or agencies who participate in research. This practice should eliminate the access barrier and provide a trigger to clinical practice change. The research question guiding this study was, “Does receiving individualized feedback about the findings of a research study that the hospice participated in affect clinical practice?” Three issues were examined: (1) Did anyone at the hospice recall receiving the research results? (2) Were the finding shared with the hospice staff? and (3) Did the findings influence clinical practice in the hospice?
Methods
Parent study: Hospice materials to prepare families for dying in the home8
The purpose of the parent study was to describe how written materials are used by hospices to assist staff in preparing families for death and to describe the content of those materials. The Hospice Materials study included a randomized sample of 400 hospices in the United States that were surveyed and asked to submit the written materials they used to help prepare families for death. The parent study used conventional content analysis to identify the signs of impending death included in the materials. The response rate was 45.3%. The most common signs of impending death were; decreased fluid intake (93.5%), decreased food intake (93.5%), breathing pattern changes (92.9%), cold extremities (92.4%), and mottling (92.4%). The signs least frequently addressed were; pain (28.2%), dyspnea (19.4%), bed-bound state (18.2%), skin changes (18.2%), vital sign changes (17.1%), surge of energy (11.8%), and mandibular breathing (5.9%).
Follow-Up Study
Sample and setting
The sample for this study was the 170 hospices that participated in the Hospice Materials study. Participating hospices were from all regions of the United States, including Alaska and Hawaii.
Procedure
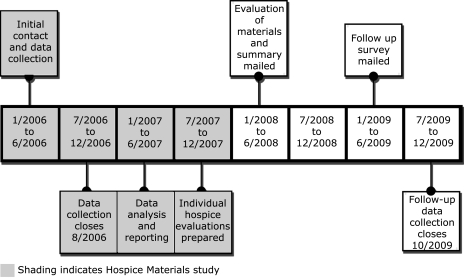
The initial contact with the hospices in the first study was in January 2006. Details of the timeline can be found in Figure 1. Data collection in the initial study was closed in August 2006. As the data was analyzed, the results from the evaluation of the materials for each hospice were compiled separately from the aggregated results.
FIG. 1.
Timeline of contacts with hospices.
In April 2008, the hospices were sent a packet that included a summary of the overall findings, and a grid that showed the signs of impending death and the domains of information that were present in the hospice's materials (Table 1). The 27 signs of impending death included decreased food intake, decreased fluid intake, pain, dypsnea, and vital sign changes. The domains were derived from Johnson's self-regulation theory of coping with illness9 and included information about: (1) the patient's sensory experiences, (2) the family's sensory experiences, (3) causes of the sensations, (4) environmental changes, (5) temporal aspects, and (6) what the family could do.
Table 1.
Example of Information Provided to Hospices
| Type of information Symptoms addressed | Patient sensory | Family sensory | Temporal | Environmental | Causes | What to do | Sub scores |
|---|---|---|---|---|---|---|---|
| Audible respiratory secretions | 1 | 1 | 1 | 3 | |||
| Bed-bound care | 0 | ||||||
| Breathing changes | 1 | 1 | 2 | ||||
| Cold extremities | 1 | 1 | 2 | ||||
| Coma | 1 | 1 | |||||
| Decreased socialization | 1 | 1 | 2 | ||||
| Disorientation | 1 | 1 | |||||
| Dysphagia | 0 | ||||||
| Dyspnea | 0 | ||||||
| Emotional/spiritual | 1 | 1 | |||||
| Fluid intake | 1 | 1 | |||||
| Food intake | 1 | 1 | 2 | ||||
| Incontinence | 1 | 1 | |||||
| Increased central body temperature | 1 | 1 | 2 | ||||
| Mandibular breathing | 1 | 1 | |||||
| Mottling | 1 | 1 | 1 | 3 | |||
| Overall decline | 1 | 1 | |||||
| Pain | 0 | ||||||
| Restlessness | 1 | 1 | 1 | 3 | |||
| Saying good bye | 0 | ||||||
| Sensory changes | 0 | ||||||
| Skin changes | 1 | 1 | 1 | 3 | |||
| Sleeping | 1 | 1 | 2 | ||||
| Surge of energy | 1 | 1 | 1 | 3 | |||
| Time of death | 1 | 1 | |||||
| Unusual communications | 1 | 1 | 2 | ||||
| Urine output decreased | 1 | 1 | |||||
| Visions | 1 | 1 | 1 | 3 | |||
| Vital sign changes | 1 | 1 | 2 | ||||
| Subscores | 1 | 15 | 21 | 0 | 5 | 1 | 43 |
Scaled score = (43/174)*100 = 24.7 (0–100 range, mean score of all 150 documents = 22.4).
In May 2009, a follow-up survey was mailed to explore whether: (1) hospices recalled receiving the results, (2) the results were shared with staff, (3) changes were made to how families were prepared, including changing the materials, and (4) receiving the results of the research affected the changes that were made.
Questionnaire Design
The 1-page questionnaire was developed by the authors, reviewed by three content experts (a nurse researcher with experience in survey design, a hospice nurse manager, and a hospice social worker) and modified according to their suggestions. The questions were: (1) Did you receive the follow-up letter in 2008 with the study results and evaluation of your agency's materials? (2) Did you share the results with your clinical staff and/or administration? (3) Has your agency made any changes in the materials you provide since 2007? (4) Has your agency made any formal changes in how staff prepares families for death since 2007? (5) Were any of these changes related to the evaluation of your agency's materials?
Analysis
SPSS (version 16.0, SPSS Inc., Chicago, IL) was used for calculation. Frequencies and percents were calculated for the responses to the questions on the follow-up survey. Demographic data for the hospices were extracted from the data that those hospices provided for the parent study in 2006. Updates of demographic data were not obtained. Comparative analysis of the demographic data of hospices who responded to the follow-up survey and those who did not was completed.
Results
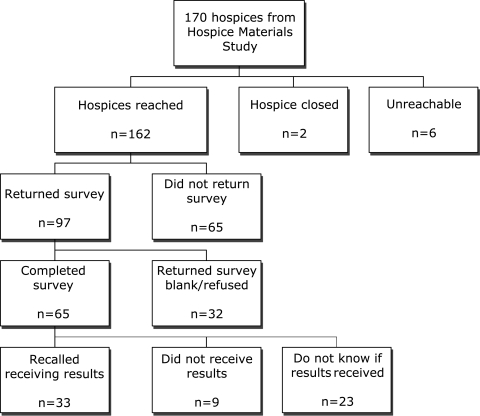
Of the initial 170 hospices, 2 agencies had closed and 6 were unreachable. Ninety-seven of the remaining 162 hospices responded (59.9%), and 65 hospices (40.1%) completed the survey (Fig. 2).
FIG. 2.
Participation flow diagram.
The hospices that responded were not significantly different from those who did not respond to this follow up study in regards to number of offices, average daily census, number of patients served per year, location (urban, rural, or mixed) or race/ethnicity (Table 2). The responding hospices were primarily rural or mixed and were of greatly varying size and ethnic distribution (Table 2).
Table 2.
Demographics of Hospices and Patients and Comparison of Those who Responded to the Follow-Up Survey and Those who Did Not
| |
Demographics of hospices and patients in the hospices that responded to the follow-up survey |
Difference between hospices/patients who completed follow-up survey and those who did not |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Percent | Mean | SD | Min | Max | Mann-Whitney U | Wilcoxon W | Z | Asymp Sig (2-tailed) | |
| Urban setting n = 49a | 6.1% | 1753.50 | 2929.50 | −1.63 | 0.104 | ||||
| Rural setting n = 54a | 48.15% | 2209.50 | 5779.50 | −0.08 | 0.933 | ||||
| Mixed setting (urban and rural) n = 53a | 39.62% | 2030.00 | 5600.00 | −0.83 | 0.407 | ||||
| Average daily census n = 55a | 80.15 | 179.71 | 0 | 1250 | 2273.00 | 6189.00 | −0.43 | 0.665 | |
| Total patients served annually n = 54a | 433.96 | 656.91 | 0 | 3145 | 2097.50 | 592550 | −0.895 | 0.371 | |
| Hispanic or Latino n = 23a | 4.88% | 7.55 | 0% | 30% | 239.0 | 515.00 | −1.24 | 0.216 | |
| Non-Hispanic n = 21a | 92.20% | 21.65 | 1% | 100% | 279.50 | 510.50 | −0.30 | 0.761 | |
| White n = 44a | 91.32% | 11.17 | 50% | 100% | |||||
| Black n = 44a | 4.59% | 8.52 | 0% | 50% | |||||
| Asian n = 44a | 0.58% | 1.71 | 0% | 10% | |||||
| Native American or Eskimo n = 42a | 0.02% | 0.15 | 0% | 1% | |||||
| Other ethnic or racial group n = 44a | 1.61% | 3.30 | 0% | 14% | |||||
| Mixed ethnic or racial group n = 53a | 0.40% | 0.49 | 0% | 1% | |||||
Number used for analysis varies based on whether information was provided by hospice.
Of the hospices that completed the survey 33 (50.8%) said they received the results of the previous research, 9 (13.8%) said they did not, and 23 (35.4%) said they did not know. The 32 hospices that did not receive results or did not know were offered the opportunity to have the results resent. Six hospices requested their results and they were resent in every instance. None of these were included in the rest of the study since they did not have time to act on the results. Hospices that did not recall receiving the information were surveyed, but they uniformly did not complete the rest of the survey.
All hospices who recalled receiving data shared it with the staff. Most shared it with both administration and clinical staff (15, 45.5%), 10 (30.3%) shared it only with clinical staff and five (15.2%) only with administration. Three hospices shared the information with unspecified others.
Of the 33 hospices that recalled receiving the data, 26 (78.8%) said that they made some change to how they prepare families for the patient's death; 12 made changes to both their written materials and their clinical practice, 7 changed their written materials only and 7 changed only their clinical practice. Of the 26 hospices that made changes, 11 (42.3% of those who made changes) said the changes were related to the research results they received. An additional 11 agencies did not know if the changes made were related to the research results.
Discussion
This study is unique in following participating agencies to determine whether receiving individualized feedback from research was used to improve clinical practice. The number of hospices that responded to a follow-up and recalled receiving the results was low. Yet of those who did receive the results and responded, all shared the data, which helps to break down the barrier of lack of access to research data. Most of the hospices who recalled receiving the results made changes either to their written materials or to their agency's clinical practice. Almost half of those who did make changes attributed the change to receiving the results. While the number of agencies who attributed their clinical practice change to the results was small, it is a promising option for future studies.
Limitations
There were a number of limitations to this study. The study is of limited generalizability since the 33 hospices that recalled receiving feedback may not be representative of the more than 3500 hospices in the United States. While there were no significant demographic differences between those hospices that completed the follow up and those who did not, there may be other factors that make these groups different. Also, only 33 of the 170 hospices who participated in the original study (19.4% or 8.25% of the original sample of 400) recalled receiving the results. Some hospices explained that there had been staff changes that may have affected their recall.
This study did not explore in-depth what types of practice change occurred. The results are based on information received from clinical managers who responded based only on agency wide changes. It is not known what changes might have been initiated by individuals.
Clinical and Research Implications
The results of this study, while modest in scope, clearly indicate that providing research results may trigger clinical practice change on an organizational level. When we have little empiric knowledge of a topic, provision of research results may be important in moving both science and clinical practice forward at a more rapid rate. The information that was provided to the hospices was basic and was simple to prepare. Only the results of the content analysis were supplied. Each agency decided what to do with the findings in terms of sharing information and using it to improve practice. The primary issues for researchers intending to do similar follow up include scheduling follow up from the beginning of the study, budgeting for additional follow-up, and keeping contact information up dated to facilitate future communication.
Conclusions
The findings of this study suggest that providing feedback to agencies or individuals who participate in some descriptive studies may be used to promote improvements in clinical care. Most of the agencies that recalled receiving the results of the study reported changes to practice, and many of those changes were directly influenced by the results that they received. We need to explore how provision of individualized research results may aid in improving clinical practice.
Acknowledgments
This study was supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Author Disclosure Statement
The authors have no competing financial interests.
References
- 1.Ellis J. Mulligan I. Rowe J. Sackett DL. Inpatient general medicine is evidence based. A-Team, Nuffield Department of Clinical Medicine. Lancet. 1995;346:407–410. [PubMed] [Google Scholar]
- 2.Gill P. Dowell AC. Neal RD. Smith N. Heywood P. Wilson AE. Evidence based general practice: A retrospective study of interventions in one training practice. BMJ. 1996;312:819–821. doi: 10.1136/bmj.312.7034.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenna HP. Ashton S. Keeney S. Barriers to evidence-based practice in primary care. J Adv Nurs. 2004;45:178–189. doi: 10.1046/j.1365-2648.2003.02879.x. [DOI] [PubMed] [Google Scholar]
- 4.Green ML. Ruff TR. Why do residents fail to answer their clinical questions? A qualitative study of barriers to practicing evidence-based medicine. Acad Med. 2005;80:176–182. doi: 10.1097/00001888-200502000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Funk SG. Tornquist EM. Champagne MT. Barriers and facilitators of research utilization. An integrative review. Nurs Clin North Am. 1995;30:395–407. [PubMed] [Google Scholar]
- 6.Carroll DL. Greenwood R. Lynch KE. Sullivan JK. Ready CH. Fitzmaurice JB. Barriers and facilitators to the utilization of nursing research. Clin Nurs Spec. 1997;11:207–212. doi: 10.1097/00002800-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Humphris D. Littlejohns P. Victor C. O'Halloran P. Peacock J. Implementing evidence-based practice: Factors that Influence the use of research evidence by occupational therapists. Br J Occup Ther. 2000;63:516–522. [Google Scholar]
- 8.Kehl KA. Kirchhoff KT. Finster MP. Cleary JF. Materials to prepare hospice families for dying in the home. J Palliat Med. 2008;11:969–972. doi: 10.1089/jpm.2008.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JE. Self-regulation theory and coping with physical illness. Res Nurs Health. 1999;22:435–448. doi: 10.1002/(sici)1098-240x(199912)22:6<435::aid-nur2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]