Abstract
Purpose
The primary objective of this study was to determine if children with localized ependymoma experience a decline in verbal or visual-auditory learning after conformal radiation therapy (CRT). The secondary objective was to investigate the impact of age and select clinical factors on learning before and after treatment.
Methods and Materials
Learning in a sample of 71 patients with localized ependymoma was assessed with the California Verbal Learning Test (CVLT-C) and the Visual-Auditory Learning Test (VAL). Learning measures were administered before CRT, at six months and then yearly for a total of five years.
Results
There was no significant decline on measures of verbal or visual-auditory learning after CRT; however, younger age, more surgeries and CSF shunting did predict lower scores at baseline. There were significant longitudinal effects (improved learning scores after treatment) among older children on the CVLT-C and children that did not receive pre-CRT chemotherapy on the VAL.
Conclusion
There was no evidence of global decline in learning after CRT in children with localized ependymoma. Several important implications from the findings include: 1) identification of and differentiation among variables with transient versus long-term effects on learning; 2) demonstration that children treated with chemotherapy prior to CRT had greater risk of adverse visual-auditory learning performance, and; 3) establishment of baseline and serial assessment as critical in ascertaining necessary sensitivity and specificity for the detection of modest effects.
Keywords: ependymoma, brain tumor, radiotherapy, memory, pediatrics
INTRODUCTION
Advances in conformal radiation therapy (CRT) have lowered the risk of cognitive decline in pediatric brain tumor patients by minimizing exposure of healthy brain tissue to irradiation.1-4 CRT uses 3-dimensional imaging and specialized software to concentrate the prescribed dose to the tumor while sparing surrounding healthy tissue.3 Research has linked higher doses of radiation to longitudinal decline in IQ, memory, attention and academic achievement.2-4 These adverse effects are more pronounced with conventional radiation therapy versus CRT.5-8 Cognitive decline after irradiation has been attributed to a slower rate of knowledge acquisition versus loss of intellectual skill.9,10
A prior study examining neurocognitive outcome in children with ependymoma treated with CRT found that children under 3 years of age at the time of CRT had significantly lower intellectual function scores at baseline compared to children over 3 years.3 However, these differences were no longer significant at the 48 month follow-up time point since the younger cohort exhibited an increase in IQ over time.3 No significant declines in memory, academic achievement or adaptive behavior were found at the 24 month follow-up time point; although only a portion of the cohort completed all measures of memory, learning and academic achievement.3
CRT has demonstrated the potential to reduce global cognitive decline following treatment; however, the possibility of decline in other neurocognitive domains remains to be determined.2,3,11-15 Preliminary evidence of subtle difficulties in reading, attention and memory following irradiation in the treatment of ependymoma have been reported.15-16 We recently found that children with ependymoma experienced modest declines in reading but not math or spelling after CRT.16 Another study of children treated with conventional irradiation for infratentorial ependymoma revealed reading difficulties after treatment, as well as visuospatial, memory and attention deficits, despite the lack of significant deterioration in aggregate IQ scores following treatment.15
Childhood ependymoma typically emerges at an earlier age than other localized tumors such as craniopharyngioma and astrocytoma, which may create additional risk as young age is a robust predictor of adverse neurocognitive outcome.4,17-21 Studies investigating outcome in children with ependymoma have found young age to predict lower mean scores on measures of intellectual function, although young age has not necessarily been found to predict decline in intellectual function after treatment.3,8,15
For the current study, verbal and visual-auditory learning were serially assessed in children with localized ependymoma. Patients were treated with smaller-than-conventional volumes with the objective of sparing normal tissue. It was our hypothesis that the use of CRT would not result in significant longitudinal decline in verbal or visual-auditory learning after treatment. A secondary goal was to examine the effect of age and other select clinical factors on neurocognitive outcome. Despite advances in CRT, age was predicted to exert a modest but significant effect on rate of learning after treatment. Hypothetically, older children were expected to exhibit a more rapid rate of learning over time than younger children. To our knowledge, no prior studies have examined and compared rate of longitudinal verbal and visual-auditory learning in a prospective manner over a five year span. Recent advances in treatment have reduced the likelihood of more pervasive and conspicuous deficits in the form of global neurocognitive decline(s) after treatment.2-10 Therefore, future studies will need to adapt to treatment advances by employing investigative methods more sensitive to the detection of specific and less conspicuous side effects. The investigative methods used in this study could serve as a potential benchmark for future studies.
PATIENTS AND METHODS
Patients
Seventy-one pediatric patients with localized primary ependymoma were enrolled on a phase II trial of CRT between July 1997 and August 2007. The study was approved by the Institutional Review Board and written informed consent was obtained prior to participation. Criteria for enrollment included diagnosis of localized ependymoma without metastasis, age range between 1 and 21 years at the time of irradiation, no prior irradiation, no ongoing chemotherapy and adequate performance status (ECOG performance grade 0-2).22 Participants ranged from 1 to 15 years of age at the start of CRT (mean age = 5.59 ± 3.82). The median length of follow-up was 59.6 months (range = 11.7 - 92.0). No participants were excluded due to motor, vision or hearing impediments that would have prohibited testing. Patients who experienced treatment failure were not included in the study. Clinical and demographic characteristics are in Table 1.
Table 1.
Patient Demographic and Clinical Characteristics (N = 71)
| N | % | |
|---|---|---|
| Gender | ||
| Female | 35 | 49.3 |
| Male | 36 | 50.7 |
| Tumor Location | ||
| Infratentorial | 52 | 73.2 |
| Supratentorial | 19 | 26.8 |
| Hydrocephalus | ||
| No | 23 | 32.4 |
| Yes | 48 | 67.6 |
| CSF Shunting | ||
| No | 49 | 69.0 |
| Yes | 22 | 31.0 |
| Pre-CRT Chemotherapy | ||
| No | 56 | 78.9 |
| Yes | 15 | 21.1 |
| Pre-CRT Extent of Resection | ||
| GTR or NTR | 65 | 91.6 |
| STR | 6 | 8.5 |
| Total Number of Pre-CRT Surgeries | ||
| 1 | 44 | 62.0 |
| 2-4 | 27 | 38.0 |
| Tumor Laterality | ||
| Midline | 49 | 69.0 |
| Left | 9 | 12.7 |
| Right | 13 | 18.3 |
| Mean ± SD | Range | |
|---|---|---|
| Age at CRT (Years) | 5.59 ± 3.82 | 1.06 – 15.37 |
| Time from Diagnosis to CRT (Years) | 0.54 ± 1.07 | 0.05 – 5.80 |
Acronyms: CSF= cerebrospinal fluid; CRT= conformal radiation therapy; GTR = gross-total resection; NTR = near-total resection; STR = sub-total resection.
CRT
All patients received CRT, including intensity-modulated radiation therapy, over six weeks with dosage fractionated to 1.8 Gy per day. Total dose ranged from 54 Gy to 59.4 Gy; children ≤18 months received 54 Gy after gross-total resection. Target volume definitions and treatment parameters are reviewed in prior studies.3,12
Clinical Variables
All patients underwent systematic tumor resection before CRT. Patients that developed progressive disease were removed from the study. Approximately 20% of patients received chemotherapy prior to irradiation (typically a multiagent regimen of cyclophosphamide, cisplatin, carboplatin, etoposide, and/or vincristine). Hydrocephalus was identified on neuroimaging performed at the time of diagnosis.
Measures of Verbal and Visual Auditory Learning
Neurocognitive testing was performed before CRT, six months after CRT, and yearly after CRT. Verbal learning was assessed with the California Verbal Learning Test — Children’s Version (CVLT-C).23 This task presents a word list over five trials; the total recall score for trials 1-5 was used. Age-standardized scores were derived with a mean of 50 and standard deviation of 10. Higher scores indicate better performance. Visual learning was assessed with the Visual-Auditory Learning Test (VAL) from the Woodcock-Johnson Tests of Cognitive Ability: Revised.24 The VAL is an associative learning task of word-symbol pairings. Age-standardized scores are derived with a mean of 100 and standard deviation of 15. Higher scores indicate better performance.
Assessment of Intellectual Function
Intellectual function estimates were obtained using the mental index of the Bayley scales or the three subtest administration (Block Design, Information and Similarities subtests) of the age-appropriate Wechsler scale.25-27 The mean baseline IQ score for the sample was in the average range (Mean = 102).
Statistical Analyses
All Table 1 variables were included in regression analyses, except for tumor laterality as the majority of participants had midline tumors. Linear models best fit the data. Baseline scores and mean rate of longitudinal change on the CVLT-C and VAL were examined using linear mixed-effects models with random coefficients.28 The intercept of the regression line is the baseline score and slope is the average rate of change per month (Table 2). One set of analyses was completed for the CVLT-C and another for the VAL. To correct for multiple comparisons, the significance level was set at p ≤.01, with p ≤ .05 considered statistical trends.
Table 2.
Demographic and Clinical Predictors of CVLT-C and VAL (N = 71)
| Variable | Subgroup | N | Baseline Intercept |
Change per month | Estimated 5 year score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SE | p-value | Slope | SE | p-value | Score | SE | p-value | ||||
| California Verbal Learning Test — Children’s Version (CVLT-C) | |||||||||||
| Age at CRT | ≥ 5 years | 34 | 45.56 | 2.00 | ns | .154 | .051 | <.01 | 54.79 | 2.38 | .01 |
| < 5 years | 37 | 43.50 | 2.46 | ns | .025 | .075 | ns | 44.97 | 3.06 | ns | |
| Diagnosis to CRT | ≥ 2 months | 33 | 43.12 | 2.28 | ns | .067 | .064 | ns | 47.13 | 2.85 | ns |
| < 2 months | 38 | 44.77 | 2.05 | ns | .148 | .058 | .01 | 53.65 | 2.65 | ns | |
| Gender | Female | 35 | 44.83 | 2.25 | ns | .116 | .065 | ns | 51.82 | 2.87 | ns |
| Male | 36 | 43.26 | 2.08 | ns | .100 | .060 | ns | 49.25 | 2.82 | ns | |
| Tumor Location | Infratentorial | 52 | 45.50 | 1.76 | ns | .067 | .050 | ns | 49.53 | 2.33 | ns |
| Supratentorial | 19 | 40.74 | 2.73 | ns | .203 | .080 | .01 | 52.92 | 3.88 | ns | |
| Number of Surgeries | 1 | 44 | 46.41 | 1.81 | .04 | .071 | .053 | ns | 50.68 | 2.51 | ns |
| ≥ 2 | 27 | 40.35 | 2.36 | ns | .159 | .069 | .02 | 49.86 | 3.31 | ns | |
| Hydrocephalus | No | 23 | 41.64 | 2.60 | ns | .178 | .075 | .02 | 52.34 | 3.49 | ns |
| Yes | 48 | 45.31 | 1.88 | ns | .072 | .054 | ns | 49.65 | 2.47 | ns | |
| Shunt | No | 49 | 44.88 | 1.79 | ns | .124 | .052 | .01 | 52.31 | 2.41 | ns |
| Yes | 22 | 42.03 | 2.83 | ns | .083 | .079 | ns | 47.02 | 3.51 | ns | |
| Pre CRT Chemotherapy | No | 56 | 44.99 | 1.66 | ns | .093 | .048 | .05 | 50.60 | 2.24 | ns |
| Yes | 15 | 40.12 | 3.49 | ns | .180 | .109 | ns | 50.93 | 4.88 | ns | |
| Visual Auditory Learning (VAL) | |||||||||||
| Age at CRT | ≥ 5 years | 34 | 97.93 | 3.29 | ns | .178 | .065 | <.01 | 108.58 | 3.32 | ns |
| < 5 years | 37 | 89.18 | 3.77 | ns | .176 | .099 | ns | 99.74 | 4.22 | ns | |
| Diagnosis to CRT | ≥ 2 months | 33 | 88.58 | 3.62 | ns | .190 | .081 | .02 | 99.95 | 3.81 | ns |
| < 2 months | 38 | 97.49 | 3.29 | ns | .175 | .074 | .02 | 108.00 | 3.55 | ns | |
| Gender | Female | 35 | 92.27 | 3.60 | ns | .149 | .080 | ns | 101.20 | 3.74 | ns |
| Male | 36 | 94.61 | 3.42 | ns | .208 | .073 | <.01 | 107.10 | 3.66 | ns | |
| Tumor Location | Infratentorial | 52 | 94.94 | 2.91 | ns | .160 | .064 | .01 | 104.52 | 3.09 | ns |
| Supratentorial | 19 | 89.56 | 4.64 | ns | .223 | .103 | .03 | 102.93 | 5.14 | ns | |
| Number of Surgeries | 1 | 44 | 97.86 | 3.02 | .02 | .147 | .068 | .03 | 106.68 | 3.26 | ns |
| ≥ 2 | 27 | 86.21 | 3.91 | ns | .226 | .089 | .01 | 99.79 | 4.27 | ns | |
| Hydrocephalus | No | 23 | 97.08 | 4.28 | ns | .208 | .094 | .02 | 109.59 | 4.51 | ns |
| Yes | 48 | 91.54 | 3.04 | ns | .169 | .068 | .01 | 101.67 | 3.20 | ns | |
| Shunt | No | 49 | 97.47 | 2.80 | .01 | .148 | .063 | .02 | 106.37 | 3.13 | ns |
| Yes | 22 | 84.46 | 4.32 | ns | .244 | .095 | .01 | 99.13 | 4.54 | ns | |
| Pre CRT Chemotherapy | No | 56 | 94.04 | 2.78 | ns | .212 | .058 | <.01 | 106.75 | 2.76 | .05 |
| Yes | 15 | 91.40 | 5.64 | ns | .037 | .134 | ns | 93.62 | 6.01 | ns | |
CRT = conformal radiation therapy; ns = not significant
Multiple regression analyses used a backward selection method whereby all variables with a p ≤.01 for the slope were retained as both an intercept and slope term. Variables were also retained if their removal significantly reduced overall model fit (p ≤ .01). Continuous covariates were split at their median value for inclusion in models (e.g., age at CRT). For multiple regression analyses, only those with infratentorial tumor location, which comprised 73% of the sample, were included to maintain necessary power and homogeneity for analyses. However, infratentorial versus supratentorial tumor location was examined with univariate analyses (Table 2). Correlations among variables in multiple regression analyses were run to help determine the presence of multicollinearity.
Given that learning measures were only administered to children 5 or older, baseline scores at the start of CRT were not available for children under 5. Therefore, adjustments were made to the simple linear model to use scores at the time of first eligible testing as baseline values for children less than age 5 years at the start of CRT (see Appendix). Univariate analyses assessed practice effects by comparing performance between participants with fewer evaluations (≤4) to those with more evaluations (>4). Analyses were conducted by the biostatistician coauthors using SAS software.29
RESULTS
Memory Outcomes
Mean scores on the CVLT-C (43.78) and VAL (91.86) were in the average range at the start of CRT. There was no evidence of decrease in CVLT-C and VAL scores over time following CRT. In contrast, a trend for increase was found on the CVLT-C (.12 ± .041 points/month; p = .03) and a significant increase on the VAL (.20 ± .054 points/month; p = <.01) over the five year follow-up time period.
Testing for Practice Effects
The results revealed no difference in the rate of improvement between those with more (>4) versus fewer assessments (≤4) on either the CVLT-C (p = .32) or the VAL (p = .66). The findings are not suggestive of practice effects.
Demographic and Clinical Predictors of Memory Scores
Univariate
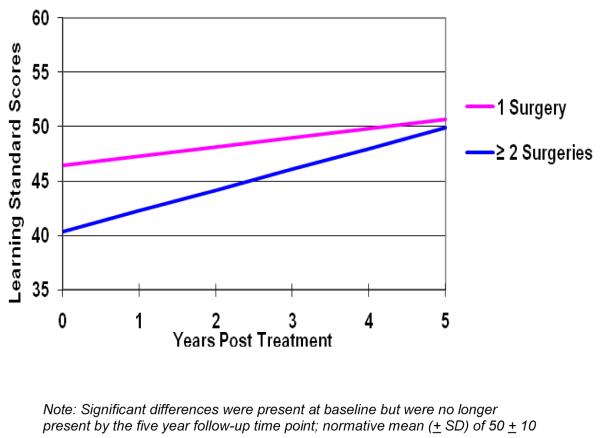
Univariate analyses tested the individual effects of all variables on the CVLT-C and VAL (Table 2). No decline in longitudinal learning scores (slope) was observed for either the CVLT-C or the VAL. For the CVLT-C, there was a trend for a greater number of surgeries to predict lower baseline scores (Figure 1a). Older age, starting CRT within 2 months of initial surgery, supratentorial tumor location, and the absence of shunt predicted a significant increase in CVLT-C scores over time. The rate of increase was greater in older children versus younger children. Older children had significantly higher CVLT-C scores five years after treatment than younger children (Figure 1b).
Figure 1a.
Longitudinal Change in Verbal Learning in Childhood Ependymoma. Significant Differences in Verbal Learning at Baseline
Figure 1b.
Longitudinal Change in Verbal Learning in Childhood Ependymoma. Significant Differences in Verbal Learning 5 years post-CRT
For the VAL, CSF shunt predicted significantly lower scores at baseline. There was a trend for a greater number of surgeries to predict lower scores at baseline. Older age, male gender, infratentorial tumor location, a greater number of surgeries, absence of pre-CRT chemotherapy, and the presence of hydrocephalus and CSF shunts predicted a significant increase in VAL scores over time. Analyses revealed that although the presence of shunt and a greater number of surgeries predicted lower scores at baseline on the CVLT-C and VAL, they no longer predicted significant differences five years after treatment since these characteristics were also associated with a more rapid rate of increase in VAL scores over time. Children who received pre-CRT chemotherapy exhibited little to no increase in their VAL scores over time. In contrast, children who did not receive pre-CRT chemotherapy exhibited a significant increase in VAL scores over time. There was a trend for those who did not receive pre-CRT chemotherapy to have higher scores on the VAL five years after treatment compared to those who received pre-CRT chemotherapy.
Multiple Regression
No clinically significant or meaningful correlations were found between predictor variables, suggesting an absence of multicollinearity between predictors. For the CVLT-C, multiple regression analyses (Table 3) found that older children (≥5) had significantly higher scores at baseline (+7.45 points) than younger children (< 5). A trend was found for children without shunt to have higher baselines scores (+5.86 points) than children with shunt. For the VAL, older children (≥ 5) had significantly higher baseline scores (+17.08 points) than younger children (< 5), and children with fewer surgeries (< 2) had significantly higher baseline scores (+13.06 points) than children with more surgeries (≥ 2). There were no significant slope effects.
Table 3.
Multiple Regression Analyses Predicting Baseline CVLT-C and VAL scores (N = 52)
| Term | Variable | Parameter | Estimate | SE | p-value |
|---|---|---|---|---|---|
| California Verbal Learning Test — Children’s Version (CVLT-C) | |||||
| Intercept | α 0 | 38.63 | 2.70 | <.01*a | |
| Age at CRT (<5 vs. ≥ 5) | α 1 | 7.45 | 2.61 | <.01* | |
| Shunt (no shunt vs. shunt) | α 2 | 5.86 | 2.68 | .03 | |
| Visual Auditory Learning (VAL) | |||||
| Intercept | α 0 | 81.22 | 3.96 | <.01*b | |
| Age at CRT (<5 vs. ≥ 5) | α 1 | 17.08 | 3.83 | <.01* | |
| Number of Surgeries (1 vs. >1) | α 2 | 13.06 | 3.87 | <.01* | |
compared with normative mean of 50
compared with normative mean of 100; baseline is defined as the start of CRT for those ≥ 5 years of age at the start of CRT, and the first evaluation after the age of 5 for those <5 years at the start of CRT.
p ≤.01.
DISCUSSION
The results of this study support the primary hypothesis that patients treated with smaller-than-conventional volumes using CRT do not exhibit longitudinal decline in verbal or visual-auditory learning after treatment. These findings are notable as longitudinal declines in learning and intellectual functioning are associated with conventional radiation therapy.5-8
Evidence supporting the secondary hypothesis that older children would exhibit a more rapid rate of learning over time than younger children was evident for verbal learning but not visual-auditory learning. Multivariate analyses revealed older children had better verbal learning at baseline, and univariate analyses revealed older children had more rapid improvement in verbal learning over time and significantly higher verbal learning scores five years after CRT than younger children. Risk factors were identified that predicted worse learning at different stages of treatment. Patients with multiple tumor resections and CSF shunts had worse verbal and visual-auditory learning at baseline. However, the effects of these specific clinical factors were temporary as they no longer predicted differences five years after treatment.
Risk factors identified near the time of diagnosis also varied in their transient versus long-term effect on learning. Specifically, younger age tended to predict lower scores at baseline as well as smaller rates of increase in learning scores over time. A greater number of surgeries and CSF shunts were associated with lower scores at baseline only, as these scores tended to improve over time, suggestive of recovery from insult associated with tumor mass effect and initial surgical management. These findings support the idea that change in learning can result from both perioperative risk factors with more acute and transient effects, as well as more stable and long-term CRT related side effects that impede learning in younger children.
Supratentorial tumor location was not a risk factor for learning outcome. Prior studies examining the effects of supratentorial versus infratentorial tumor location have been mixed. Some studies have found no increased risk for supratentorial tumor location, while others have found increased risk when the effects of CRT were investigated using detailed radiation dosimetry.2,3,13 The treatment of supratentorial tumors leaves brain areas critical to learning new information (e.g., hippocampalmammillary circuits) vulnerable to radiation exposure.30 These mixed findings are likely due to varied methodology and sample characteristics across studies.2,3,13
The findings revealed a trend for pre-CRT chemotherapy to have an adverse effect on rate of visual-auditory learning after treatment. This trend was not present for verbal learning. The aggregate effect over time resulted in the pre-CRT chemotherapy subgroup having worse visual-auditory learning five years after therapy compared to children who did not receive pre-CRT chemotherapy. These results suggest that visual auditory learning may be more vulnerable to the adverse effects of pre-CRT chemotherapy than verbal learning. Prior studies have documented increased vulnerability of visual memory to the adverse effects of chemotherapy in children with acute lymphoblastic leukemia treated with intrathecal chemotherapy only.31,32 In this study, visual-auditory learning was more susceptible to a greater number of risk factors than verbal learning (e.g., pre-CRT chemotherapy, and more surgeries adversely effected visual-auditory learning to a greater extent than verbal learning). This trend for increased risk in visual-auditory learning may help explain why children with localized ependymoma are at greater risk for reading difficulties following CRT.15-16 Given that the VAL was developed to assess skills that parallel those used in reading, with associative-paired learning akin to the pairing of letter combinations and their associated phonological representations, disruption of visual-auditory learning may, in part, help elucidate recent findings of increased risk for reading difficulties.14-16 Given that pre-CRT chemotherapy was equally distributed across both the younger and older age subgroups, pre-CRT findings cannot be attributable to age effects. The possibility of an interaction effect stemming from the use of chemotherapy prior to CRT, which left children more vulnerable to neurocognitive late effects, may have contributed to this finding and warrants investigation in future studies.33-36
The results from this study underscore the critical importance of baseline testing, particularly since learning scores were typically within the average range despite significant subgroup differences. Therefore, the modest yet significant effects found in this study would not have been detected without careful baseline measurement and comparison. The study found increased vulnerability in learning for younger children both at the start of treatment and after treatment as evidenced by diminished rate of learning following CRT. The greater susceptibility of visual-auditory learning to disruption may help explain findings of greater risk for reading deficits among children with localized ependymoma after CRT. The increased vulnerability of younger children to late effects has been suggested in the literature, with one explanation being that younger children experience greater loss of white matter volume following irradiation compared to older children.37-40 It has also been suggested that visual memory may be disproportionately affected following white matter injury early in life, particularly as the right hemisphere may contain greater volume of white matter.41-44 In sum, the findings provide strong evidence that CRT increases the likelihood of a positive neurocognitive outcome for patients with ependymoma, although some subgroups including younger children, those receiving pre-CRT chemotherapy, and those with more perioperative complications may be at increased risk and should be carefully considered during treatment planning and discussion with caregivers.
Supplementary Material
Acknowledgments
This work was supported in part by Cancer Center Support Grant CA21765 from the National Cancer Institute, by Research Project Grant RPG-99-252-01-CCE from the American Cancer Society and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dennis M, Spiegler BJ, Obonsawin MC, et al. Brain tumors in children and adolescents--III. Effects of radiation and hormone status on intelligence and on working, associative and serial-order memory. Neuropsychologia. 1992;30:257–275. doi: 10.1016/0028-3932(92)90004-6. [DOI] [PubMed] [Google Scholar]
- 2.Merchant TE, Kiehna EN, Li C, et al. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63:1546–54. doi: 10.1016/j.ijrobp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 4.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 5.Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45:137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 6.Kieffer-Renaux V, Viguier D, Raquin MA, et al. Therapeutic schedules influence the pattern of intellectual decline after irradiation of posterior fossa tumors. Pediatr Blood Cancer. 2005;45:814–819. doi: 10.1002/pbc.20329. [DOI] [PubMed] [Google Scholar]
- 7.Fuss M, Poljanc K, Hug EB. Full scale IQ (FSIQ) changes in children treated with whole brain and partial brain irradiation: A review and analysis. Strahlenther Onkol. 2000;176:573–581. doi: 10.1007/pl00002327. [DOI] [PubMed] [Google Scholar]
- 8.Mulhern RK, Hancock J, Fairclough D, et al. Neuropsychological status of children treated for brain tumors: a critical review and integrative analysis. Med Pediatr Oncol. 1992;20:181–191. doi: 10.1002/mpo.2950200302. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 10.Nagel BJ, Delis DC, Palmer SL, et al. Early patterns of verbal memory impairment in children treated for medulloblastoma. Neuropsychology. 2006;20:105–112. doi: 10.1037/0894-4105.20.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Merchant TE, Kiehna EN, Miles MA, et al. Acute effects of irradiation on cognition: changes in attention on a computerized continuous performance test during radiotherapy in pediatric patients with localized primary brain tumors. Int J Radiat Oncol Biol Phys. 2002;53:1271–1278. doi: 10.1016/s0360-3016(02)02828-6. [DOI] [PubMed] [Google Scholar]
- 12.Merchant TE, Zhu Y, Thompson SJ, et al. Preliminary results from a Phase II trial of conformal radiation therapy for pediatric patients with localised low-grade glioma and ependymoma. Int J Radiat Oncol Biol Phys. 2002;52:325–332. doi: 10.1016/s0360-3016(01)01807-7. [DOI] [PubMed] [Google Scholar]
- 13.Merchant TE, Lee H, Zhu J, et al. The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg. 2004;101:159–168. doi: 10.3171/ped.2004.101.2.0159. [DOI] [PubMed] [Google Scholar]
- 14.Moore BD. Neurocognitive outcomes in survivors of childhood cancer. Journal of Pediatric Psychology. 2005;30:51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- 15.Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 16.Conklin HM, Chenghong L, Xiaoping X, et al. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26:3965–3970. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer RJ, MacDonald T, Vezina G. Central nervous system tumors. Pediatr Clin N Am. 2008;55:121–145. doi: 10.1016/j.pcl.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Merchant TE, Happersett L, Finlay JL, et al. Preliminary results of conformal radiation therapy for medulloblastoma. Neuro Oncol. 1999;1:177–187. doi: 10.1093/neuonc/1.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchant TE. Current management of childhood ependymoma. Oncology (Williston Park) 2002;16:629–642. 644. discussion 645-646, 648. Review. [PubMed] [Google Scholar]
- 20.Hoff von K, Kieffer V, Habrand JL, et al. Impairment of intellectual functions after surgery and posterior fossa irradiation in children with ependymoma is related to age and neurologic complications. BMC Cancer. 2008;8:15. doi: 10.1186/1471-2407-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong CL, Gyato K, Awadalla AW, et al. A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol Rev. 2004;14:65–86. doi: 10.1023/b:nerv.0000026649.68781.8e. [DOI] [PubMed] [Google Scholar]
- 22.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 23.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test: Children’s Version. Harcourt, Brace, Jovanovich; New York, NY: 1994. [Google Scholar]
- 24.Woodcock R, Johnson M. The Woodcock-Johnson Tests of Cognitive Ability: Revised. Riverside Publishing; New York, NY: 1989. [Google Scholar]
- 25.Wechsler D. Wechsler Preschool and Primary Scales of Intelligence: Revised. The Psychological Corporation; San Antonio, TX: 1989. [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Scale: Revised. The Psychological Corporation; New York, NY: 1981. [Google Scholar]
- 27.Wechsler D. Wechsler Intelligence Scale for Children: Third Edition. The Psychological Corporation; New York, NY: 1991. [Google Scholar]
- 28.Littell RC, Milliken GA, Stroup WW, et al. SAS system for mixed models. SAS Institute; Cary, NC: 1996. [Google Scholar]
- 29.SAS . SAS/STAT user’s guide. ed 9.1 SAS Institute; Cary, NC: 2004. [Google Scholar]
- 30.Armstrong CL, Stern CH, Corn BW. Memory performance used to detect effects of cognitive functioning. Appl Neuropsychol. 2001;8:129–139. doi: 10.1207/S15324826AN0803_1. [DOI] [PubMed] [Google Scholar]
- 31.Hill DE, Ciesielski KT, Sethre-Hofstad Lisa. Visual and verbal short term memory deficits in childhood leukemia survivors after intrathecal chemotherapy. J Ped Psychol. 1997;22:861–870. doi: 10.1093/jpepsy/22.6.861. [DOI] [PubMed] [Google Scholar]
- 32.Jansen CE, Miaskowski C, Dodd M, et al. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104:2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 33.Waber DP, Tarbell NJ, Fairclough D, et al. Cognitive sequelae of treatment in childhood acute lymphoblastic leukemia: Cranial radiation requires an accomplice. J Clin Oncol. 1995;13:2490–2496. doi: 10.1200/JCO.1995.13.10.2490. [DOI] [PubMed] [Google Scholar]
- 34.Mullenix PJ, Kernan WJ, Schunior A, et al. Interactions of steroid, methotrexate, and radiation determine neurotoxicity in an animal model to study therapy for childhood leukemia. Pediatr Res. 1994;35:171–178. doi: 10.1203/00006450-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Montour-Proulx I, Kuehn SM, Keene DL, et al. Cognitive changes in children treated for acute lymphoblastic leukemia with chemotherapy only according to the Pediatric Oncology Group 9605 protocol. J Child Neurol. 2005;20:129–133. doi: 10.1177/08830738050200020901. [DOI] [PubMed] [Google Scholar]
- 36.Waber DP, Turek J, Catania L, et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: Findings from Dana-Farber Cancer Institute ALL consortium protocol 95-01. J Clin Oncol. 2007;25:4914–4921. doi: 10.1200/JCO.2007.10.8464. [DOI] [PubMed] [Google Scholar]
- 37.Reddick WE, Laningham FM, Glass JO, et al. Quantitative morphologic evaluation of magnetic resonance imaging during and after treatment of childhood leukemia. Neuroradiology. 2007;49:889–904. doi: 10.1007/s00234-007-0262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shan ZY, Liu JZ, Glass JO, et al. Quantitative morphologic evaluation of white matter in survivors of childhood medulloblastoma. Magn Reson Imaging. 2006;24:1015–1022. doi: 10.1016/j.mri.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19:472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- 40.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 41.Carey ME, Haut MW, Reminger SL, et al. Reduced frontal white matter volume in long-term childhood leukemia survivors: a voxel-based morphometry study. AJNR Am J Neuroradiol. 2008;29:792–797. doi: 10.3174/ajnr.A0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schatz J, Craft S, Koby M, et al. Associative learning in children with perinatal brain injury. J Int Neuropsychol Soc. 1997;3:521–527. [PubMed] [Google Scholar]
- 43.Rourke BP, Ahmad SA, Collins DW, et al. Child clinical/pediatric neuropsychology: some recent advances. Annu Rev Psychol. 2002;53:309–339. doi: 10.1146/annurev.psych.53.100901.135204. [DOI] [PubMed] [Google Scholar]
- 44.Kaemingk KL, Carey ME, Moore IM, et al. Math weaknesses in survivors of acute lymphoblastic leukemia compared to healthy children. Child Neuropsychol. 2004;10:14–23. doi: 10.1076/chin.10.1.14.26240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.