Abstract
Social functioning deficits are a prominent feature of many neurological and psychiatric conditions, and may include disruption in the acquisition or application of basic or complex social skills. Such disturbances are often resistant to treatment, and individuals with such conditions are often faced with lifelong difficulties in maintaining personal relationships, employment, and independent living. In recent years, a number of psychosocial treatments have been developed to address this growing problem. In this article, we review studies investigating the use of psychosocial training interventions in individuals with acquired brain injuries, which frequently require intervention for impairments in cognitive and social functioning. We then discuss limitations of these studies and highlight specific areas in which such treatments might be improved in the future.
Key words: brain injury, neuroplasticity, rehabilitation, social cognition, social skills
Introduction
Humans are social beings. We live within a broad spectrum of social relationships and roles, which draw on a diverse set of cognitive processes that may be disrupted to varying degrees by brain dysfunction. Impairments in social functioning are among the most devastating consequences of brain dysfunction and are common across a multitude of neurological and psychiatric conditions, including traumatic brain injury (TBI), stroke, schizophrenia, and autism. Such deficits can place enormous strain on interpersonal relationships and severely limit one's ability to function independently in society. A number of studies have documented efforts to improve social functioning through training in skills that are likely to be important in everyday social interactions, such as engaging in conversation or making eye contact during interactions. Other studies have focused on the treatment of deficits in social cognition, which can be defined as cognitive processes that are recruited in processing information about ourselves and others in support of social interactions. These include the abilities to perceive and interpret the emotions of other people, or to attribute mental states (e.g., beliefs or intentions) to others, also referred to as “theory of mind” (Premack and Woodruff, 1978). Most of this work has been carried out in the context of neuropsychiatric disorders like schizophrenia and autism spectrum disorders (ASD), which are commonly characterized by impairments in social functioning (American Psychiatric Association, 1994).
The head injury sustained by railroad worker Phineas Gage over 150 years ago serves as one of the earliest and most vivid glimpses into the neural basis of social behavior (Damasio, 1994). More recent studies of complex social behavior both in humans (Baron-Cohen et al., 1985; Fletcher et al., 1995; Goel et al., 1995; Rizzolatti and Craighero, 2004), and in non-human animals, have yielded further insights into the brain areas at work in cognition (Fig. 1). In recent decades the development of brain imaging methodologies such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) has given rise to the burgeoning field of social cognitive neuroscience. Recent work in this field suggests that social cognition can be divided into two broad categories. Explicit processes involve awareness and volitional control, and are thought to rely heavily on the prefrontal cortex (PFC), while implicit processes have been characterized as relatively fast and inflexible routines that primarily engage posterior cortical and subcortical brain regions (Forbes and Grafman, 2010; Frith and Frith, 2008; Lieberman, 2007).
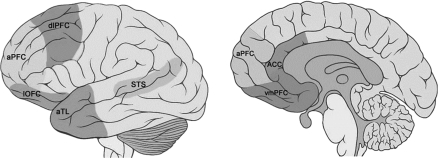
FIG. 1.
Brain regions that support social cognitive processing in humans (aPFC, anterior prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; lOFC, lateral orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex; ACC, anterior cingulate cortex; aTL, anterior temporal lobe; STS, superior temporal sulcus).
In recent years, a growing body of research has begun to address the remediation of impairments in social behavior resulting from acquired brain damage. TBI commonly affects regions in the PFC and temporal lobe that have been linked to the processing of social and emotional information (Adolphs, 1999, 2001; Forbes and Grafman, 2010; Lezak et al., 2004; Lieberman, 2007; Zahn et al., 2009). It has been well documented that TBI can result in a variety of chronic disturbances in social functioning, including social withdrawal, inappropriate behavior, and an inability to establish or maintain meaningful relationships (Hoofien et al., 2001). Further, impairment in psychosocial functioning is one of the strongest determinants of long-term outcome in individuals who have sustained a brain injury (Levin et al., 1987; Schwab et al., 1993), and represents one of the greatest challenges facing providers of rehabilitation services. Further work is needed to better establish how to treat social impairments resulting from brain injury. However, despite an extensive and growing body of literature on the remediation of cognitive impairments in individuals with TBI (Rohling et al., 2009), progress in developing effective methods of treatment for social functioning deficits has been quite limited. The purpose of this article is threefold: (1) to stress the importance of these efforts in improving outcomes following brain injury; (2) to review current research findings regarding therapeutic behavioral interventions aimed at enhancing social functioning, with an emphasis on studies of individuals with acquired brain injuries (ABI); and (3) to outline recommendations for advancing research in this area.
Methods
For the current review, we searched for studies evaluating social training interventions in adults with ABI published in peer-reviewed journals in neuropsychology, brain injury, and head trauma rehabilitation. The search was conducted using PubMed, PsycINFO, and Google Scholar, with the following combination of search terms: traumatic brain injury, stroke, acquired brain injury, training, social rehabilitation, social impairment, social cognition, virtual reality training, remediation, social functioning, theory of mind, attributional style, emotion perception, social skills, and social cognitive neuroscience. Articles were then reviewed to exclude reports based on the following criteria: (1) non-ABI or non-adult samples; (2) reports without adequate methodological details or empirical data; (3) single-case reports; (4) non-social training papers; (5) theoretical articles; (6) pharmacological interventions; and (7) non-English-language papers. The reference lists in these articles were also reviewed to identify other relevant articles. Through this screening process, we selected a total of 12 articles for inclusion in the review. Each article was then evaluated according to the levels of evidence described by Cicerone and colleagues (Cicerone et al., 2000; Table 1). Briefly, “Class I” consisted of prospective randomized controlled trials (RCTs); “Class II” consisted of prospective cohort studies, retrospective case-control studies, or controlled clinical series; and “Class III” included uncontrolled clinical series or single-subject studies. Based on these criteria, six studies were rated as Class I (Bornhofen and McDonald, 2008b, 2008c; Dahlberg et al., 2007; Helffenstein and Wechsler, 1982; McDonald et al., 2008; Radice-Neumann et al., 2009), one as Class II (Johnson and Newton, 1987), and five as Class III (Braunling-McMorrow et al., 1986; Brotherton et al., 1988; Gajar et al., 1984; Guercio et al., 2004; O'Reilly et al., 2000). In most of the studies reviewed here, participants were reported to be medically stable, and classification of injury severity was based on Glasgow Coma Scale scores or duration of post-traumatic amnesia.
Table 1.
Overview of Social Skills Training Programs in Individuals with Brain Injury
| Study | Sample | Design | Study type | Training | Outcome |
|---|---|---|---|---|---|
| Helffenstein and Wechsler, 1982 | ABI (n = 16) | RCT | Class I | Social skills (IPR) | Improvement in interpersonal and communication skills; limited maintenance at 1-month follow-up |
| Gajar et al., 1984 | TBI (n = 2) | Multiple baseline | Class III | Use of feedback and self-monitoring during conversation | Improvement in positive conversational behaviors |
| Braunling-McMorrow et al., 1986 | Severe TBI (n = 3) | Pre-post test | Class III | Social skills | Improvement in all targeted social skills |
| Johnson and Newton, 1987 | Severe TBI (n = 10) | Pre-post test | Class II | Social skills | No significant improvement in treatment group |
| Brotherton et al., 1988 | Severe TBI (n = 4) | Multiple baseline | Class III | Social skills | Limited improvement or maintenance at 1-year follow-up |
| O'Reilly et al., 2000 | TBI (n = 2) | Multiple baseline | Class III | Work-related problem solving/social skills | Improvement that was maintained at 6-week follow-up |
| Guercio et al., 2004 | TBI (n = 3) | Pre-post test | Class III | Emotion recognition | Improved facial-emotion recognition |
| Dahlberg et al., 2007 | Moderate to severe TBI (n = 52) | RCT | Class I | Social skills | Improvement and maintenance at 3-, 6-, and 9-month follow-up |
| McDonald et al., 2008 | ABI (n = 51) | RCT | Class I | Social skills | Limited improvement in social skills |
| Bornhofen and McDonald, 2008b | Severe TBI (n = 12) | RCT | Class I | Emotion perception | Improved emotion perception and social inferences |
| Bornhofen and McDonald, 2008c | Severe TBI (n = 18) | RCT | Class I | Emotion perception (EL or SIT) | More improvement in EL group |
| Radice-Neumann et al., 2009 | Severe ABI (n = 19) | RCT | Class I | Emotion perception (FAR or SEI) | More improvement in FAR group |
TBI, traumatic brain injury; ABI, acquired brain injury; RCT, randomized controlled trial; IPR, interpersonal process recall; EL, errorless learning; SIT, self-instruction training; FAR, facial affect recognition; SEI, stories of emotional inference.
Interventions to Improve Social Functioning in Acquired Brain Injury
There have been numerous reports of impairments in social cognitive abilities such as emotion perception and theory of mind following TBI (Bibby and McDonald, 2005; Bornhofen and McDonald, 2008a; Prigatano and Pribram, 1982), and a variety of methods have been developed for the treatment of such disturbances (Struchen, 2005). In addition, several studies to date have evaluated the effects of social training interventions in individuals with ABI (Bornhofen and McDonald, 2008b, 2008c; Braunling-McMorrow et al., 1986; Brotherton et al., 1988; Dahlberg et al., 2007; Gajar et al., 1984; Helffenstein and Wechsler, 1982; Johnson and Newton, 1987; McDonald et al., 2008; O'Reilly et al., 2000; Radice-Neumann et al., 2009). The training studies discussed in this review primarily focused on two functional domains: social communication skills and emotion perception. All studies included individuals with ABI, most of whom were individuals with TBI studied at least 6 months post-injury.
Impairments in social communicative abilities can disrupt the ability to successfully maintain relationships and employment (Ylvisaker et al., 2001). Most studies that have evaluated the efficacy of social training interventions in individuals with ABI indicate that such training is generally well tolerated, and that improvements in targeted social skills can be achieved through several weeks of practice in either an individual or group format, compared to those who did not receive training (Johnson and Newton, 1987). Consistent with anecdotal reports (Guercio et al., 2004; Helffenstein and Wechsler, 1982), recent findings also indicate that such training may help to improve some aspects of everyday social functioning (Bornhofen and McDonald, 2008b, 2008c; Radice-Neumann et al., 2009), though additional studies are needed to further address this issue.
To date, three RCTs have addressed the efficacy of social skills training in individuals with ABI (Dahlberg et al., 2007; Helffenstein & Wechsler, 1982; McDonald et al., 2008). Helffenstein and Wechsler (1982) examined the effectiveness of 20 h of interpersonal process recall (IPR) training (versus non-therapeutic attention) in 16 individuals (13 male, 3 female) with ABI. IPR involves the recording of social interactions and subsequent review of the recorded interactions with feedback to gain further insights into the interactions. Observer ratings of the participants' interactions indicated that those who received the IPR training showed greater improvement in interpersonal skills, and this improvement was also observed at a follow-up session 1 month later.
Dahlberg and colleagues (2007) evaluated the effects of social skills training in a group of 52 adults (85% male) with TBI. Participants in the training group completed a 12-week group training program with weekly 1.5-h sessions that targeted a variety of social communication skills, such as listening, problem solving with others, and nonverbal communication. Those who received the treatment exhibited significant improvements in a variety of social communication skills at the end of the program, that were also maintained at 6- and 9-month follow-up sessions. Similarly, a study by McDonald and colleagues (2008) evaluated the effects of a 12-week social skills training program (compared to social activity or wait-list control) in a group of 51 adults (40 male, 11 female) with ABI. Participants who received the training showed gains in a few specific behaviors, though there was no clear evidence of improved social functioning following the treatment.
Facial expressions are one of the primary sources of information that we rely on to infer the emotional states of others (Mehrabian and Epstein, 1972). It has been observed that brain injury can disrupt the ability to identify and discriminate facial emotions, despite intact face recognition ability (Green et al., 2004; Ietswaart et al., 2008; Jackson and Moffat, 1987; Milders et al., 2003; Spell and Frank, 2000). To date, numerous studies evaluating treatments for deficits in emotion perception have reported significant gains. While most of these studies have focused on individuals with schizophrenia (Combs et al., 2006, 2007; Frommann et al., 2003; Penn et al., 2000; Wölwer et al., 2005), a number of studies of ASD (Bölte et al., 2002, 2006; Solomon et al., 2004), and in intellectual disability (McAlpine et al., 1992; McKenzie et al., 2000), have also reported improvements in emotion perception ability following training. Moreover, recent work suggests that training can be effective in improving emotion perception in individuals with ABI (Bornhofen and McDonald, 2008b, 2008c; Guercio et al., 2004; Radice-Neumann et al., 2009). Guercio and colleagues used a matching-to-sample approach to train three men with ABI (two with TBI and one with encephalitis) to recognize basic emotions from pictures of faces. While the authors reported significant improvement in this ability from pre-test to post-test, the small sample and use of the same stimuli for training and testing limit the generalizability of these findings beyond this study.
Bornhofen and McDonald (2008b) trained 12 participants (11 male, 1 female) with severe TBI to investigate whether social skills training could improve emotion perception in this population. Half of the participants were randomly assigned to treatment and the other half to a wait-list control group. The training was administered for 8 weeks, in 3-h periods. Participants were asked to identify emotions from facial expressions of people in everyday social situations (e.g., a birthday party), to identify emotions from faces in still photographs or video clips, and to make social inferences. Significant gains were observed for all tasks, but not for identifying emotions from still photographs. A second study from the same authors (Bornhofen and McDonald, 2008c) compared two training approaches (errorless learning and self-instruction training) for treating emotion perception deficits in 18 individuals (17 male, 1 female) with TBI. The stimuli presented were similar to those used in the previous experiment. Participants exhibited improved ability to recognize emotion from still photographs using either strategy, while social inferences were improved only through self-instruction training.
Radice-Neumann and colleagues (2009) evaluated the effectiveness of two training interventions intended to improve the processing of emotions following ABI. One of these approaches (facial affect recognition [FAR]) was intended to enhance the recognition of emotions from facial features (e.g., the eyes or mouth), while the second approach (stories of emotional inference [SEI]) trained participants to infer emotions from a series of short stories. Both types of training were conducted for 1 h per day across nine sessions. In addition, changes in executive and socioemotional functioning were indexed using the Brock Adaptive Functioning Questionnaire, which has been found to help in predicting employment status following TBI (Simpson and Schmitter-Edgecombe, 2002). The authors reported that participants who received the SEI training showed improvement in making emotional inferences, while the FAR training was associated with gains in recognizing facial emotions, in making emotional inferences, and in caregiver ratings of socioemotional behavior.
Limitations and Directions for Future Research
The studies discussed above, particularly those providing Class I evidence (i.e., RCTs), are encouraging in that they suggest that social functioning deficits in individuals with ABI can be ameliorated to some degree through a training intervention. However, there are a number of significant limitations within this body of research that should be highlighted. First, studies evaluating the effects of social training interventions vary considerably with regard to their design, including the specific skills or abilities that are targeted, characteristics of study participants (e.g., age and severity of deficits), lesion characteristics (e.g., age of onset and injury severity), and the outcome measures employed, making it difficult to draw clear conclusions regarding the effectiveness of individual approaches to treatment. Comparisons across studies have been further limited by inadequate detail regarding aspects of study methodology or data presentation, including characterization of study participants and the reporting of effect size estimates. Another issue that remains to be clarified concerns the intensity or “dosage” of training interventions (i.e., how much training is needed to produce meaningful changes), as there are currently no agreed-upon guidelines regarding the level of intensity and duration of training interventions.
Second, a large number of studies are based on relatively small samples, and some of these lack adequate comparison conditions, making it difficult to disentangle specific training effects from other factors (e.g., the passage of time). Also, in studies that involve training in multiple domains of social functioning, it is not entirely clear what features of the treatment are most responsible for observed changes in behavior. Greater progress in identifying the active ingredients of such interventions may strengthen future studies by enabling the design of more appropriate comparison conditions, and will allow the development of more efficient and targeted training programs. Further, psychosocial training studies have predominantly focused on developmental disorders such as ASD or schizophrenia, or adults (particularly men) with TBI. On the one hand, this focus is not surprising, given the prominence of social disturbances in these populations. However, further work is needed to develop interventions that address other clinical populations (e.g., pediatric TBI and stroke), that are commonly faced with lifelong impairments in social functioning.
Another major limitation relates to the psychometric properties of the training and assessment materials used in previous studies. As others have pointed out (Guercio et al., 2004), many of the measures adopted in interventional studies lack sufficient sensitivity to detect changes in social functioning, and the small number of stimuli often contained in these measures imposes practical limits on their applicability to a rehabilitation setting. Further, the training tools or outcome measures employed in some cases may not be sufficiently adapted to the population under study. For instance, ceiling effects have been frequently encountered in studies regarding theory of mind in adults using measures that can easily be mastered by children (Baron-Cohen et al., 1997). There are also very limited data supporting the application of experimental measures across clinical populations and across a range of severity of deficits. Hence, further empirical studies are needed to better understand the limits of current training and assessment tools and how they might be improved in the future.
Fourth, social skills training programs to date have mostly been limited to the treatment of deficits in social communication and emotion recognition, though studies in other populations with similar social functioning deficits point to other approaches that should be explored for treating individuals with ABI. For example, it has been observed that training in some areas of social cognition, such as theory of mind, can be improved in schizophrenia (Kayser et al., 2006), and in ASD (Fisher and Happé, 2006; Swettenham, 1996; Turner-Brown et al., 2008). Several studies evaluating the social cognitive and interaction training (SCIT) program in individuals with schizophrenia, for instance, have reported improvements in social cognitive functions (Combs et al., 2007; Penn et al., 2005, 2007). Given that individuals with brain injury commonly exhibit similar types of impairments (Lundgren et al., 2007), further work is needed to determine whether approaches that show effectiveness in other populations might also benefit individuals with acquired brain damage.
As discussed above, there is also very limited empirical support regarding the generalizability of training-related improvements in social skills or social cognition to other functional domains. In particular, a growing number of studies have reported improvements in social skills or in more specific aspects of social cognition following training, although few of them have examined the extent to which training in one domain enhances other abilities (e.g., executive functions), or the degree to which such improvements extend to real-life functioning. The vast majority of training studies to date have relied on pictures or other static stimuli, and it has been argued that dynamic training stimuli (e.g., film clips or virtual reality environments) may provide greater generalization to everyday social settings (Bornhofen and McDonald, 2008a; Parsons and Mitchell, 2002). Virtual reality environments have also been discussed as an approach to rehabilitation that may help to increase the generalization of treatment effects to the real world (Burdea, 2003). Role-play in such interactive environments can be used to approximate real-life social settings in a flexible and relatively non-threatening manner, and given the repetitive nature of rehabilitation, such approaches could potentially help to increase patient motivation during treatment. To date, studies using virtual reality have been carried out in individuals undergoing motor rehabilitation (Henderson et al., 2007; Merians et al., 2002), and in the treatment of social impairments in ASD (Parsons and Mitchell, 2002). Further work in this area may play an important role in clarifying the potential of laboratory training procedures for improving real-life functional outcomes in individuals with brain dysfunction. In addition, as a large proportion of individuals with brain injury are unable to maintain long-term employment following their injury (van Velzen et al., 2009), one important goal for future research is to develop training interventions that are capable of improving return to work and other real-life outcomes in individuals with brain injury.
One of the most striking limitations of this literature, however, is the limited amount of attention paid to the sustainability of training-related improvements over time. Only a handful of the studies reviewed above (Brotherton et al., 1988; Dahlberg et al., 2007; Helffenstein and Wechsler, 1982; O'Reilly et al., 2000; Radice-Neumann et al., 2009) addressed the duration of training effects, and these studies generally involved relatively short-term post-training evaluations (i.e., less than 1 year later). Further studies in large samples and over longer time periods are needed to more convincingly demonstrate their clinical utility. The development of training methods that involve the patient caregiver in addition to the patient (Togher et al., 2009) represents one approach that may help to promote target behaviors over longer periods.
Behavioral training interventions may also benefit from advances in our ability to study and modify brain activity. Within the last decade, brain stimulation techniques such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) have become more widely adopted to explore the mechanisms of cortical plasticity within brain networks (Siebner and Rothwell, 2003). From these studies, there is evidence that modulation of cortical activity can induce short-term gains in cognitive functioning, and may play a role in improving the treatment of aphasia, unilateral neglect, and other neurological disorders (Miniussi et al., 2008). A better understanding of the applicability of these tools in TBI could be instrumental in the development and application of effective clinical rehabilitation interventions to promote the recovery of social skills and improve outcomes in these populations.
There is also much that can be gained from existing structural and functional neuroimaging tools to develop improved methods for treating deficits in social functioning. Such methods have contributed enormously to our understanding of the neural systems that support social interactions, and have informed current thinking about the brain mechanisms that underlie social cognitive deficits in disorders like autism and schizophrenia (Critchley et al., 2000; Pinkham et al., 2008). Further, a growing number of studies have demonstrated that the neural circuitry supporting a variety of functions can be modified in response to injury or during learning (Raymont and Grafman, 2006). Several studies using PET or fMRI in humans have demonstrated activity changes in different brain areas after a training intervention (Bölte et al., 2006; DeGutis et al., 2007; Kawashima and Fukuda, 1994; Schlaug et al., 1994; Vitali et al., 2007), though no study to date has used brain-imaging methodologies to investigate the neuroplastic changes associated with social cognitive training in ABI. A better understanding of the conditions that stimulate such changes, particularly those associated with functional improvements, will no doubt be an important step toward improving future rehabilitation efforts.
Furthermore, a better understanding of genetic factors that support neuroplasticity may help to identify potential targets for therapeutic efforts. A recent study of military veterans with TBI examined the possible role of the val66met polymorphism of the BDNF gene in recovery of cognitive functioning following brain injury (Krueger et al., 2010). It was observed that carriers of the met allele exhibited evidence of greater recovery of executive functioning than those with the val allele, suggesting that the met allele may actually serve a protective role in recovery of function following a brain injury. In the future, studies using similar approaches may lead to improved triage and treatments for impairments in social functioning.
Conclusion
Considerable progress has been made toward understanding the long-term consequences of brain dysfunction with regard to social functioning. A large and growing body of research suggests that such impairments can be effectively remediated in many individuals with TBI and other forms of brain damage, though in general, empirical support for these interventions has not been strong enough to justify their use on a large scale. Given the growing numbers of civilians and military personnel who are surviving significant brain injuries, it is clear that effective treatments for such impairments are needed now more than ever.
In this paper, we have highlighted several areas in which training intervention studies might be improved (Table 2). In particular, more work is needed to develop methodologically sound techniques capable of producing reliable improvements in real-life social functioning that can be sustained for long periods. Based on the body of literature reviewed here, it is likely that rehabilitation efforts that can be tailored to an individual's unique pattern of deficits, and can be reinforced through the support of family and caregivers, will move us closer to achieving this goal. We hope this new decade heralds a sustained research effort to establish useful evidence-based techniques for maximizing social skills remediation and outcome in individuals with ABI.
Table 2.
Recommendations for Improving Future Training Studies
| Limitations | Recommendations |
|---|---|
| • Limited empirical support for different training approaches | • Greater focus on identifying limits and active ingredients of training approaches |
| • Methodological weaknesses (e.g., small sample size, inadequate controls) | • More randomized controlled trials and studies in larger samples |
| • Limited study of the effects of training in social cognitive abilities (e.g., theory of mind) | • Further study of effects of training in social cognition • Greater emphasis on sustainability of training-related improvements and transfer of learning to other functions |
| • Limited attention to generalizability and sustainability of training-related improvements | • Further study of neural and genetic factors that may influence recovery of function following brain injury |
Acknowledgments
This work was supported by funding from the U.S. National Institute of Neurological Disorders and Stroke intramural research program, and the Center for Neuroscience and Regenerative Medicine.
Author Disclosure Statement
No competing financial interests exist.
References
- Adolphs R. Social cognition and the human brain. Trends Cogn. Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. APA; Washington, DC: 1994. Text Revision. [Google Scholar]
- Baron-Cohen S. Jolliffe T. Mortimore C. Robertson M. Another advanced test of theory of mind: Evidence from very high-functioning adults with autism or Asperger syndrome. J. Child Psychol. Psychiatry. 1997;38:813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Leslie A.M. Frith U. Does the autistic child have a ‘‘theory of mind’’? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bibby H. McDonald S. Theory of mind after traumatic brain injury. Neuropsychologia. 2005;43:99–114. doi: 10.1016/j.neuropsychologia.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Bölte S. Feineis-Matthews S. Leber S. Dierks T. Hubl D. Poustka F. The development and evaluation of a computer-based program to test and to teach the recognition of facial effect. Int. J. Circumpolar Health. 2002;2:61–68. doi: 10.3402/ijch.v61i0.17503. [DOI] [PubMed] [Google Scholar]
- Bölte S. Hubl D. Feineis-Matthews S. Prvulovic D. Dierks T. Poustka F. Facial affect recognition training in autism: Can we animate the fusiform gyrus? Behav. Neurosci. 2006;120:211–216. doi: 10.1037/0735-7044.120.1.211. [DOI] [PubMed] [Google Scholar]
- Bornhofen C. McDonald S. Comparing strategies for treating emotion perception deficits in traumatic brain injury. J. Head Trauma Rehabil. 2008c;23:103–115. doi: 10.1097/01.HTR.0000314529.22777.43. [DOI] [PubMed] [Google Scholar]
- Bornhofen C. McDonald S. Emotion perception deficits following traumatic brain injury: A review of the evidence and rationale for intervention. J. Int. Neuropsychol. Soc. 2008a;14:511–528. doi: 10.1017/S1355617708080703. [DOI] [PubMed] [Google Scholar]
- Bornhofen C. McDonald S. Treating deficits in emotion perception following traumatic brain injury. Neuropsychol. Rehabil. 2008b;18:22–44. doi: 10.1080/09602010601061213. [DOI] [PubMed] [Google Scholar]
- Braunling-McMorrow D. Lloyd K. Fralish K. Teaching social skills to head injured adults. J. Rehabil. 1986;52:39–44. [Google Scholar]
- Brotherton F.A. Thomas L.L. Wisotzek I.E. Milan M.A. Social skills training in the rehabilitation of patients with traumatic closed head injury. Arch. Phys. Med. Rehabil. 1988;69:827–832. [PubMed] [Google Scholar]
- Burdea G.C. Virtual rehabilitation-benefits and challenges. Methods Inf. Med. 2003;42:519–523. [PubMed] [Google Scholar]
- Cicerone K.D. Dahlberg C. Kalmar K. Langenbahn D.M. Malec J.F. Bergquist T.F. Felicetti T. Giacino J.T. Harley J.P. Harrington D.E. Herzog J. Kneipp S. Laatsch L. Morse P.A. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch. Phys. Med. Rehabil. 2000;81:1596–1615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- Combs D.R. Adams S.D. Penn D.L. Roberts D. Tiegreen J. Stem P. Social Cognition and Interaction Training (SCIT) for inpatients with schizophrenia spectrum disorders: Preliminary findings. Schizophr. Res. 2007;91:112–116. doi: 10.1016/j.schres.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Combs D.R. Tosheva A. Wanner J. Basso M.R. Remediation of emotion perception deficits in schizophrenia: The use of attentional prompts. Schizophr. Res. 2006;87:340–341. doi: 10.1016/j.schres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. Daly M.E. Bullmore E.T. Williams S.C.R. Van Amelsvoort T. Robertson D.M. Rowe A. Phillips M. McAlonan G. Howlin P. Murphy D.G.M. The functional neuroanatomy of social behaviour. Brain. 2000;123:2203–2012. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dahlberg C.A. Cusick C.P. Hawley L.A. Newman J.K. Morey C.E. Harrison-Felix C.L. Whiteneck G.G. Treatment efficacy of social communication skills training after traumatic brain injury: A randomized treatment and deferred treatment controlled trial. Arch. Phys. Med. Rehabil. 2007;88:1561–1573. doi: 10.1016/j.apmr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Damasio A.R. Descartes' Error: Emotion, Reason, and the Human Brain. Quill; New York: 1994. [Google Scholar]
- DeGutis J.M. Bentin M. Robertson L.C. D'Esposito M. Functional plasticity in ventral temporal cortex following cognitive rehabilitation of a congenital prosopagnosia. J. Cogn. Neurosci. 2007;19:1790–1802. doi: 10.1162/jocn.2007.19.11.1790. [DOI] [PubMed] [Google Scholar]
- Fisher N. Happé F. A training study of theory of mind and executive function in children with autistic spectrum disorders. J. Autism Dev. Disord. 2006;35:757–771. doi: 10.1007/s10803-005-0022-9. [DOI] [PubMed] [Google Scholar]
- Fletcher P.C. Happe F. Frith U. Baker S.C. Dolan R.J. Frackowiak R.S. Frith C.D. Other minds in the brain: A functional imaging study of ‘‘theory of mind’’ in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Forbes C.E. Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu. Rev. Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Frith C.D. Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60:503–510. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Frommann N. Streit M. Wölwer W. Remediation of facial affect recognition in patients with schizophrenia: A new training program. Psychiatry Res. 2003;117:281–284. doi: 10.1016/s0165-1781(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Gajar A. Schloss P.J. Schloss C.N. Thompson C.K. Effects of feedback and self-monitoring on head trauma youths' conversation skills. J. Appl. Behav. Anal. 1984;17:353–358. doi: 10.1901/jaba.1984.17-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V. Grafman J. Sadato N. Hallett M. Modeling other minds. Neuroreport. 1995;6:1741–1746. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Green R. Turner G.R. Thompson W.F. Deficits in facial emotion perception in adults with recent traumatic brain injury. Neuropsychologia. 2004;42:133–141. doi: 10.1016/j.neuropsychologia.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Guercio J.M. Podolska-Schroeder H. Rehfeldt R.A. Using stimulus equivalence technology to teach emotion recognition to adults with acquired brain injury. Brain Inj. 2004;18:593–601. doi: 10.1080/02699050310001646116. [DOI] [PubMed] [Google Scholar]
- Helffenstein D.A. Wechsler F.S. The use of interpersonal process recall (IPR) in the remediation of interpersonal and communication skill deficits in the newly-brain injured. Arch. Clin. Neuropsychol. 1982;4:139–142. [Google Scholar]
- Henderson A. Korner-Bitensky N. Levin M. Virtual reality in stroke rehabilitation. A systematic review of its effectiveness for upper limb motor recovery. Top. Stroke Rehabil. 2007;14:52–61. doi: 10.1310/tsr1402-52. [DOI] [PubMed] [Google Scholar]
- Hoofien D. Gilboa A. Vakil E. Donovick P.J. Traumatic brain injury (TBI) 10–20 years later: A comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj. 2001;15:189–209. doi: 10.1080/026990501300005659. [DOI] [PubMed] [Google Scholar]
- Ietswaart M. Milders M. Crawford J.R. Currie D. Scott C.L. Longitudinal aspect of emotion recognition in patients with traumatic brain injury. Neuropsychologia. 2008;46:148–159. doi: 10.1016/j.neuropsychologia.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Jackson H.F. Moffat N.J. Impaired emotional recognition following severe head injury. Cortex. 1987;23:293–300. doi: 10.1016/s0010-9452(87)80039-4. [DOI] [PubMed] [Google Scholar]
- Johnson D.A. Newton A. Social adjustment and interaction after severe head injury: II. Rationale and bases for intervention. Br. J. Clin. Psychol. 1987;26:289–298. doi: 10.1111/j.2044-8260.1987.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Kawashima R. Fukuda H. Functional organization of the human primary motor area: An update on current concepts. Rev. Neurosci. 1994;5:347–354. doi: 10.1515/revneuro.1994.5.4.347. [DOI] [PubMed] [Google Scholar]
- Kayser N. Sarfati Y. Besche C. Hardy-Bayle M.C. Elaboration of a rehabilitation method based on a pathogenetic hypothesis of ‘theory of mind’ impairment in schizophrenia. Neuropsychol. Rehabil. 2006;16:83–95. doi: 10.1080/09602010443000236. [DOI] [PubMed] [Google Scholar]
- Krueger F. Pardini M. Huey E.D. Raymont V. Solomon J. Lipsky R.H. Hodgkinson C.A. Goldman D. Grafman J. The role of the met66 BDNF allele in the recovery of executive functioning following combat-related traumatic brain injury. J. Neurosci. 2010 doi: 10.1523/JNEUROSCI.1399-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H.S. Grafman J. Eisenberg H.M. Neurobehavioral Recovery From Head Injury. Oxford University Press; New York: 1987. [Google Scholar]
- Lezak M.D. Howieson D.B. Loring D.W. Neuropsychological Assessment. Oxford University Press; New York: 2004. [Google Scholar]
- Lieberman M.D. Social cognitive neuroscience: A review of core processes. Annu. Rev. Clin. Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lundgren K. Brownell H. Cayer-Meade C. Spitzer J. Training theory of mind following right hemisphere damage: A pilot study. Brain Lang. 2007;103:209–210. [Google Scholar]
- McAlpine C. Singh N.N. Kendall K.A. Ellis C.R. Recognition of facial expressions of emotion by persons with mental retardation. A matched comparison study. Behav. Modif. 1992;16:543–558. doi: 10.1177/01454455920164006. [DOI] [PubMed] [Google Scholar]
- McDonald S. Tate R. Togher L. Bornhofen C. Long E. Gertler P. Bowen R. Social skills treatment for people with severe, chronic acquired brain injuries: A multicenter trial. Arch. Phys. Med. Rehabil. 2008;89:1648–1659. doi: 10.1016/j.apmr.2008.02.029. [DOI] [PubMed] [Google Scholar]
- McKenzie K. Matheson E. McKaskie K. Hamilton L. Murray G.C. Impact of group training on emotion recognition in individuals with a learning disability. Br. J. Learning Disabilities. 2000;28:143–147. [Google Scholar]
- Mehrabian A. Epstein N. A measure of emotional empathy. J. Pers. 1972;40:525–543. doi: 10.1111/j.1467-6494.1972.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Merians A.S. Jack D. Boian R. Tremaine M. Burdea G.C. Adamovich S.V. Recce M. Poizner H. Virtual reality–augmented rehabilitation for patients following stroke. Phys. Ther. 2002;82:898–915. [PubMed] [Google Scholar]
- Milders M. Fuchs S. Crawford J.R. Neuropsychological impairments and changes in emotional and social behaviour following severe traumatic brain injury. J. Clin. Exp. Neuropsychol. 2003;25:157–172. doi: 10.1076/jcen.25.2.157.13642. [DOI] [PubMed] [Google Scholar]
- Miniussi C. Cappa S.F. Cohen L.G. Floel A. Fregni F. Nitsche M.A. Oliveri M. Pascual-Leone A. Paulus W. Priori A. Walsh A. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008;1:326–336. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- O'Reilly M.F. Lancioni G.E. Kane N.O. Using a problem-solving approach to teach social skills to workers with brain injuries in supported employment settings. J. Vocat. Rehabil. 2000;14:187–194. [Google Scholar]
- Parsons S. Mitchell P. The potential of virtual reality in social skills training for people with autistic spectrum disorders. J. Intellect. Disabil. Res. 2002;46:430–443. doi: 10.1046/j.1365-2788.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- Penn D.L. Combs D.R. Ritchie M. Francis J. Cassisi J. Morris S. Townsend M. Emotion recognition in schizophrenia: Further investigation of generalized versus specific deficit models. J. Abnorm. Psychol. 2000;109:512–516. [PubMed] [Google Scholar]
- Penn D.L. Roberts D.L. Combs D. Sterne A. Best practices: The development of the social cognition and interaction training program for schizophrenia spectrum disorders. Psychiatr. Serv. 2007;58:449–451. doi: 10.1176/ps.2007.58.4.449. [DOI] [PubMed] [Google Scholar]
- Penn D. Roberts D.L. Munt E.D. Silverstein E. Jones N. Sheitman B. A pilot study of Social Cognition and Interaction Training (SCIT) for schizophrenia. Schizophr. Res. 2005;80:357–359. doi: 10.1016/j.schres.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Pinkham A.E. Hopfinger J.B. Pelphrey K.A. Piven J. Penn D.L. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr. Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D.G. Woodruff G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978;1:515–526. [Google Scholar]
- Prigatano G.P. Pribram K.H. Perception and memory of facial affect following brain injury. Percept. Mot. Skills. 1982;54:859–869. doi: 10.2466/pms.1982.54.3.859. [DOI] [PubMed] [Google Scholar]
- Radice-Neumann D. Zupan B. Tomita M. Willer B. Training emotional processing in persons with brain injury. J. Head Trauma Rehabil. 2009;24:313–323. doi: 10.1097/HTR.0b013e3181b09160. [DOI] [PubMed] [Google Scholar]
- Raymont V. Grafman J. Cognitive neural plasticity during learning and recovery from brain damage. Brain Res. 2006;157:199–206. doi: 10.1016/S0079-6123(06)57013-X. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G. Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rohling M. Faust M.E. Beverly B. Demakis G. Effectiveness of cognitive rehabilitation following acquired brain injury: A meta-analytic re-examination of the Cicerone et al. (2002, 2005) systematic reviews. Neuropsychology. 2009;23:20–39. doi: 10.1037/a0013659. [DOI] [PubMed] [Google Scholar]
- Schlaug G. Knorr U. Seitz R.J. Inter-subject variability of cerebral activations in acquiring a motor skill: A study with positron emission tomography. Exp. Brain Res. 1994;98:523–534. doi: 10.1007/BF00233989. [DOI] [PubMed] [Google Scholar]
- Schwab K. Grafman J. Salazar A.M. Kraft J. Residual impairments and work status 15 years after penetrating head injury: Report from the Vietnam Head Injury Study. Neurology. 1993;43:95–103. doi: 10.1212/wnl.43.1_part_1.95. [DOI] [PubMed] [Google Scholar]
- Siebner H.R. Rothwell J. Transcranial magnetic stimulation: New insights into representational cortical plasticity. Exp. Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Simpson A. Schmitter-Edgecombe M. Prediction of employment status following traumatic brain injury using a behavioural measure of frontal lobe functioning. Brain Inj. 2002;16:1075–1091. doi: 10.1080/02699050210155249. [DOI] [PubMed] [Google Scholar]
- Solomon M. Goodlin-Jones B.L. Anders T.F. A social adjustment enhancement intervention for high functioning autism, Asperger's syndrome, and pervasive developmental disorder NOS. J. Autism Dev. Disord. 2004;34:649–668. doi: 10.1007/s10803-004-5286-y. [DOI] [PubMed] [Google Scholar]
- Spell L.A. Frank E. Recognition of nonverbal communication of affect following traumatic brain injury. J. Nonverbal Behav. 2000;24:285–300. [Google Scholar]
- Struchen M.A. In: Social communication interventions, in: Rehabilitation of Traumatic Brain Injury. W.M. High., editor; A.M. Sander., editor; M.A. Struchen., editor; K.A. Hart., editor. Oxford University Press; New York: 2005. pp. 88–117. [Google Scholar]
- Swettenham J. Can children with autism be taught to understand false belief using computers? J. Child Psychol. Psychiatry. 1996;37:157–165. doi: 10.1111/j.1469-7610.1996.tb01387.x. [DOI] [PubMed] [Google Scholar]
- Togher L. McDonald S. Tate R. Power E. Rietdijk R. Training communication partners of people with traumatic brain injury: Reporting the protocol for a clinical trial. Brain Impair. 2009;10:188–204. [Google Scholar]
- Turner-Brown L.M. Perry D.T. Dichter G.S. Bodfish J.W. Penn D.L. Brief report: Feasibility of social cognition and interaction training for adults with high functioning autism. J. Autism Dev. Disord. 2008;38:1777–1784. doi: 10.1007/s10803-008-0545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen J.M. van Bennekom C.A. Edelaar M.J. Sluiter J.K. Frings-Dresen M.H. How many people return to work after acquired brain injury? A systematic review. Brain Inj. 2009;23:473–488. doi: 10.1080/02699050902970737. [DOI] [PubMed] [Google Scholar]
- Vitali P. Abutalebi J. Tettamanti M. Danna M. Ansaldo A. Perani D. Joanette Y. Cappa S.F. Training-induced brain remapping in chronic aphasia: A pilot study. Neurorehabil. Neural Repair. 2007;21:152–160. doi: 10.1177/1545968306294735. [DOI] [PubMed] [Google Scholar]
- Wölwer W. Frommann N. Halfmann S. Piaszek A. Streti M. Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: Efficacy and specificity of a new training program. Schizophr. Res. 2005;80:295–303. doi: 10.1016/j.schres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Ylvisaker M. Todis B. Glang A. Urbanczyk B. Franklin C. DePompei R. Feeney T. Maxwell N.M. Pearson S. Tyler J.S. Educating students with TBI: Themes and recommendations. J. Head Trauma Rehabil. 2001;16:76–93. doi: 10.1097/00001199-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Zahn R. Moll J. Paiva M. Garrido G. Krueger F. Huey E.D. Grafman J. The neural basis of human social values: Evidence from functional MRI. Cereb. Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]