Abstract
Mutations in human copper-zinc superoxide dismutase (SOD1) cause an inherited form of the fatal neurodegenerative disease amyotrophic lateral sclerosis (ALS). Here, we present structures of the pathogenic SOD1 variants D124V and H80R, both of which demonstrate compromised zinc-binding sites. The disruption of the zinc-binding sites in H80R SOD1 leads to conformational changes in loop elements, permitting non-native SOD1-SOD1 interactions that mediate the assembly of these proteins into higher order filamentous arrays. Analytical ultracentrifugation sedimentation velocity experiments indicate that these SOD1 variants are more prone to monomerization than is the wild type enzyme. Although D124V and H80R SOD1 proteins appear to have fully functional copper-binding sites, inductively coupled plasma mass spectrometery (ICP-MS) and anomalous scattering X-ray diffraction analyses reveal that zinc (not copper) occupies the copper-binding sites in these variants. The absence of copper in these proteins, together with the results of covalent thiol modification experiments in yeast strains with and without the gene encoding the copper chaperone for SOD1 (CCS), suggest that CCS may not fully act on newly translated forms of these polypeptides. Overall, these findings lend support to the hypothesis that immature mutant SOD1 species contribute to toxicity in SOD1-linked ALS.
Copper-zinc superoxide dismutase (SOD1) detoxifies superoxide anion, a byproduct of cellular respiration, to molecular oxygen and hydrogen peroxide [2O2− + 2H+ → H2O2 + O2] (1). In the early 1990s, mutations in the human gene encoding SOD1 were linked to the fatal, progressive neurodegenerative disease amyotrophic lateral sclerosis (ALS, Lou Gehrig’s disease) (2, 3). Today, more than 100 distinct pathogenic mutations have been documented (reviewed in refs (4) and (5)), with most resulting in single amino acid substitutions and a few in truncations of the polypeptide.
Seminal studies in transgenic mice have established that pathogenic SOD1 proteins give rise to motor neuron dysfunction through the gain of a toxic property and not a loss of enzymatic function. Mice expressing human fALS SOD1 polypeptides in addition to their endogenous active SOD1 develop paralytic symptoms (6–8), while SOD1 knockout mice do not (9). The appearance of inclusions enriched in mutant SOD1 in cell culture model systems, in ALS-SOD1 transgenic mice, and in fALS patients, has led to the suggestion that SOD1- linked ALS toxicity is related to misfolding and/or aggregation of these polypeptides (reviewed in (10–12)). However, the precise molecular mechanisms underlying mutant SOD1 toxicity remain unknown, and it is unclear whether the observed proteinaceous inclusions cause or arise from motor neuron dysfunction.
The appearance of insoluble, higher order assemblies of pathogenic SOD1 proteins in patients and in murine models of the disease strongly suggests that these SOD1 variants possess properties distinct from the wild type enzyme that lead to their aggregation in vivo. Previous studies on wild type SOD1 have highlighted the stabilizing roles of both metal ion binding and the presence of an oxidized intrasubunit disulfide bond (13–15). Both of these stabilizing posttranslational modifications of nascent SOD1 are facilitated by a helper protein called the copper chaperone for SOD1 (CCS) (16–18). Pathogenic SOD1 mutations that alter CCS/SOD1 protein-protein interactions or prevent proper CCS action could result in destabilized, immature SOD1 proteins that are prone to non-native self-association (4, 5, 19).
Here, we characterize the human pathogenic SOD1 variants D124V and H80R using single crystal X-ray diffraction, analytical ultracentrifugation, and covalent thiol modification. The findings: 1) reinforce previous observations that the absence of metal ions in the zinc-binding sites of SOD1 variants can result in non-native intermolecular interactions that give rise to higher order filamentous arrays (20); 2) reveal that both the D124V and H80R SOD1 homodimers are more prone to dissociation than is the wild type enzyme; and 3) suggest that unlike the wild type enzyme, CCS may not be able to interact productively with these pathogenic SOD1 variants in vivo. The resulting metal-deficient, destabilized mutant SOD1 proteins can essentially be considered as trapped folding intermediates that may be prone to oligomerization in motor neurons.
EXPERIMENTAL PROCEDURES
Materials
Monobasic and dibasic potassium phosphate, yeast extract, peptone, dextrose (glucose), EDTA, sodium chloride, acetic acid and sodium acetate (used for demetallation) were obtained from Fischer Scientific. Ammonium sulfate was purchased from US Biological. Tris base [Tris(hydroxymethyl)amino ethane] was purchased from Research Products International. Sodium malonate came from Fluka. AMS [4-acetamido-4'-maleimidylstilbene-2, 2'-disulfonic acid] was purchased from Invitrogen. Agarose, glycerol [ethylene glycol], and the protease inhibitor cocktail were obtained from Sigma. Pfu DNA polymerase and deoxyribonucleotides were purchased from Stratagene. Glass beads were obtained from Biospec. Crystallization screening kits were obtained from Qiagen and crystal growth trays were purchased from Hampton Research. Phenyl Sepharose, DEAE Sephadex, and Sephadex G-75 resin beads were purchased from Pharmacia. All solutions unless otherwise noted in the text were prepared using de-ionized water passed through a Millipore ultra purification system.
Protein Expression and Purification
The human D124V and H80R SOD1 proteins used in this study were expressed, purified, stripped of metals (for analytical ultracentrifugation), and characterized by inductively coupled plasma mass spectrometry (ICP-MS) and electrospray ionization mass spectrometry as described previously (14).
Crystallization, Data Collection, Structure Determination, and Refinement
All crystals were grown at room temperature using the hanging drop vapor diffusion method. Protein concentrations for the D124V and H80R SOD1 samples used in crystallization trials were 8.0 and 18.7 mg/mL, respectively. Because D124V SOD1 tends to precipitate from solution over time, it could not be stored for later use and was therefore used in crystallization experiments immediately after upon purification and concentration. These SOD1 variants in 2.25 mM potassium phosphate, pH 7.0, 160 mM sodium chloride, were mixed with an equal volume of reservoir solution containing 2.4 M sodium malonate, pH 7.0 for D124V, and 2.3 M ammonium sulfate, 100 mM Tris, pH 8.0 for H80R. Teardrop-shaped crystals of D124V SOD1 grew in space group P212121 within one week. Slender, rod-like crystals of H80R SOD1 grew in space group P21, also within one week, while block-like prisms of H80R SOD1 grew in space group P212121 after ~two months in the same crystallization drop as the monoclinic form. Specimens of each crystal form suitable for single crystal X-ray diffraction work were soaked in a cryoprotectant consisting of reservoir solution made 15% (v/v) in ethylene glycol before flash-cooling by plunging into liquid nitrogen. Native diffraction data from the D124V and H80R orthorhombic crystal forms were collected at resolutions of 1.55 Å and 1.65 Å, respectively, at the Advanced Light Source, Berkeley, CA, beam line 8.2.2 on an Area Detector Systems Corporation Q315R CCD detector. Native diffraction data from the monoclinic H80R crystal form were collected at a resolution of 1.85Å at the X-ray Crystallography Core Laboratory at the University of Texas health Science Center at San Antonio on a Rigaku FR-D High Flux X-ray generator equipped with Osmic purple optics and a Rigaku HTC imaging plate detector. All diffraction data were processed using the HKL2000 program suite (21).
The identities of metal ions bound in the D124V and H80R SOD1 proteins “as purified” were determined by ICP-MS and by collecting diffraction data from D124V and H80R crystals using X-rays of wavelengths (energies) tuned to the copper and zinc absorption edges as described previously for the pathogenic SOD1 variant G85R (22). The scattering of X-rays is altered by a metal ion at its absorption edge (anomalous scattering), and this property causes the metal to be illuminated in an electron density map, revealing both its location as well as an estimate of the amount (occupancy) bound at each site. The energy of X-rays tuned to the copper edge illuminates zinc only very weakly, providing a means to discriminate between the two metal ions. Anomalous diffraction data from the D124V and H80R orthorhombic crystal forms were collected at beam line 8.2.1 at the Advanced Light Source (Lawrence Berkeley National Laboratory) on an Area Detector Systems Corporation Q315R CCD detector. The first data sets were collected at the zinc absorption edge and above the copper absorption edge, giving anomalous diffraction data with copper f" = 3.4 e and zinc f" = 3.9 e. The second data sets were collected at a wavelength of 1.38 Å, at the copper and below the zinc absorption edges, giving anomalous data with copper f" = 3.9 e and zinc f" = 0.6 e. The wavelengths for optimal copper and zinc anomalous diffraction at the Advanced Light Source were determined by monitoring the X-ray fluorescence of the crystals at an angle normal to the incident X-ray beam immediately prior to data collection.
The D124V and H80R SOD1 structures were determined by molecular replacement with the program MOLREP (23) using the structure of the human SOD1 variant G37R (Protein Data Bank entry 1AZV) (24) as the search model. Once positioned, the models were subjected to alternating cycles of refinement in the program PHENIX (25) followed by manual adjustment into σA-weighted electron density maps (26) with the program COOT (27). No stereochemical restraints were applied to the metal-ligand distances or bond angles during refinement.
Analytical Ultracentrifugation
All analytical ultracentrifugation experiments were performed with a Beckman Optima XL-I centrifuge in the Center for Analytical Ultracentrifugation of Macromolecular Assemblies at the University of Texas Health Science Center at San Antonio. Sedimentation velocity data were analyzed with the ULTRASCAN software (28) version 9.9 (29). All calculations were performed on the Lonestar cluster at the Texas Advanced Computing Center at the University of Texas at Austin, and on the Jacinto cluster at the Bioinformatics Core Facility at the University of Texas Health Science Center at San Antonio. Velocity measurements were made at 230 nm in a buffer containing 2.25 mM sodium phosphate, pH 7.0, 160 mM NaCl. The samples were centrifuged at 20.4 °C, and at 60,000 rpm, using standard epon 2-channel centerpieces. Hydrodynamic corrections for buffer density, viscosity, and partial specific volume were applied according to methods outlined by Laue (30) as implemented in ULTRASCAN (28). The partial specific volume was determined to be 0.725 cm3/g for wild type human SOD1 and for H80R SOD1, whereas the value for D124V SOD1 was 0.727 cm3/g. All data were first analyzed by 2-dimensional spectrum analysis (2DSA) (31) with simultaneous removal of time-invariant noise, and then by genetic algorithm refinement (32), followed by Monte Carlo analysis (33).
Sedimentation velocity data for freshly isolated D124V SOD1 preparations were also fit to a nonlinear model for a reversible monomer-dimer equilibrium using the Adaptive Space-Time Finite Element Solution for Multicomponent-Reacting Systems (ASTFEM-RA) (34) to obtain equilibrium constants and kinetic rate constants. Extinction coefficients at 280 nm for SOD1 were determined by the method of Gill and von Hippel (35) to be 6170 OD mol−1 cm−1. Absorption profiles from multiple concentrations ranging between 220 – 340 nm were fit with ULTRASCAN (28) to a sum of five Gaussian functions. The extinction fit was calibrated at 280 nm with the extinction coefficient determined above to derive an extinction coefficient at 230 nm of 58596.1 OD mol−1cm−1. Bead models were calculated with the ULTRASCAN SOlutionMOdeler (SOMO) software (36) which is available for free download from http://www.ultrascan.uthscsa.edu.
Covalent Modification of Free Thiols in SOD1
4-acetamido-4'-maleimidylstilbene-2, 2'-disulfonic acid (AMS) was used to covalently modify free thiols in D124V and H80R SOD1 proteins coming from yeast with and without the ccs1 gene as previously described (37) in order to monitor the degree to which the SOD1 intrasubunit disulfide bond is oxidized in these variants.
Figure Preparation
All figures were created using the program PyMOL (DeLano, W.L. The PyMOL Molecular Graphics System, 2002, DeLano Scientific, San Carlos, CA, USA. http://www.pymol.org).
RESULTS
Structures of D124V and H80R SOD1
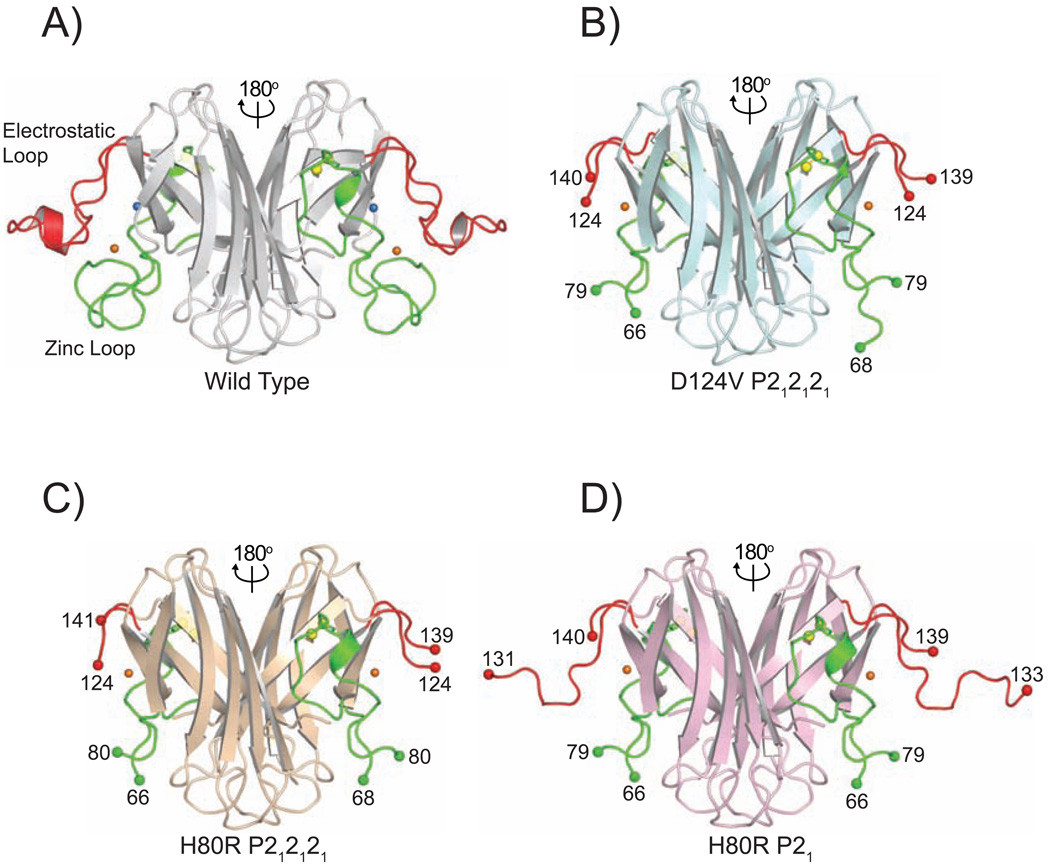
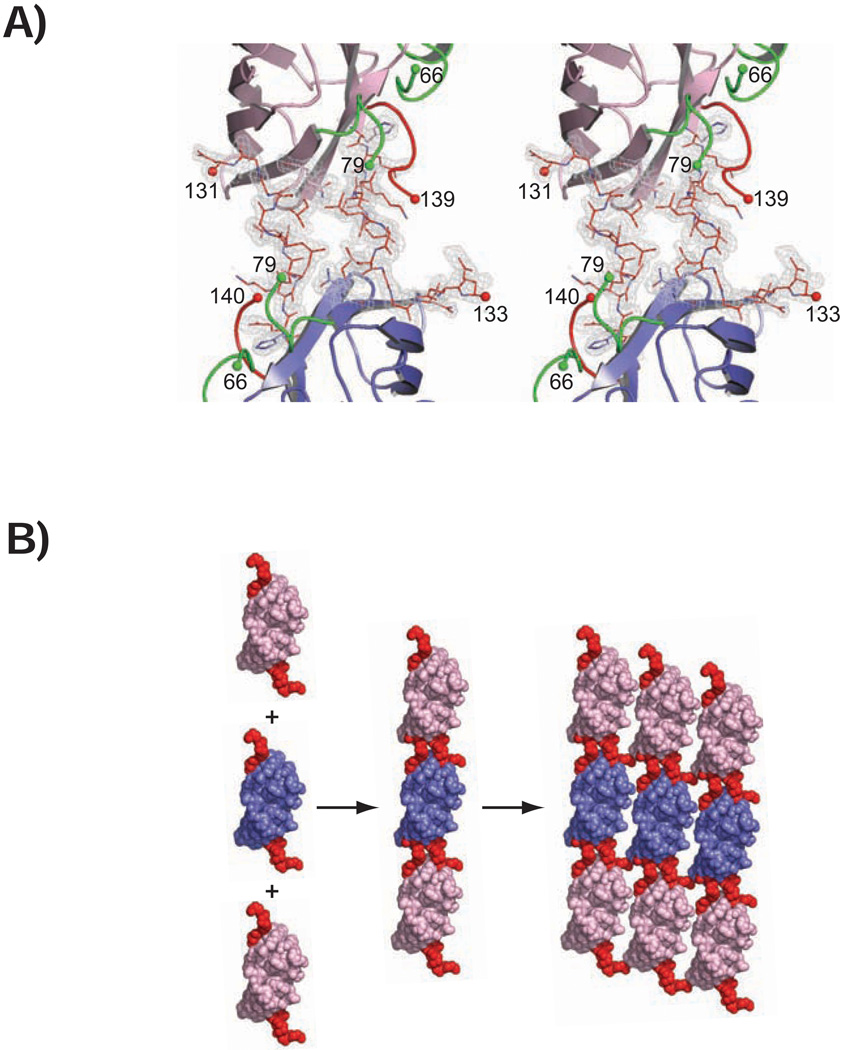
The crystal structures of the pathogenic D124V and H80R SOD1 proteins were determined and refined at resolutions ranging from 1.55 – 1.85 Å. X-ray diffraction data collection and protein structure refinement statistics are given in Table 1. Figure 1 reveals that while the Greek key β-barrels of D124V and H80R SOD1 remain similar in structure to that of the wild type enzyme, the D124V and H80R substitutions give rise to substantial disorder in loops IV (the “zinc loop”, residues 50–83) and VII (the “electrostatic loop”, residues 121–142). Figure 2A shows that the electrostatic loop elements in adjacent metal-deficient H80R SOD1 dimers in the monoclinic crystal form can engage in reciprocal, non-native SOD1-SOD1 interactions at edge strands in the Greek key β-barrel that become accessible due to the inability of this variant to bind metal in the zinc-binding site. As shown in Figure 2B, these non-native SOD1-SOD1 contacts occur at opposite ends of the H80R SOD1 homodimer, permitting them to propagate bidirectionally over the entire length of the crystal to form higher order filamentous arrays.
Table 1.
X-ray Diffraction Data and Refinement Statistics
| D124V P212121 3H2P |
H80R P212121 3H2R |
H80R P21 3H2Q |
D124V P212121 | H80R P212121 | |||
|---|---|---|---|---|---|---|---|
| A | B | C | Copper edge | Zinc edge | Copper edge | Zinc edge | |
| Data collection | |||||||
| Cell Dimensions | |||||||
| a, b, c (Å) | 39.92, 57.97, 105.02 | 40.25, 58.43, 104.83 | 35.24, 136.80, 56.36 | ||||
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 90, 104.58, 90 | ||||
| Resolution (Å) | 50.0-1.55 (1.61-1.55)* | 50.0-1.65 (1.71-1.65) | 30.0-1.85 (1.92-1.85) | 1.80 (1.86-1.80) | 1.80 (1.86-1.80) | 1.65 (1.71-1.65) | 1.65 (1.71-1.65) |
| λ (Å) | 1.0000 | 1.2820 | 1.5418 | 1.3781 | 1.2826 | 1.3786 | 1.2820 |
| Rsym (on I) (%) | 6.7 (56.6) | 6.4 (38.5) | 9.0 (53.1) | 9.0 (52.5) | 9.0 (48.6) | 6.3 (41.4) | 6.4 (38.5) |
| I/ σI | 18.7 (2.3) | 23.2 (5.5) | 21.1 (3.4) | 17.5 (6.2) | 17.1 (6.7) | 23.1(3.2) | 23.2 (5.5) |
| Completeness (%) | 99.1 (96.0) | 99.9 (99.5) | 94.5 (95.3) | 99.8 (100) | 99.9 (99.9) | 99.2 (93.7) | 99.9 (99.5) |
| Redundancy | 5.9 (5.1) | 7.2 (6.3) | 6.8 (6.5) | 6.7 (6.0) | 6.6 (6.1) | 6.7 (3.5) | 7.2 (6.3) |
| Refinement | |||||||
| Resolution (Å) | 50.0-1.55 | 50.0-1.65 | 30.0-1.85 | ||||
| No. reflections | 35,624 | 56,897 | 41,462 | ||||
| Rwork/ Rfree (%) | 15.28/19.96 | 14.33/18.42 | 18.24/22.33 | ||||
| Number of dimers/ | 1 | 1 | 2 | ||||
| Number of atoms | |||||||
| Protein | 1856 | 1804 | 3772 | ||||
| Metal ions | 3 (Zn) | 2 (Zn) | 4 (Zn) | ||||
| Water | 259 | 188 | 370 | ||||
| Ligands | 14 (Malonate) | 10 (Sulfate) | 25 (Sulfate) | ||||
| R.m.s deviations | |||||||
| Bond lengths (Å) | 0.004 | 0.004 | 0.006 | ||||
| Bond angles (°) | 0.900 | 0.850 | 1.033 | ||||
Values in parentheses represent the highest resolution bin.
Figure 1.
Structures of human wild type, D124V, and H80R SOD1. A) Human wild type SOD1 [pdb code 2c9v (39)]. The relationship between the two subunits in the SOD1 dimer is indicated. The Greek key β-barrel is shown in gray, the zinc loop (loop IV, residues 50–83) is green, and the electrostatic loop (loop VII, residues 121–142) is red. Copper, zinc, and the sulfur atoms of C57 and C146 that form the disulfide bond in each subunit are shown as blue, orange, and yellow spheres, respectively. B) The human D124V SOD1 variant in the same orientation as the wild type enzyme shown in panel A). The color coding is the same as in panel A) except the Greek key β-barrel is shown in cyan. Green and red spheres represent the last residues visible in the electron density for the zinc and electrostatic loop elements, respectively. Zinc occupies the copper-binding site and there is no metal in the zinc-binding site. C) The human H80R SOD1 variant in space group P212121. The color coding is the same as for the wild type enzyme except the Greek key β-barrel is shown in gold. Zinc occupies the copper-binding site and there is no metal in the zinc-binding site. D) The human H80R SOD1 variant in space group P21. The color coding is the same as for the wild type enzyme except the Greek key β-barrel is shown in pink. Zinc occupies the copper-binding site and there is no metal in the zinc-binding site.
Figure 2.
Divergent stereo view of the non-native contacts formed between adjacent H80R SOD1 homodimers in the P21 crystal form. Residues of the zinc and electrostatic loop elements are shown in green and red, respectively. The Greek key β-barrel of a subunit coming from one SOD1 homodimer is shown in pink and that of another subunit coming from an adjacent SOD1 homodimer is shown in blue. Green and red spheres represent the last residues visible in the electron density in the zinc and electrostatic loops, respectively. Residues 121–131 of the electrostatic loop from one H80R dimer interact with a depression in the β-barrel of a neighboring H80R dimer in the crystal lattice. The σA-weighted electron density, with coefficients 2mFo-DFc is contoured at 1.5σ. B) Space-filling representation of H80R SOD1 proteins assembling into higher order filamentous arrays in the P21 crystal form. The color coding as the same in panel A) except the zinc loop has not been colored green to more easily visualize the reciprocal interactions mediated by the electrostatic loop and β-barrel elements of adjacent H80R SOD1 dimers in the crystal lattice.
Disrupted Zinc-Binding Sites
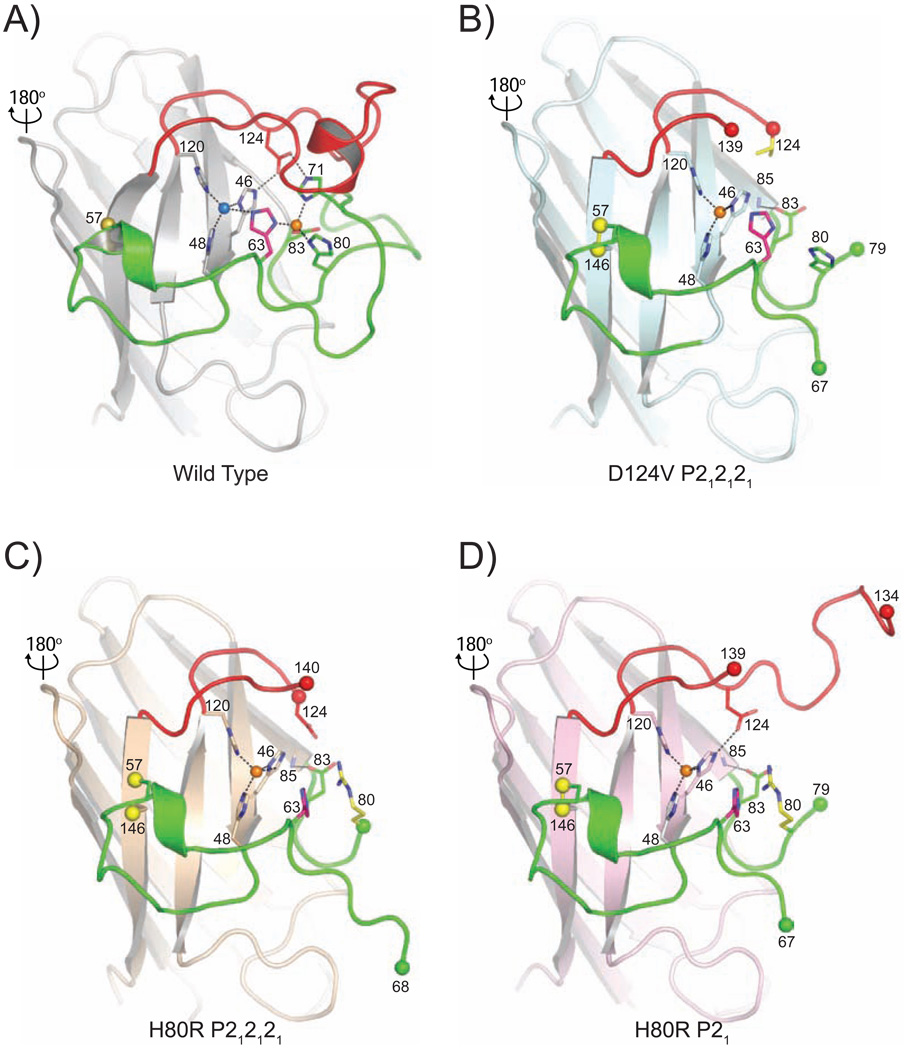
Figure 3 shows that the D124V and H80R pathogenic SOD1 substitutions disrupt the zinc-binding sites in these variants. The degree of disorder of the zinc-binding sites is quite similar overall in all three structures, with an absence of electron density for H71 and only very poor electron density for H80 in each case.
Figure 3.
The metal-binding sites in human wild type, D124V, and H80R SOD1. The color coding of the zinc and electrostatic loop elements is as in Figure 1. A) Human wild type SOD1 [pdb code 2c9v (39)]. Metal-ligand bonds and hydrogen bonds between D124 and the nonliganding imidazole nitrogen atoms of copper ligand H46 and H71 are shown as dotted lines. H63, the bridging imidazolate, is shown in magenta. B) The human D124V SOD1 variant in the same orientation as the wild type enzyme shown in panel A). Zinc occupies the copper-binding site and there is no metal in the zinc-binding site. H63 is shown in magenta and the D124V mutation is shown in yellow. Metal-ligand bonds and the hydrogen bond between D83 and N85 are shown as dotted lines. C) The human H80R SOD1 variant in space group P212121. Zinc occupies the copper-binding site and there is no metal in the zinc-binding site. H63 is shown in magenta and the H80R mutation is shown in yellow. Metal-ligand bonds and the hydrogen bond between D83 and N85 are shown as dotted lines. D) The human H80R SOD1 variant in space group P21. Zinc occupies the copper-binding site and there is no metal in the zinc-binding site. H63 is shown in magenta and the H80R mutation is shown in yellow. Metal-ligand bonds and the hydrogen bonds between D83 and N85 and D124 and H46 are shown as dotted lines.
Zinc in the Copper-Binding Sites
ICP-MS analyses performed on freshly purified D124V and H80R SOD1 variants prior to use in crystallization or in analytical ultracentrifugation experiments indicated that although these proteins were completely devoid of copper, they contained between 1.5 – 1.7 equivalents of zinc per dimer. Measurement of the fluorescence of D124V and H80R SOD1 crystals as they were scanned with X-rays at energies spanning the zinc and copper absorption edges, followed by inspection of anomalous difference Fourier electron density maps, confirmed the presence of zinc and the absence of copper in these pathogenic SOD1 variants. Supplementary Figure 1 reveals sharp transitions in the fluorescence spectra measured from D124V and H80R SOD1 crystals when the energy of the X-rays illuminating the crystals reaches the zinc absorption edge. In contrast, no sharp transitions are evident when the energy of the X-rays illuminating the crystals coincide with the copper absorption edge. Supplementary Figure 2 shows zinc- and copper-edge anomalous difference Fourier electron density maps superimposed on the refined D124V SOD1 structure. The zinc edge anomalous difference electron density peaks are very strong, persisting at contour levels greater than 60 σ, while the anomalous difference Fourier electron density peaks coming from the small contribution of zinc at the copper edge (f" = 0.6 e) disappears between 12 and 13 σ. As a comparison, the anomalous difference Fourier electron density peaks coming from the sulfur atoms of the four cysteine residues in each SOD1 subunit are no longer visible at contour levels greater than approximately 10 σ. Nearly identical results were obtained using anomalous difference Fourier electron density maps obtained from the H80R SOD1 orthorhombic crystal form.
As shown in Figure 3, the zinc ions occupying the copper-binding sites in the D124V and H80R SOD1 variants are coordinated by H46, H48, and H120, at distances of ~2.0 Å. Depending upon which was present in the crystallization mother liquor, the oxygen atoms of malonate or sulfate anions act as a fourth ligand to the zinc (not shown in Figure 3 for clarity). The zinc coordination geometry in the D124V and H80R SOD1 proteins is best described as distorted tetrahedral, with the zinc ion displaced ~0.4 Å from the plane formed by the nitrogen atoms of the three liganding histidine ligands. This coordination geometry is quite similar to that observed for zinc in the copper-binding site in the 1.4 Å resolution structure of the pathogenic D125H SOD1 variant, which was crystallized in the presence of sulfate anion (38).
Oxidation of Cys111
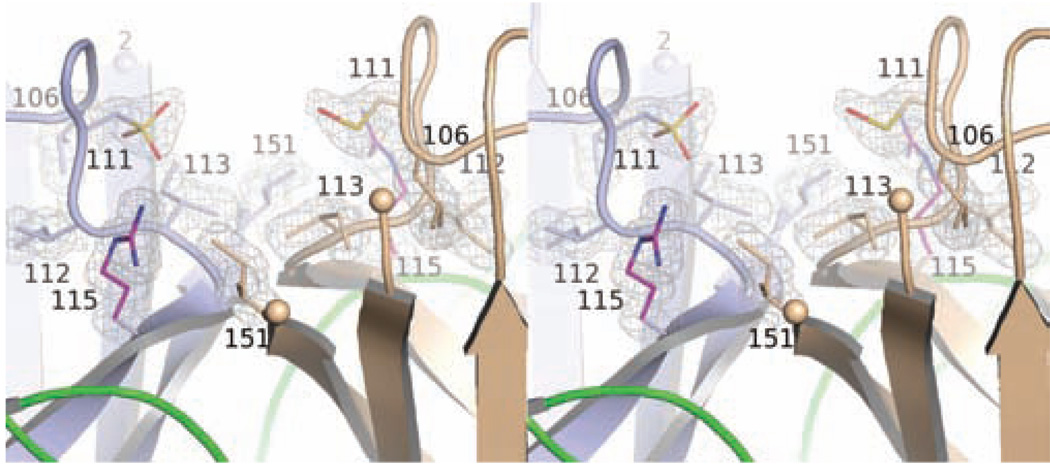
Figure 4 shows σA-weighted electron density with coefficients 2mFo-DFc superimposed on the C111 residues in the H80R P212121 crystal structure. The C111 Sγ atoms appear to be oxidized to sulfonic acid (Cys111-SO3H) in subunit A and to sulfenic acid (Cys111-SOH) in subunit B.
Figure 4.
Divergent stereo view of the dimer interface in the H80R P212121 structure showing the oxidation of C111 residues. Subunits A and B of the H80R homodimer are shown in blue and gold, respectively. Residues of the zinc loop are shown in green. The molecular model is superimposed on σA-weighted electron density of the form 2mFo-DFc contoured at 1.5σ. The Sγ of C111 is oxidized to sulfonic acid in subunit A and sulfenic acid in subunit B. Except for R115, the environment immediately surrounding C111 is quite apolar. The proximity of R115 to C111 in this environment may lower the pKa of C111, causing it to be “reactive” (see text).
Covalent Thiol Modification
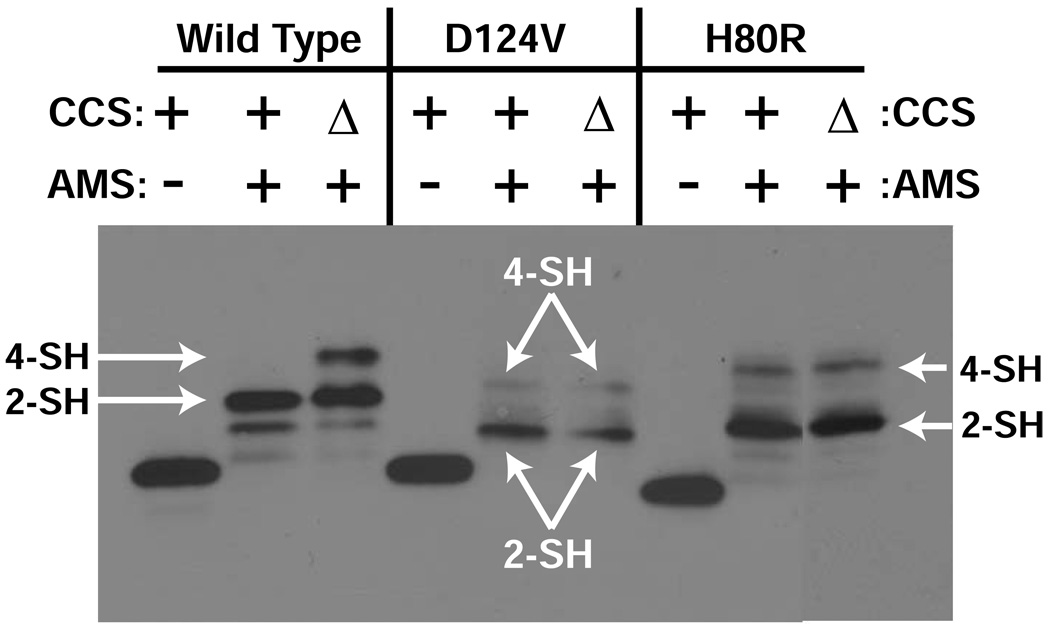
Cysteine residues are found in human SOD1 at positions 6, 57, 111, and 146. C57 and C146 participate in the intrasubunit disulfide bond in the mature wild type enzyme. To probe whether CCS can affect the redox status of this intrasubunit disulfide bond in the D124V and H80R SOD1 variants in cells, covalent thiol modification experiments were performed on these proteins after expression in ccs1+ and ccs1Δ yeast as previously described (19, 37). Figure 5 reveals that when AMS is added to fresh lysates of ccs1Δ yeast expressing wild type, D124V, or H80R SOD1, in each case, a fraction is observed to possess four free thiols that are covalently modified by AMS. When expressed in ccs1+ yeast, this fraction of wild type SOD1 is converted to a species with only two free thiols, suggesting that CCS facilitates the oxidation of the C57–C146 disulfide bond. In contrast, the four thiol fractions of the D124V and H80R SOD1 proteins remain unaltered when these proteins are expressed in ccs1+ yeast, suggesting that CCS is unable to interact productively with these particular SOD1 variants.
Figure 5.
AMS covalent thiol modification of D124V and H80R SOD1 variants coming from ccs1Δ and ccs1+ yeast. The SOD1 monomer contains four cysteine residues, two of which participate in a conserved disulfide bond within each SOD1 subunit. ccs1Δ and ccs1+yeast cells expressing D124V and H80R SOD1 are lysed in the presence of AMS, and the lysates are run on SDS-PAGE and probed with antibody specific for SOD1 as described (55, 56). AMS-modified thiols produce gel-shifts to higher molecular weight such that two are modified in the disulfide oxidized enzyme and four are modified in the disulfide reduced enzyme. The disulfide status of the D124V and H80R SOD1 variants is predominantly oxidized, and is unaffected by CCS.
Analytical Ultracentrifugation
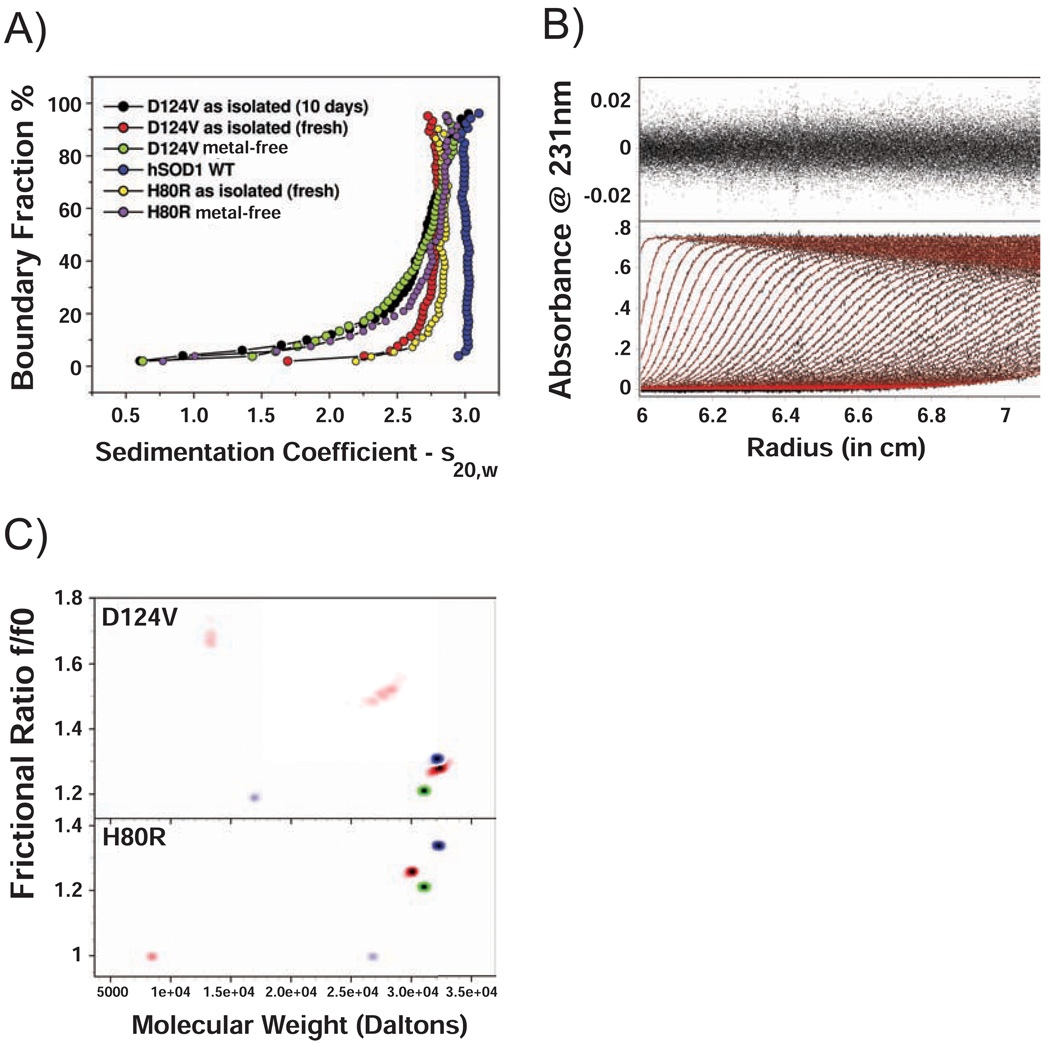
A comparison of the sedimentation coefficient distributions of D124V and H80R SOD1 with wild type SOD1 is shown in Figure 6A. Plots of boundary fraction versus s20,w will be vertical if the sample is homogeneous, and will have a positive slope if the sample is heterogeneous. Although the wild type SOD1 protein sediments as a single, homogeneous species with an S value of 3.03, the D124V and H80R SOD1 proteins form a distribution of species ranging from ~1.7–2.6 S, consistent with a reversible monomer-dimer equilibrium (14). After metal ion removal, the D124V and H80R SOD1 variants show a heterogeneous distribution ranging from 0.5 – 3.0 S. The D124V sample, when examined in the analytical ultracentrifuge after ten days of storage at 4°C, displays a distribution identical to the distribution observed for the metal-free sample, suggesting that it loses its bound metal over time.
Figure 6.
Analytical ultracentrifugation of wild type, and D124V and H80R SOD1 variants. A) van Holde & Weischet integral distribution plot of sedimentation coefficients for wild type hSOD1 (blue) and D124V and H80R mutants. Freshly isolated D124V and H80R mutants are shown as red and yellow, respectively, while their metal-free forms are green and purple, respectively. D124V is also shown after 10 days of storage at 4°C (black). After 10 days, the sedimentation coefficient distribution appears identical to the apo form, suggesting loss of metal from the D124V SOD1 protein over time. Wild type hSOD1 sediments as a homogeneous species with a sedimentation coefficient of 3.03 S, consistent with a homodimer. The D124V and H80R mutant SOD samples showed various degrees of heterogeneity. B) D124V SOD1 sedimentation velocity data fitted with a reversible monomer-dimer model based on the ASTFEM-RA solution (34). The equilibrium constant, shape factors and rate constants are listed in Table 3. The residuals of the fit are shown in the top panel, and are randomly distributed, suggesting that the monomer-dimer model is a good representation of these data. The lower panel shows the experimental sedimentation velocity data in black, the ASTFEM-RA model overlay is shown in red. C) Results for the Monte Carlo fit of sedimentation velocity data from the metal-free form (red) and the freshly isolated sample (blue) of D124V mutant (top) and H80R mutant (bottom). Wild type hSOD1 is shown in green for comparison in both panels. Both mutants show an increase in frictional ratio for the dimer relative to the wild type enzyme.
To further characterize the mass and shape distributions of these samples, the sedimentation velocity data were analyzed using whole boundary fitting to the Adaptive Space-Time Finite Element Solution for Multi-Component Reacting Systems (34). Consistent with the results of the above analysis, wild type SOD1 sediments as a single species with a sedimentation coefficient of 3.03 S, a frictional ratio of 1.21, and a molecular weight of 31.0 kDa, the latter of which is in excellent agreement with the theoretically determined molecular weight of the homodimer (31.95 kDa) (Figure 6A and 6C). The sedimentation velocity results for wild type SOD1 were also compared to the hydrodynamic parameters derived from bead modeling of the X-ray crystal structure (PDB code 2C9V (39)) using the UltraScan SOMO software (36). SOMO predicted the sedimentation coefficient for wild type to be 3.06 S for the homodimer, and the frictional ratio to be 1.22, which is also in excellent agreement with the values obtained from the sedimentation velocity experiments.
The monomer-dimer fit of the sedimentation data for freshly isolated D124V SOD1 is shown in Figure 6B. The monomer-dimer equilibrium model parameters are listed in Table 3. The D124V SOD1 variant dissociates to monomers with an equilibrium constant of approximately 650 nM. The kinetics of this dissociation are quite slow with a koff rate of only 1.03 × 10−4 s−1. A similar equilibrium constant of 660 nM is calculated when the data are fit to a non-interacting model (Table 3 and Figure 6C, top panel).
Table 3.
Results of Analytical Ultracentrifugation Analyses
| D124V | Reversible Monomer-Dimer Self- Associating Model |
Non-Interacting Model |
|---|---|---|
| Monomer MW | 16.1 (15.8, 16.4) kDa | 16.8 (16.6, 17.1) kDa |
| Dimer MW | Constrained to 2 × Monomer MW | 31.8 (31.6, 3.23) kDa (unconstrained) |
| Monomer f/f0 | 1.12 (1.09, 1.14) | 1.19 (1.18, 1.20) |
| Dimer f/f0 | 1.29 (1.28, 1.31) | 1.31 (1.30, 1.31) |
| K Dissociation | 0.65 µM (0.49 µM, 0.96 µM) | 0.66 µM (based on partial concentrations) |
| Koff rate const. | 1.03 × 10−4 (0.96 × 10−4, 1.11 × 10−4) s−1 | n/a |
| Kon rate const. | 6.69 × 10−5 M s−1 | n/a |
| H80R | ||
| Monomer MW | 16.9 (16.8, 17.0) kDa | 29.1 (26.9, 29.7) kDa * |
| Dimer MW | Constrained to 2 × Monomer MW | 30.6 (30.1, 32.8) kDa (unconstrained) |
| Monomer f/f0 | * | 1.0 (1.0, 1.0) |
| Dimer f/f0 | 1.32 (1.31, 1.32) | 1.27 (1.25, 1.36) |
| K Dissociation | 0.143 µM (0.142 µM, 0.144 µM) | * |
| Koff rate const. | * | n/a |
| Kon rate const. | * | n/a |
Fitting parameters for the freshly isolated mutant D124V and H80R SOD1 samples. Values in parenthesis represent 95% confidence intervals determined in the Monte Carlo analysis. Both the reversible self-association model and the non-interacting models give very similar results, and are fitted equally well to the data.
The second species determined in this fit did not correspond to the monomer molecular weight, and therefore equilibrium constant cannot be calculated.
The sedimentation velocity data for freshly isolated H80R SOD1 were also fit to a monomer-dimer equilibrium model, which resulted in a binding constant of 143 nM (see Table 3). However, the genetic algorithm-Monte Carlo analysis reveals a species that does not correspond to a monomer-dimer molecular weight (see Figure 6C, bottom). While the dimer molecular weight is as expected (32.2 kDa), the second, much less abundant species appears to be globular with a molecular weight of 26.7 kDa. This result suggests that the second species may not be a reversible self-associating monomer, but instead might be a degradation product. However, the concentration of the second species is too low to reliably determine its identity. In fact, in both the D124V and H80R metal-free SOD1 samples, several minor degradation products were detected whose concentration is too low to permit reliable identification. However, the increase in frictional ratio for the dimeric forms of both pathogenic SOD1 mutants relative to the wild type strongly suggests that both the D124V and H80R mutants are more extended or unfolded than the wild type enzyme, a suggestion consistent with the disordered regions of loops IV and VII shown in Figures 1 and 3.
DISCUSSION
The D124V and H80R Mutations Disrupt the Zinc-Binding Site
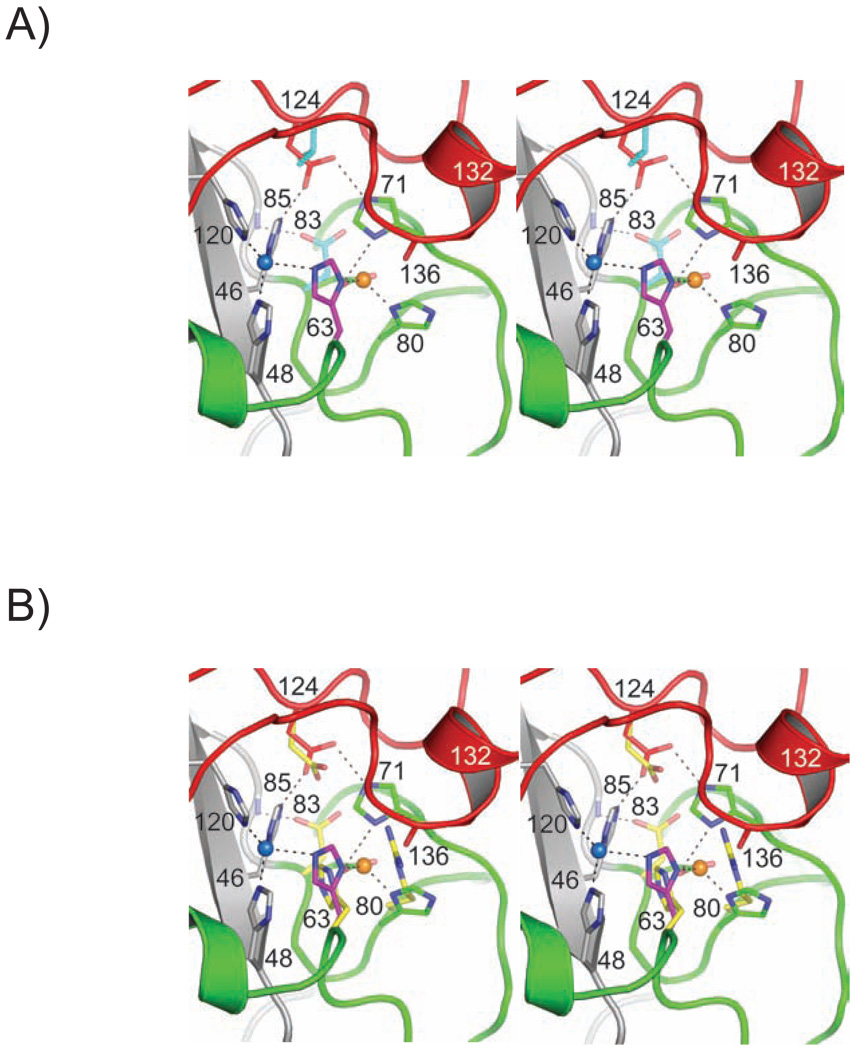
The crystal structures determined in this work suggest why the D124V and H80R pathogenic substitutions adversely affect the ability of these variants to bind zinc in the zinc-binding site. Figures 3A and 7A reveal that D124 is located in the electrostatic loop (loop VII, residues 121–142), where its side chain stabilizes both the copper- and zinc-binding sites by accepting hydrogen bonds simultaneously from the nonliganding imidazole nitrogen atoms of copper ligand H46 and zinc ligand H71. These two D124-mediated hydrogen bonds are the primary interactions linking the electrostatic loop to the zinc loop, as well as anchoring these loop elements to the SOD1 β-barrel. The importance of the D124 side chain for proper metal binding to the zinc-binding site in SOD1 was noted previously when D124N and D124G SOD1 mutants were found to be severely zinc-deficient, even after extensive dialysis against 0.5 M ZnCl2 at neutral pH (40). Figure 3B shows that the elimination of the hydrogen bonding interactions with H46 and H71 by the D124V substitution results in severely disordered electrostatic and zinc loop elements, the latter of which house the zinc ligands H63, H71, H80, and D83. Figure 7A shows that the side chain of the zinc ligand D83 is oriented away from the zinc-binding site such that its Oδ1 atom, which coordinates the zinc ion in the wild type enzyme, instead accepts a hydrogen bond from the amide nitrogen of G85.
Figure 7.
The metal-binding site of human wild type SOD1 on the D124V and H80R SOD1 variants. The color coding of the zinc and electrostatic loop elements is as in Figure 1. A) D124V SOD1 superimposed on human wild type SOD1 [pdb code 2c9v (39)]. Only the side chains of residues 83 and 124 are shown coming from the D124V structure. B) H80R SOD1 superimposed on human wild type SOD1 [pdb code 2c9v (39)]. Only the side chains of residues 63, 80, 83, and 124 are shown coming from the H80R structure. The R80 side chain clashes with the carbonyl oxygen of K136, disrupting the a-helix formed by residues 132–136 of the H80R electrostatic loop (see text).
Figures 3C, 3D and 7B show that the H80R pathogenic SOD1 substitution disrupts the zinc-binding site by sterically preventing zinc-binding and by inducing conformational disorder in the electrostatic and zinc loop elements. Figure 7B shows that in addition to being a poor zinc ligand relative to histidine, the lengthy side R80 side chain occupies the position held by the carbonyl oxygen of K136 in the wild type enzyme, which prevents residues 132–136 of the electrostatic loop from forming the short α-helix that normally to acts as a “lid” to shield the zinc-binding site from bulk solvent. As can be inferred from Figures 3C and 3D, the R80-induced displacement of this helical “lid” permits the zinc loop to become conformationally dynamic, resulting in the displacement of the zinc ligand H71 such that it no longer resides within 15 Å of its original position in wild type SOD1. The bridging imidazolate, H63, adopts a conformation such that it is unable to coordinate metal ions in either metal binding site. Finally, as is also observed in the D124V structure, the zinc ligand, D83 adopts a conformation such that it points away from the position occupied by zinc in the wild type enzyme.
Non-Native Higher Order Assembly of H80R SOD1
Although the ability of metal-deficient, pathogenic SOD1 proteins to assemble into higher order filamentous arrays was first described over six years ago in structures of the pathogenic SOD1 variants S134N and H46R (20, 41), the notion that the edges of β-strands 5 and 6 and the cleft between them may act as a “hot-spot” for non-native pathogenic SOD1 self-association has recently gained momentum. For example, non-native intermolecular SOD1 interactions nearly identical to those shown in Figure 2A were observed in structures of wild type human SOD1 proteins engineered to possess ablated zinc-binding sites (42, 43). Recent work by Nordlund, Oliveberg, and colleagues is particularly notable because the non-native SOD1-SOD1 interactions highlighted in Figure 2A were also observed in the structure an SOD1 protein engineered to be exclusively monomeric, which led them to suggest that the interactions at this interface may initiate aggregation of pathogenic SOD1 proteins in vivo (42, 43). In each of these cases, the absence of metal ions in the zinc-binding site appears to be necessary for these non-native intermolecular SOD1 interactions to occur, consistent with the notion that immature SOD1 proteins may represent the noxious entities in SOD1-linked ALS (4, 5, 19).
C111 Oxidation
The electron density superimposed on C111 residues at the homodimeric interface in H80R SOD1 shown in Figure 4 strongly suggests that the sulfur atoms are oxidized to sulfonic acid (C111-SO3H) in subunit A and to sulfenic acid (C111-SOH) in subunit B. Interestingly, these adducts are clearly observed on the H80R SOD1 variant in the P212121 crystal form but not in the P21 crystal form, even though these crystals were grown from the same crystallization drop. A similar partitioning of SOD1 species upon crystallization was observed previously for G85R SOD1 and for H46R/H48Q SOD1. G85R SOD1 was found to crystallize in four distinct crystal forms such that each crystal form contained a metal-bound species distinct from those in the other crystal forms (22), while H46R/H48Q SOD1 was observed to crystallize in two distinct crystal forms in which one displayed diatomic covalent adducts to C111 while the other did not (19).
Several reports have implicated C111 in human SOD1 as a “reactive” cysteine. In addition to the oxidation products described above and in (44), there have been reports of covalent modification of C111 by glutathione (45) and by sulfur to form persulfide (46, 47), trisulfide (48), and more recently, a heptasulfane bridge linking the two SOD1 subunits at the homodimeric interface (57). We speculate that the reactive nature of C111 may arise from the nature of its local environment. Figure 4 shows that C111 is tucked into a recessed cavity located the SOD1 dimer interface that is lined with apolar residues except for R115. The proximity of the positive charge coming from the R115 guanidinium moiety in an apolar environment that is partially protected from the solvent might lower the pKa of the C111 thiol, enhancing its reactivity.
Dissociation of D124V and H80R SOD1 Dimers
As shown in Figure 6C, freshly purified D124V and H80R SOD1 dimers with zinc in the copper-binding site demonstrate greater f/f0 ratios than the wild type SOD1 homodimer, suggesting that the differences in s20,w between the ~2.6 S D124V and H80R SOD1 dimers and the ~3.0 S metal-bound wild type SOD1 dimers shown in Figure 6A are due to differences in shape rather than mass. The observation of a greater frictional ratio for the D124V and H80R SOD1 variants is consistent with the observation of disordered zinc and electrostatic loop elements observed in their crystal structures as shown in Figure 3 because conformational disorder of these loop elements would be expected to enhance the drag of these molecules, thereby decreasing their S values relative to the wild type dimer with its well-ordered zinc and electrostatic loops.
The binding of metal ions to SOD1 has long been known to have an enormous stabilizing effect on the SOD1 homodimer [reviewed in (14)]. When metal-free, however, disulfide-oxidized human wild type SOD1 proteins still do not fractionally dissociate into monomers under even the most dilute concentrations used in sedimentation equilibrium experiments (2 µM) (14), which is indicative of a dimerization Kd of 10 nM or less. In contrast, despite the fact that the D124V and H80R SOD1 proteins possess an oxidized disulfide bond (Figure 5) and bind metal ions in their copper-binding sites (Figure 3), they remain significantly weaker dimers than metal-free, disulfide-oxidized wild type SOD1 as indicated by the positive slopes of their van Holde & Weischet plots shown in Figure 6A, as well as their calculated dissociation constants of 650 and 143 nM, respectively (Table 3). A potential implication of these differences in dissociation behavior of D124V and H80R SOD1 proteins relative to wild type is with regard to CCS action is explored more fully below.
Hindered CCS-Mediated Maturation and SOD1-Linked ALS
When expressed in sod1Δ/ccs1+ yeast, wild type SOD1 proteins rescue the oxygen sensitive phenotype of these yeast and contain substantial amounts of both copper and zinc when purified (24, 50, 51). In contrast, H46R, D124V and H80R SOD1 proteins expressed in these yeast are unable to rescue the oxygen-sensitive phenotype, an observation that is consistent with ICP-MS and anomalous scattering analyses indicating that these SOD1 variants are completely devoid of copper (20). The results of the covalent thiol modification experiments shown in Figure 5 suggest that CCS has no apparent effect on the disulfide bond status of the D124V and H80R variants. Taken together these observations might be indicative of an inability of CCS to interact with newly translated D124V and H80R variants in a productive fashion.
In contrast to the D124V and H80R SOD1 proteins studied here, crystal structures of human H46R/H48Q SOD1 reveal zinc bound to the zinc-binding sites and ablated copper-binding sites (19). H46R/H48Q SOD1 proteins were observed to form stable heterocomplexes with CCS under reducing conditions in native gel elecetrophoresis experiments, but in AMS covalent thiol modification experiments analogous to those in the present study, the H46R/H48Q SOD1 remained disulfide-reduced, even when expressed in ccs1+ yeast (19). Thus, in the case of the H46R/H48Q SOD1 variant, interaction with CCS is not sufficient for CCS to catalyze the oxidation of the intrasubunit disulfide bond. Because copper delivery and disulfide bond oxidation of SOD1 proteins by CCS are postulated to be coupled processes, the inability of H46R/H48Q SOD1 to accept copper from CCS might also prevent CCS-mediated oxidation of its disulfide bond (16, 18, 52).
The fact that CCS fails to activate D124V and H80R SOD1 proteins with copper, even though these variants possess what appear to be normal copper-binding sites, is somewhat puzzling. As mentioned above, one possibility might be that interactions between CCS and these variants is weakened, a notion supported by the observation of an enhanced propensity of D124V and H80R SOD1 proteins to dissociate relative to the wild type enzyme. The weakened affinity of D124V and H80R SOD1 subunits for themselves in the homodimer might also translate into a weakened affinity for the SOD1-like domain 2 of CCS that is responsible for the specificity of CCS/SOD1 interactions. Although this seem like a plausible suggestion, additional experiments are necessary in order to test this hypothesis.
We and others have demonstrated that the affinity of CCS for disulfide-oxidized forms of SOD1 is substantially lower than for disulfide-reduced forms (19,18, 53, 54), and the data in Figure 5 reveal that the D124V and H80R SOD1 proteins are predominantly disulfide-oxidized whether or not CCS is present in yeast cells expressing these variants. This observation is intriguing in itself because if CCS cannot productively engage D124V and H80R SOD1, the fraction of disulfide-reduced D124V and H80R proteins in these experiments might be expected to be at least as large as that observed for the wild type enzyme or for the H46R/H48Q variant. A possible explanation for these somewhat puzzling observations might be that oxidation of the SOD1 intrasubunit disulfide bond in yeast may depend more on whether a metal is bound at the copper-binding site than on the identity of the metal ion per se, as long as oxygen and/or superoxide are present. In this context, the reduced disulfide bond observed in H46R/H48Q SOD1 in previous AMS thiol modification experiments (19) might arise from the inability of this variant to bind any metal ion at its copper-binding site, while the predominantly oxidized disulfide bond observed in the D124V and H80R SOD1 variants could arise from the fact that they can bind a metal (zinc) in their respective copper-binding sites. In summary, the results presented here on the structural and biophysical properties of the D124V and H80R pathogenic SOD1 variants, together with previous observations on the H46R/H48Q SOD1 variant, are consistent with the suggestion that incomplete posttranslational modification of nascent SOD1 polypeptides may be a characteristic associated with toxicity in SOD1-linked ALS..
Supplementary Material
Table 2.
Disordered regions and identity of metal ions in the metal-binding sites
| Metal binding sites | ||||||
|---|---|---|---|---|---|---|
| Monomer | Disordered regions |
1° Bridge (H63) |
2° Bridge (D 124) |
D83 Orientation |
Copper site (occupancy) |
Zinc site (occupancy) |
| D124V P212121 | ||||||
| A | 67–78 | Broken | Broken | Flipped | Zinc (0.8) | Zinc (0.15) |
| 125–139 | ||||||
| B | 69–77 | Broken | Broken | Flipped | Zinc (0.9) | Empty |
| 125–138 | ||||||
| H80R P212121 | ||||||
| A | 67–78 | Broken | Intact | Wild type | Zinc (0.7) | Empty |
| 131–138 | ||||||
| B | 69–78 | Broken | Intact | Flipped | Zinc (0.9) | Empty |
| 125–138 | ||||||
| H80R P21 | ||||||
| A | 67–78 | Broken | Intact | Flipped | Zinc (0.7) | Empty |
| 134–138 | ||||||
| B | 67–78 | Intact | Intact | Flipped | Zinc (0.6) | Empty |
| 133–139 | ||||||
| C | 67–79 | Intact | Intact | Flipped | Zinc (0.2) | Empty |
| 125–140 | ||||||
| D | 67–78 | Broken | Intact | Flipped | Zinc (0.7) | Empty |
| 126–138 | ||||||
Acknowledgements
We acknowledge the support provided by the staff at the Texas Advanced Computing Center at the University of Texas at Austin, and by Jeremy Mann at the Bioinformatics Core Facility at the University of Texas Health Science Center at San Antonio. Support for the X-ray Crystallography Core Laboratory and the Center for Analytical Ultracentrifugation of Macromolecular Assemblies by the UTHSCSA Executive Research Committee and the San Antonio Cancer Institute is also gratefully acknowledged
This work was supported by grants NIH-NS39112 (PJH), NIH-RR022200 (BD), NIH-NS04913 (JSV), NIH-GM50016 (VCC), NSF-TG-MCB070038 (BD), and the JHU NIEHS center (VCC). DDW and LJW were supported in part by an American Foundation for Aging Research predoctoral fellowship. XC and SVS were supported in part by the Judith and Jean Pape Adams Charitable Foundation and the William and Ella Owens Medical Research Foundation.
ABBREVIATIONS AND TEXTUAL FOOTNOTES
- SOD1
copper-zinc superoxide dismutase
- ALS
amyotrophic lateral sclerosis
- fALS
familial amyotrophic lateral sclerosis
- D124V
copper-zinc superoxide dismutase with D124 substituted with Val
- H80R
copper-zinc superoxide dismutase with H80 substituted with Arg.
- CCS
copper chaperone for SOD1
- 2DSA
2-dimensional spectrum analysis
- SOMO
solution modeler
- ASTFEM-RA
adaptive space-time finite element solution for multicomponent-reacting systems
- ICP-MS
inductively coupled plasma mass spectrometry
- AMS
4-acetamido-4'-maleimidylstilbene-2, 2'-disulfonic acid
- EDTA
ethylenediaminetetraacetic acid
Footnotes
Supporting Information Available
Supplementary Figure 1: Fluorescence of D124V and H80R SOD1 crystals measured normal to the X-ray beam at energies spanning the zinc and copper edges. Supplementary Figure 2: Anomalous difference Fourier electron density calculated at various contour levels with diffraction data taken from orthorhombic D124V SOD1 crystals with X-rays tuned to the zinc and copper absorption edges superimposed on the refined D124V SOD1 structure. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989;264:7761–7764. [PubMed] [Google Scholar]
- 2.Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, Warner C, Deng G, Soriano E, Smyth C, Parge HE, Ahmed A, Roses AD, Hallewell RA, Pericak-Vance MA, Siddique T. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 3.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH., Jr Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 4.Winkler DD, Prudencio M, Karch CM, Borchelt DR, Hart PJ. Copper-Zinc Superoxide Dismutase, its Copper Chaperone, and Familial Amyotrophic Lateral Sclerosis. In: Dobson CM, Kelly JW, Ramirez-Alvarado M, editors. Protein Misfolding Diseases: Current and Emerging Principles and Therapies. Hoboken: John Wiley & Sons, Inc.; 2009. (in press) [Google Scholar]
- 5.Seetharaman SV, Prudencio M, Karch C, Holloway SP, Borchelt DR, Hart PJ. Immature Copper-Zinc Superoxide Dismutase and Familial Amyotrophic Lateral Sclerosis. Exp. Biol. Med. 2009 doi: 10.3181/0903-MR-104. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 7.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Xu G, Li H, Gonzales V, Fromholt D, Karch C, Copeland NG, Jenkins NA, Borchelt DR. Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: alphaB-crystallin modulates aggregation. Hum Mol Genet. 2005;14:2335–2347. doi: 10.1093/hmg/ddi236. [DOI] [PubMed] [Google Scholar]
- 9.Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 10.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 11.Hart PJ. Pathogenic superoxide dismutase structure, folding, aggregation and turnover. Curr Opin Chem Biol. 2006;10:131–138. doi: 10.1016/j.cbpa.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 13.Arnesano F, Banci L, Bertini I, Martinelli M, Furukawa Y, O'Halloran TV. The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279:47998–48003. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 14.Doucette PA, Whitson LJ, Cao X, Schirf V, Demeler B, Valentine JS, Hansen JC, Hart PJ. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J Biol Chem. 2004;279:54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 15.Lindberg MJ, Normark J, Holmgren A, Oliveberg M. Folding of human superoxide dismutase: disulfide reduction prevents dimerization and produces marginally stable monomers. Proc Natl Acad Sci U S A. 2004;101:15893–15898. doi: 10.1073/pnas.0403979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown NM, Torres AS, Doan PE, O'Halloran TV. Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu,Zn superoxide dismutase. Proc Natl Acad Sci U S A. 2004;101:5518–5523. doi: 10.1073/pnas.0401175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa Y, O'Halloran TV. Posttranslational modifications in Cu,Zn-superoxide dismutase and mutations associated with amyotrophic lateral sclerosis. Antioxid Redox Signal. 2006;8:847–867. doi: 10.1089/ars.2006.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler D, Schuermann J, Cao X, Holloway S, Borchelt D, Carroll M, Proescher J, Culotta V, Hart P. Structural and Biophysical Properties of the Pathogenic SOD1 Variant H46R/H48Q. Biochemistry. 2009 doi: 10.1021/bi8021735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elam JS, Taylor AB, Strange R, Antonyuk S, Doucette PA, Rodriguez JA, Hasnain SS, Hayward LJ, Valentine JS, Yeates TO, Hart PJ. Amyloid-like filaments and water-filled nanotubes formed by SOD1 mutant proteins linked to familial ALS. Nat Struct Biol. 2003;10:461–467. doi: 10.1038/nsb935. [DOI] [PubMed] [Google Scholar]
- 21.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter CW, Sweet RM, editors. Methods Enzymol. New York: Academic Press; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 22.Cao X, Antonyuk SV, Seetharaman SV, Whitson LJ, Taylor AB, Holloway SP, Strange RW, Doucette PA, Valentine JS, Tiwari A, Hayward LJ, Padua S, Cohlberg JA, Hasnain SS, Hart PJ. Structures of the G85R variant of SOD1 in familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:16169–16177. doi: 10.1074/jbc.M801522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vagin AA, Teplyakov A. MOLREP: an automated program for molecular replacement. J. Appl. Cryst. 1997;30:1022–1025. [Google Scholar]
- 24.Hart PJ, Liu H, Pellegrini M, Nersissian AM, Gralla EB, Valentine JS, Eisenberg D. Subunit asymmetry in the three-dimensional structure of a human CuZnSOD mutant found in familial amyotrophic lateral sclerosis. Protein Sci. 1998;7:545–555. doi: 10.1002/pro.5560070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 26.Read RJ. Acta Crystallogr. 1986;A42:140–149. [Google Scholar]
- 27.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 28.Demeler B. UltraScan A Comprehensive Data Analysis Software Package for Analytical Ultracentrifugation Experiments. In: Scott D, Harding S, Rowe A, editors. Modern Analytical Ultracentrifugation: Techniques and Methods. Royal Society of Chemistry (UK); 2005. pp. 210–229. [Google Scholar]
- 29.Demeler B. UltraScan - An comprehensive data analysis software package for analytical ultracentrifugation experiments. 2009 < http://www.ultrascan.uthscsa.edu/>.
- 30.Laue TM, Shah BD, Ridgeway TM, Pelletier SL. Computer-aided interpretation of analytical sedmentation data for proteins. In: Harding SE, Rowe AJ, Horton JC, editors. Analytical Ultracentrifugation in Biochemistry and Polymer Science. Cambridge: Royal Society of Chemistry; 1992. pp. 90–125. [Google Scholar]
- 31.Brookes E, Cao W, Demeler B. A two-dimensional spectrum analysis for sedimentation velocity experiments of mixtures with heterogeneity in molecular weight and shape. Eur Biophys J. 2009 doi: 10.1007/s00249-009-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brookes E, Demeler B. Parsimonious Regularization using Genetic Algorithms Applied to the Analysis of Analytical Ultracentrifugation Experiments. GECCO Proceedings ACM 978-1-59593-697-4/07/0007. 2007 [Google Scholar]
- 33.Demeler B, Brookes E. Monte Carlo analysis of sedimentation experiments. Colloid Polym Sci. 2008;286:129–137. [Google Scholar]
- 34.Cao W, Demeler B. Modeling analytical ultracentrifugation experiments with an adaptive space-time finite element solution for multicomponent reacting systems. Biophys J. 2008;95:54–65. doi: 10.1529/biophysj.107.123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 36.Brookes E, Demeler B, Rosano C, Rocco M. The implementation of SOMO (SOlution MOdeller) in the UltraScan analytical ultracentrifugation data analysis suite: enhanced capabilities allow the reliable hydrodynamic modeling of virtually any kind of biomacromolecule. Eur Biophys J. 2009 doi: 10.1007/s00249-009-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll MC, Outten C, Proescher JB, Rosenfeld L, Watson WH, Whitson LJ, Hart PJ, Jensen LT, Culotta VC. The effects of glutaredoxins and copper activation pathways on the disulfide and stability of Cu/Zn superoxide dismutase. J Biol Chem (submitted) 2006 doi: 10.1074/jbc.M600138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elam JS, Malek K, Rodriguez JA, Doucette PA, Taylor AB, Hayward LJ, Cabelli DE, Valentine JS, Hart PJ. An alternative mechanism of bicarbonate-mediated peroxidation by copper-zinc superoxide dismutase: rates enhanced via proposed enzyme-associated peroxycarbonate intermediate. J Biol Chem. 2003;278:21032–21039. doi: 10.1074/jbc.M300484200. [DOI] [PubMed] [Google Scholar]
- 39.Strange RW, Antonyuk SV, Hough MA, Doucette PA, Valentine JS, Hasnain SS. Variable metallation of human superoxide dismutase: atomic resolution crystal structures of Cu-Zn, Zn-Zn and as-isolated wild-type enzymes. J Mol Biol. 2006;356:1152–1162. doi: 10.1016/j.jmb.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 40.Banci L, Bertini I, Cabelli DE, Hallewell RA, Tung JW, Viezzoli MS. A characterization of copper/zinc superoxide dismutase mutants at position 124. Zinc-deficient proteins. Eur J Biochem. 1991;196:123–128. doi: 10.1111/j.1432-1033.1991.tb15794.x. [DOI] [PubMed] [Google Scholar]
- 41.Antonyuk S, Elam JS, Hough MA, Strange RW, Doucette PA, Rodriguez JA, Hayward LJ, Valentine JS, Hart PJ, Hasnain SS. Structural consequences of the familial amyotrophic lateral sclerosis SOD1 mutant His46Arg. Protein Sci. 2005;14:1201–1213. doi: 10.1110/ps.041256705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nordlund A, Leinartaite L, Saraboji K, Aisenbrey C, Grobner G, Zetterstrom P, Danielsson J, Logan DT, Oliveberg M. Functional features cause misfolding of the ALS-provoking enzyme SOD1. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0812046106. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts BR, Tainer JA, Getzoff ED, Malencik DA, Anderson SR, Bomben VC, Meyers KR, Karplus PA, Beckman JS. Structural characterization of zinc-deficient human superoxide dismutase and implications for ALS. J Mol Biol. 2007;373:877–890. doi: 10.1016/j.jmb.2007.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujiwara N, Nakano M, Kato S, Yoshihara D, Ookawara T, Eguchi H, Taniguchi N, Suzuki K. Oxidative modification to cysteine sulfonic acid of Cys111 in human copper-zinc superoxide dismutase. J Biol Chem. 2007;282:35933–35944. doi: 10.1074/jbc.M702941200. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox KC, Zhou L, Jordon JK, Huang Y, Yu Y, Redler RL, Chen X, Caplow M, Dokholyan NV. Modifications of Superoxide Dismutase (SOD1) in Human Erythrocytes: A POSSIBLE ROLE IN AMYOTROPHIC LATERAL SCLEROSIS. J Biol Chem. 2009;284:13940–13947. doi: 10.1074/jbc.M809687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calabrese L, Federici G, Bannister WH, Bannister JV, Rotilio G, Finazzi-Agro A. Labile sulfur in human Superoxide dismutase. Eur J Biochem. 1975;56:305–309. doi: 10.1111/j.1432-1033.1975.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 47.de Beus MD, Chung J, Colon W. Modification of cysteine 111 in Cu/Zn superoxide dismutase results in altered spectroscopic and biophysical properties. Protein Sci. 2004;13:1347–1355. doi: 10.1110/ps.03576904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okado-Matsumoto A, Guan Z, Fridovich I. Modification of cysteine 111 in human Cu,Zn-superoxide dismutase. Free Radic Biol Med. 2006;41:1837–1846. doi: 10.1016/j.freeradbiomed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Bertini I, Mangani S, Viezzoli MS. Structure and Properties of Copper-Zinc Superoxide Dismutase. Adv. Inorg. Chem. 1998;45:127–251. [Google Scholar]
- 50.Goto JJ, Gralla EB, Valentine JS, Cabelli DE. Reactions of hydrogen peroxide with familial amyotrophic lateral sclerosis mutant human copper-zinc superoxide dismutases studied by pulse radiolysis. J Biol Chem. 1998;273:30104–30109. doi: 10.1074/jbc.273.46.30104. [DOI] [PubMed] [Google Scholar]
- 51.Goto JJ, Zhu H, Sanchez RJ, Nersissian A, Gralla EB, Valentine JS, Cabelli DE. Loss of in vitro metal ion binding specificity in mutant copper-zinc superoxide dismutases associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2000;275:1007–1014. doi: 10.1074/jbc.275.2.1007. [DOI] [PubMed] [Google Scholar]
- 52.Furukawa Y, Torres AS, O'Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. Embo J. 2004;23:2872–2881. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamb AL, Torres AS, O'Halloran TV, Rosenzweig AC. Heterodimer formation between superoxide dismutase and its copper chaperone. Biochemistry. 2000;39:14720–14727. doi: 10.1021/bi002207a. [DOI] [PubMed] [Google Scholar]
- 54.Lamb AL, Torres AS, O'Halloran TV, Rosenzweig AC. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat Struct Biol. 2001;8:751–755. doi: 10.1038/nsb0901-751. [DOI] [PubMed] [Google Scholar]
- 55.Carroll MC, Outten CE, Proescher JB, Rosenfeld L, Watson WH, Whitson LJ, Hart PJ, Jensen LT, Cizewski Culotta V. The effects of glutaredoxin and copper activation pathways on the disulfide and stability of Cu,Zn superoxide dismutase. J Biol Chem. 2006;281:28648–28656. doi: 10.1074/jbc.M600138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Proescher JB, Son M, Elliott JL, Culotta VC. Biological effects of CCS in the absence of SOD1 enzyme activation: implications for disease in a mouse model for ALS. Hum Mol Genet. 2008;17:1728–1737. doi: 10.1093/hmg/ddn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.You Z, Cao X, Taylor AB, Hart PJ, Levine RL. Characterization of a covalent polysulfane bridge in copper-zinc superoxide dismutase. Biochemistry. 2010;49:1191–1198. doi: 10.1021/bi901844d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.