Abstract
The influence of psychosocial factors on the development and progression of cancer has been a longstanding hypothesis since ancient times. In fact, epidemiological and clinical studies over the past 30 years have provided strong evidence for links between chronic stress, depression and social isolation and cancer progression. By contrast, there is only limited evidence for the role of these behavioral factors in cancer initiation. Recent cellular and molecular studies have identified specific signaling pathways that impact cancer growth and metastasis. This article provides an overview of the relationship between psychosocial factors, specifically chronic stress, and cancer progression.
Keywords: cancer, catecholamine, metastasis, signaling pathway, stress
The major cause of death from cancer is metastasis that is resistant to conventional therapy [1]. Primary neoplasms are biologically heterogeneous and the process of metastasis consists of a series of sequential and selective steps that few cells can successfully complete. The outcome of cancer metastasis depends on multiple interactions between metastatic cells and homeostatic mechanisms that are unique to a given organ micro environment [2]. Therefore, the treatment of metastasis should be targeted not only against cancer cells, but also against the host factors that contribute to and support the progressive growth and survival of metastatic cancer cells. Clinical and epidemiological studies over the last 30 years have identified psychosocial factors including stress, chronic depression and lack of social support as risk factors for cancer progression [3-6]. Whereas evidence for the role of psychosocial factors in cancer initiation is limited and some-what contradictory [7-10], support is stronger for links between psychological factors such as stress, depression and social isolation and disease progression [11,12]. Chronicity of negative affect, as manifested by depressed mood or hopelessness, appears to have stronger relationships with outcomes than do stressful events, suggesting that sustained activation of negative affective pathways may provide the strongest links to cancer progression [13-16]. Moderators of stress, such as social support, have been frequently studied with respect to cancer outcomes. Social support refers to an individual’s perceived satisfaction with social relationships and is thought to play a major role in buffering psychological and biological stress responses [17]. Several studies have linked high levels of social support to improved clinical outcomes in cancer patients. For example, in breast cancer patients, social support has been related to longer survival in several large-scale studies [18-20], although negative findings were noted in some studies [21-23]. Collectively, emerging evidence has shown stress and specific psychosocial factors to be associated with key elements of the metastatic cascade in both animal and human models. In this article, we review the biological processes affected by chronic stress and the related pathways and discuss implications for cancer management.
Stress-mediated neuroendocrine response
Stress & the CNS
Stress is a complex process including environmental and psychosocial factors that initiate a cascade of information processing in both the peripheral nervous system and CNS [24]. Stress can be acute (short-lived) or chronic (repetitive or occurring over an extended period of time) [25]. Under chronic stress conditions, the body remains in a constant state of ‘overdrive’, with deleterious downstream effects on regulation of stress response systems, as well as many organ systems [26]. Both norepinephrine (NE) and epinephrine (E) are known to be elevated in individuals with acute or chronic stress [27-29]. Furthermore, dopamine (DA) levels are increased in the brain during acute stress [30]. However, under chronic stress, DA levels are lower as a consequence of decreased release of DA [31]. A variety of stressors, including severe trauma, marital discord and bereavement, as well as depression and social isolation, have been associated with dysregulation or alterations in various neuroendocrine hormones, particularly catecholamines and cortisol [32-40].
The physiological stress response is thought to be one of the likely mediators of the effects of psychosocial factors on cancer progression. The overall stress response involves activation of several body systems including the autonomic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis. The ‘fight or flight’ response is elicited by the production of mediators such as NE and E from the sympathetic nervous system (SNS) and the adrenal medulla. The HPA response includes release of corticotropin -releasing hormone from the hypothalamus, which induces secretion of adrenocorticotrophic hormone from the anterior pituitary, resulting in downstream release of glucocorticoids (GCs) such as cortisol from the adrenal cortex [41]. Additional neuroendocrine factors are also modu lated following stress, including DA, prolactin, NGF, substance P and oxytocin [42,43].
Role of neuroendocrine mediators in peripheral organs
Neuroendocrine mediators can modulate cellular function in many of the peripheral tissue sites most relevant to cancer onset and progression. For example, neurotransmitters from the SNS (i.e., NE and E) play physiologically relevant roles in regulating the microenvironment of peripheral organs. The ovary provides an example that is highly relevant to cancers of the reproductive system. Overall concentrations of catecholamines are substantially higher in the ovaries than in plasma [44]. Moreover, catecholamine levels in the ovaries are known to be increased in response to stress owing to increased sympathetic activity, which has been shown to result in the appearance of precystic follicles [45-48]. Similarly, catecholamines are present at substantially higher levels in the bone marrow microenvironment and are secreted from both nerve endings and bone marrow cells [49]. Functionally, neuroendocrine factors can modulate hematopoiesis within the bone marrow microenvironment. With respect to catecholamines, the presence of α1B adrenoreceptors has been shown in pre-B-cells, and their activation by catecholamines results not only in suppressed myelopoiesis in vitro, but also in protection in vivo against supralethal doses of carboplatin [49].
The effects of catecholamines are mediated through adrenergic receptors (ADRs), which are the most extensively studied classes of G-protein-coupled receptors. Genomic and/or cDNA clones for nine types of ADRs have been obtained: two types of α1-ADRs (ADRA1), three types of α2-ADRs (ADRA2), β1-ADR (ADRB1), β2-ADR (ADRB2), β3-ADR (ADRB3) and the avian β-receptors [50]. These receptors are coupled to G-proteins, which act as molecular switches to control downstream pathways. In the G-protein switching mechanism of control, ADRs can bind the stimulatory G-protein, Gs, or the inhibitory G-protein, Gi. Binding of Gs mediates activation of the cAMP-dependent PKA system and results in downstream activation of several pathways, resulting in growth and migration of cells [51,52], while binding of Gi controls multiple signaling cascades, among them the mitogen-activated protein kinase pathway, which is frequently overactivated in cancers. Stimulation of the ADRA1 expressed in cultured cells leads to the activation of phospholipase C, whereas stimulation of ADRA2 leads to inhibition of adenylyl cyclase and the activation of phospholipase C. This may be due to either the ability of a receptor to activate more than one type of G-protein or the ability of the Gs subunit to activate more than one effector system. The cellular response to stimulation of a specific G-protein-coupled receptor may also vary in different cells. ADRAs are also expressed in diverse types of cancer cells, including human colon and prostate cancer cells [53,54]. Doxazosin, a selective ADRA1 antagonist used to treat hypertension, has been shown to inhibit prolif er ation of prostate and pituitary cancer cells [54].
β-adrenergic receptors (ADRBs) mediate many effects of catecholamines on target cells and have been identified on several cancer cell types, including breast [55] and ovarian cancer cells [56]. ADRBs are G-protein-coupled receptors whose primary function is the transmission of information from the extracellular environment to the interior of the cell [57]. Three distinct ADRB subtypes have been identified – ADRB1, ADRB2 and ADRB3 [58-60] – and all three can signal by coupling to the stimulatory G-protein Gαs, leading to activation of adenylyl cyclase and accumulation of the second messenger cAMP. The ADRB2 subtype is the dominant present ADR and can also bind to Gi [50]. Our group has recently demonstrated that both NE and E are elevated in a sustained fashion in ovarian and other peritoneal tissues in preclinical models of chronic stress [61]. These hormonal increases were related to greater tumor burden, which was mediated by increased angiogenesis. The ADRB–cAMP signaling pathway was identified as the underlying signaling pathway to promote angiogenesis in these malignant ovarian tumors (Figure 1).
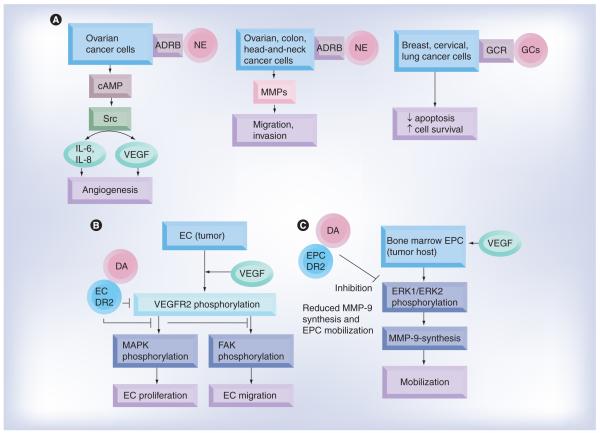
Figure 1. Norepinephrine-, dopamine- and glucocorticoid-mediated signaling pathways in tumor, endothelial and endothelial progenitor cells.
(A) In ovarian cancer cells, NE acting through ADRBs induces synthesis and release of the proangiogenic cytokines IL-6, IL-8 and VEGF. NE also induces synthesis of MMPs and stimulates migration and invasion of ovarian, colon and head and neck cancer cells; in breast, cervical and lung cancer cells, GCs acting through the GC receptor inhibit chemotherapy-induced apoptosis and promote cancer cell survival. (B) DA acting through DR2 receptors in tumor endothelial cells inhibits proliferation of these cells by inhibiting phosphorylation of VEGFR-2, MAPK and FAK. (C) DA decreases ERK1/ERK2-mediated MMP-9 synthesis and release by endothelial progenitor cells and thereby inhibits their mobilization from the bone marrow. Thus, DA prevents the participation of EPCs in tumor neovascularization.
ADRB: β-adrenergic receptor; DA: Dopamine; EC: Endothelial cell; EPC: Endothelial progenitor cell; FAK: Focal adhesion kinase; GC: Glucocorticoid; GCR: Glucocorticoid receptor; MAPK: Mitogen-activated protein kinase; MMP: Matrix metalloproteinase; NE: Norepinephrine; VEGFR: VEGF receptor.
Glucocorticoids are a class of steroid hormones that bind to the GC receptor (GCR), which is present in almost every vertebrate animal cell. GCs are part of the feedback mechanism that modulates immune activity and inflammatory responses. Pharmacologic doses of GCs are frequently used to treat conditions that are caused by an overactive immune system. GCs also interfere with various abnormal mechanisms in cancer cells, so they are used in high doses to treat certain malignancies [62]. GCs and mineralocorticoids are both produced by the adrenal cortex but differ in their specific receptors, target cells and effects [62]. The GCR is located in the cytosol and is activated by ligand binding. After binding, the newly formed receptor–ligand complex translocates itself into the nucleus, where it binds to GC response elements in the promoter region of the target genes, resulting in the regulation of gene expression [63]. This process is commonly referred as transactivation. An opposite mechanism also mediated by GCs is called transrepression. The activated hormone receptor interacts with specific transcription factors (such as AP1 and NF-κB) and prevents the transcription of targeted genes. GCs are able to prevent the transcription of proinflammatory genes, including interleukins IL-1B, IL-4, IL-6 and IL-8, chemokines, cytokines and TNFA genes [63]. Through interaction with its GCR, GCs are also able to upregulate the expression of anti-inflammatory proteins and downregulate the expression of proinflammatory proteins [64].
Cortisol is the most important human GC. It is essential for life and it regulates a variety of important cardiovascular, metabolic, immunologic and homeostatic functions [62]. Cortisol is secreted by the adrenal cortex in response to stress [25,32,65,66]. Social support and stress reduction are associated with lower cortisol levels [67-69]. A number of studies have demonstrated that stress can disrupt neuroendocrine circadian rhythms [68] in ways that favor tumor growth and metastasis. Similarly, nightshift work, which is known to disrupt endocrine rhythms, is considered a risk factor for breast and colorectal cancers [70]. GCs may have a bimodal effect on tumor cells. For example, at lower doses, dexamethasone (a synthetic GC) can stimulate tumor growth, but at higher doses it inhibits tumor growth [71]. In addition, cortisol may act in a synergistic fashion with catecholamines. For example, cortisol has been shown to potentiate the isoproterenol-induced increase in cAMP accumulation in lung cancer cells [72].
Animal models for studying the effects of stress
Animal models that mimic the pattern of human disease play an important role in understanding the effects of stress on cancer and other diseases. Restraint-stress animal models have been widely used to study the effects of chronic stress on immunity, courses of infectious diseases and cancer. In some studies, restraint-stressed mice demonstrated significantly reduced inflammatory responses to influenza virus and depressed antiviral cellular immunity. In female SKH-1 mice, the effect of stress on cutaneous wound healing showed that the reduction in inflammation and delayed healing correlated with high levels of serum corticosterone [73-77]. Physical restraint using 50-ml conical tubes for varying lengths of time has been shown to result in increased NE, E and corticosterone levels [73-77]. Restraint systems have also been demonstrated to consistently result in elevations of IL-6 [78,79]. The length of restraint in different studies has ranged from 1 to 12 h daily [74-76,78,80-82]. In C57BL/6 mice, within 3 h, there is an almost 50% increase in corticosterone levels, and at 6 h, the corticosterone levels reach a maximal increase of over 100% [74,76,78,80,82,83]. Furthermore, restraint stress has been demonstrated to raise catecholamine levels in murine models by two- to five-fold [47,82,84,85]. Restraint stress has also been shown to reduce the antitumor effects of cyclophosphamide chemotherapy in C57BL/6 mice bearing Lewis lung carcinoma [80] and to result in metallothionein induction, which may be responsible for chemotherapy resistance [86].
Other models used for stress studies include swim stress, surgical stress, social confrontation and hypothermia. These models have been shown to promote lung metastasis from injected breast cancer cells [87-90]. Most of these models have been utilized in the context of effects of stress on the immune system. In particular, the hypothermia model under thiopental anesthesia suppresses natural killer (NK)-cell activity and compromises host resistance to the formation of metastasis, possibly via adrenergic pathways [91]. Finally, a recent report described the effects of social isolation in Norway rats [92]. Socially isolated female rats have a sustained and dysregulated GC response to an acute stressor [93] and dysregulated cardiovascular responses to the everyday stressors of animal husbandry [94].
Effects of stress on cancer metastasis
Metastasis is a complex process that requires several steps to be successful, including angiogenesis, proliferation, invasion, embolization and evasion of immune system surveillance (Figure 2) [95-98]. Growth of a tumor beyond 1 mm in size requires vascularization of the tumor, which also provides a method for dissemination of metastatic cells [99]. Moreover, a tumor cell must gain the ability to break off from the main tumor, invade through the basement membrane and embolize into the bloodstream. The cell then arrests in capillary beds and must be able to extravasate from the bloodstream and adhere to parenchymal tissues. The cell must also evade immune system surveillance. Once settled, the metastatic cell interacts with its new microenvironment to grow and ultimately develop its own blood supply. Cells that fail to acquire any one of these characteristics cannot metastasize and the cascade is aborted [100]. Increasing evidence shows that the stress response can affect many parts of this cascade. Here, we examine cellular and molecular findings relating stress to processes implicated in cancer progression and metastasis.
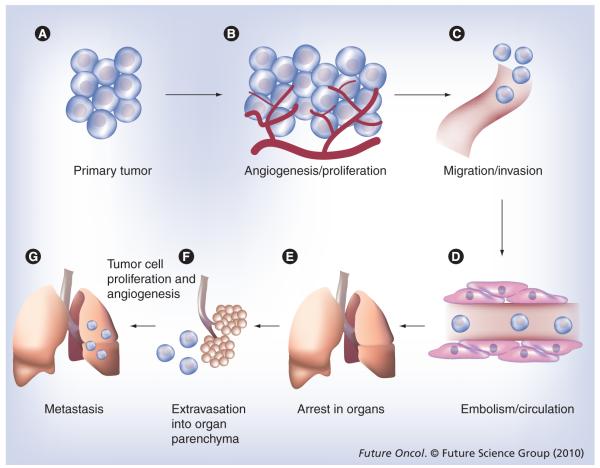
Figure 2. Main steps in the formation of a metastasis.
The process of cancer metastasis consists of sequential selective steps. The outcome of each step is influenced by the interaction of metastatic cells with homeostatic factors. Failure of a tumor cell to complete any step effectively terminates the process. Therefore, the formation of clinically relevant metastases represents the survival and growth of unique subpopulations of cells that pre-exist in primary tumors. (A) Cellular transformation and tumor growth. (B) Extensive vascularization must occur if a tumor mass exceeds 1–2 mm in diameter. The synthesis and secretion of angiogenic factors establish a capillary network from the surrounding host tissue. (C) Migration and invasion of the host stroma by some tumor cells occurs by several parallel mechanisms. Lymphatic channels offer very little resistance to penetration by tumor cells and provide the most common route for tumor cell entry into the circulation. (D) Next, detachment and embolization of single tumor cells or aggregates occurs; most circulating tumor cells are rapidly destroyed. (E) After cancer cells have survived the circulation, they become trapped in the capillary beds of distant organs by adhering either to capillary endothelial cells or to the subendothelial basement membrane. (F) Extravasation then occurs, probably by mechanisms similar to those that operate during invasion. (G) Proliferation within the organ parenchyma completes the metastatic process. To continue growing, the micrometastasis must develop a vascular network and evade destruction by host defenses. The cells can then invade blood vessels, enter the circulation and produce additional metastases.
Stress & angiogenesis
Angiogenesis is a complex and highly regulated process that is crucial for tumor growth and metastasis. The process of tumor neovascularization or angiogenesis involves the release of proangiogenic factors (e.g., VEGF, IL-6, TGF-α and -β and TNF-α [101,102]) by tumor cells to cause endothelial activation, blood vessel growth and subsequent tumor expansion [102]. Angiogenesis can also be stimulated by a disruption in the balance between pro- and anti-angiogenic factors.
VEGF is a direct angiogenic molecule that plays an essential role in embryogenesis, physiologic angiogenesis and neovascularization of malignancy [103]. VEGF stimulates endothelial cell migration, proliferation and proteolytic activity [104]. Hormones associated with SNS activation may favor angiogenic mechanisms in human tumors. NE has been shown to upregulate VEGF in adipose tissue through the ADRB–cAMP–PKA pathway [105]. Similar findings have been noted in ovarian cancer cell lines [56], and these effects were abolished by a β-blocker, propranolol, and mimicked by isoproterenol [105]. Furthermore, NE-driven increases in VEGF synthesis have been demonstrated in several human multiple myeloma cell lines (e.g., NCI-H929, MM-M1 and FLAM-76) and act via ADRB1 and ADRB2 [106].
Clinical studies have shown links between higher levels of social support and lower levels of VEGF levels in sera [107] and in tumor tissues [108] in ovarian cancer patients. In addition, depression and quality of life have been related to VEGF in colorectal cancer [109]. Whereas higher levels of preoperative depression were correlated with higher preoperative and postoperative VEGF levels (6–8 weeks following surgery), better global quality of life was related to higher levels of post-operative VEGF. Thus, various psychosocial factors have been associated with VEGF in clinical cancer settings. Furthermore, social support has been linked to lower levels of IL-6, another proangiogenic factor, both in peripheral blood and in ascites from patients with advanced ovarian cancer [110]. IL-6 serves as a major regulatory cytokine in the human body [111]. Moreover, solid tumor cells secrete high levels of IL-6, which in turn promotes fundamental processes in cancer growth and metastasis including angiogenesis, proliferation, attachment and invasion [112-114]. Increased synthesis and release of IL-6 in human ovarian tumor cell lines (e.g., SKOV3ip1, Hey-A8 and EG) was observed following treatment with NE in vitro. Furthermore, a significant increase in IL-6 mRNA synthesis and its promoter activity has been observed in these malignant ovarian cells following NE treatment [115]. Abrogation of this effect by ADRB antagonists confirmed that NE regulates IL-6 gene transcription (Figure 1) [114]. This signaling pathway also implicated Src activation in mediating the increased IL-6 mRNA synthesis through enhanced IL-6 promoter activity [15]. Since Src activation also induces other proangiogenic molecules such as VEGF and IL-8, NE and E may be responsible for regulating the synthesis of these proangiogenic molecules of ovarian cancer cells [114].
The effects of stress on tumor angiogenesis have also been examined using in vivo models. In an orthotopic mouse model of ovarian cancer, Thaker and colleagues demonstrated that chronic stress induced by daily periods of immobilization results in higher levels of tissue catecholamines, greater tumor burden and a more invasive pattern of disease [61]. Angiogenesis, as reflected by microvessel density counts, was significantly increased in tumors from stressed compared with control mice. Furthermore, VEGF mRNA and protein levels were significantly elevated in the tumor samples from mice exposed to daily stress. Continuous infusion of propranolol (a nonselective β-blocker) ameliorated the effects of stress on tumor burden and pattern of disease, thereby confirming the importance of ADRBs on ovarian cancer cells in an in vivo model. The mechanism of the NE- and E-mediated increased tumor angiogenesis has been attributed to increased VEGF synthesis [56,61] and overexpression of matrix metalloproteinase (MMP)-2 and MMP-9 [116]. The underlying signaling pathways that promote angiogenesis in these malignant ovarian tumors are summarized in Figure 1. Similar results have been found in the human nasopharyngeal carcinoma cell line HONE-1, in which NE, by acting through ADRB2, stimulated VEGF synthesis and increased production of MMP-2 and MMP-9 [116]. In addition, NE-driven increases in VEGF synthesis have now been demonstrated in several human multiple myeloma cell lines (e.g., NCI-H929, MM-M1 and FLAM-76) by acting through ADRB1 and ADRB2 [106]. Recent studies have also shown the involvement of signal transducer and activator of transcription factor (STAT)-3 in promoting stress-mediated tumor-associated angiogenesis. STAT-3 is involved in many protumorigenic pathways by activating downstream targets to promote proliferation and inhibit apoptosis. Although STAT-3 can be activated by growth factors and cytokines such as VEGF and IL-6, stress hormones such as NE and E can activate STAT-3 independent of IL-6, leading to its translocation to the nucleus and subsequent binding to DNA in order to promote transcription of genes associated with cell survival, angiogenesis and proliferation [117]. There are limited data regarding the effects of cortisol on angio genesis. In a rat glioma model, dexamethasone (a synthetic GC) treatment of cancer cells resulted in 50–60% downregulation of VEGF mRNA and this effect was dependent on GCR function [118]. However, this inhibitory effect was markedly reduced by hypoxia, which is a potent VEGF inducer. In ovarian cancer cell lines, cortisol alone had some stimulatory effects on VEGF production in vitro at lower doses, but inhibitory effects at pharmacologic doses [56]. Because stress often involves elevations in both cortisol and catecholamines, costimulation experiments were also performed. While priming with cortisol blunted NE-induced VEGF production, significant increases were still noted [56]. These findings suggest that catecholamine-induced effects are dominant in the context of angiogenic cytokine production.
Proliferation & growth of primary tumors & metastases
Tumor growth at the primary site initially depends on nutrient and oxygen diffusion. Subsequently, at metastatic sites, signals from autocrine, paracrine and/or endocrine pathways influence tumor cell proliferation, with growth dependent on the net balance of positive and negative signals [119]. There are limited data regarding the direct effects of stress hormones on cell proliferation. Most data suggest that catecholamines suppress proliferation of normal cells such as keratinocytes [120], which may contribute to impaired wound healing in the context of stress. The effects of stress-related hormones on cancer cell proliferation may depend on the type of substance and tumor type. In mammary tumors, activation of ADRBs has been related to accelerated tumor growth [55,121,122]. The cAMP responsive element-binding (CREB) protein is an important transcription factor activated by multiple signal transduction pathways in response to external stimuli, including stress hormones [123,124]. Several studies have revealed a role for the CREB family of proteins in tumor cell proliferation, migration, angiogenesis and inhibition of apoptosis [123-125]. However, in other models, catecholamines appear to inhibit tumor cell proliferation, a process that may be mediated by ADRAs [126].
Pifl and colleagues found that NE inhibited neuroblastoma cell growth, primarily in cells expressing the DA transporter [127]. In cells with DA uptake, the share of G0/G1 populations of cells was significantly increased after treatment with NE. In prostate carcinoma, treatment with agents that induce cAMP, such as E, isoproterenol, forskolin and dibutyryl cAMP, result in acquisition of neuroendocrine characteristics by epithelial prostate cancer cells [128]. The neuroendocrine characteristics were manifested by dense core granules in the cytoplasm, the extension of neuron-like processes, loss of mitogenic activity and expression of multiple neuroendocrine markers. The presence of these neuroendocrine cells has been linked to poor prognosis in prostate cancer patients [129,130]. Interestingly, the neuroendocrine cells have minimal proliferative activity, but these cells provide paracrine stimuli for the proliferation of surrounding cancer cells [131]. Among epithelial tumors, some decrease in proliferation may be reflective of a more invasive phenotype.
Effects of GC hormones on cancer cell proliferation have also been reported. Zhao and colleagues observed that cortisol and its metabolite cortisone stimulated the growth of prostate cancer cells in the absence of androgens and increased the secretion of prostate-specific antigen [132]. These cells had a mutated androgen receptor, indicating that cortisol promoted androgen-independent growth of prostate cancer cells. Simon and coworkers examined the effects of several steroid hormones on human mammary carcinoma cells and found that physiological concentrations of GCs enhanced proliferation by nearly twofold [133]. The role of GC hormones in the context of other neuroendocrine hormones remains to be studied with regard to its effects on proliferation.
Adhesion
Tumor cell adhesion to the extracellular matrix within tissues greatly influences the ability of a malignant cell to invade and metastasize to other tissues [134]. The proteins of the extra cellular matrix consist of type I and IV collagens, laminins, heparin sulfate proteoglycan, fibronectin and other noncollagenous glycoproteins [135]. Cell adhesion to these proteins is mediated in part by a group of heterodimeric transmembrane proteins called integrins, which are composed of a noncovalently associated α- and β-subunit that defines the integrin–ligand specificity [136]. The upstream factors that regulate adhesion to matrix components are not completely understood, although cAMP has been demonstrated to regulate the small GTPases, RhoA and Rac in a PKA-dependent manner [137]. cAMP is a common secondary messenger that regulates many cellular processes. Previously, PKA was thought to be the main target of cAMP in eukaryotic cells. However, Epac, a widely expressed exchange factor for the small GTPases Rap1 and Rap2, has also been shown to be a downstream signaling partner for cAMP [138,139]. Importantly, Epac controls a number of cellular processes previously attributed to PKA. cAMP controls cell adhesion in many cell types, and recently a link between cAMP, Epac–Rap1 and regulation of cell adhesion has been established [140]. Enserink and colleagues have demonstrated that isoproterenol promotes ovarian cancer cell spreading and adhesion via laminin-5, independent of PKA, but dependent on Epac1 [141]. Similarly, isoproterenol stimulated adhesion to fibronectin in a cAMP-mediated Epac–Rap1 pathway [140]. Treatment with isoproterenol induced both activation of Rap1 and phosphorylation of CREB. Isoproterenol-induced adhesion was insensitive to pretreatment with a PKA inhibitor. Thus, stress hormones may promote cell–matrix attachment of cancer cells. Such a mechanism would be particularly relevant for ovarian cancer, where cancer cells slough off the primary tumor and then implant at multiple peritoneal sites.
Migration & invasion
Another crucial step in the metastatic cascade is the ability of a tumor cell to separate from the main tumor, invade through the basement membrane and enter the bloodstream. NE and E can affect these processes by increasing MMP production [142,143] by tumor cells, as well as by acting as a chemoattractant to induce cell migration [144-146]. Sood and colleagues have examined the direct effects of NE, E and cortisol on the invasive potential (i.e., the capacity of tumor cells to penetrate the extracellular matrix) of ovarian cancer cells [147]. To measure the in vitro invasive potential of these cells, a membrane invasion culture system was used. Briefly, a polycarbonate membrane (10 μmol/l pores), uniformly coated with a defined basement membrane matrix (human laminin/type IV collagen/gelatin) was used as the intervening barrier to invasion [147]. Invasiveness was calculated as the percentage of cells that had successfully invaded through the matrix-coated membrane to the lower wells relative to the total number of cells seeded into the upper wells. Stress levels of NE increased the in vitro invasiveness of ovarian cancer cells by 89–198% [147]. E also induced significant increases in invasion of ovarian cancer cells ranging from 64 to 76%, but cortisol did not significantly affect the invasive potential of cancer cells. The β-adrenergic antagonist propranolol completely blocked the NE-induced increase in invasiveness. These findings provide direct experimental evidence that stress hormones can enhance the invasive potential of ovarian cancer cells in vitro. Additional in vivo and in vitro studies demonstrated that NE and E significantly increased production of MMP-2 and MMP-9 by ovarian cancer cells through activation of the ADRB pathway (Figure 1) [147]. Other studies have reported similar findings in several other tumor types including colon and head and neck cancers [116,142,145,148].
Clinically, both depression and stress have been related to MMP-9 secretion by tumor associated macrophages (TAMs) in ovarian cancer patients. As TAMs are now known to promote a proinflammatory tumor microenvironment, downregulate cellular immunity and enhance tumor growth and progression [149,150], effects of stress on TAMs have important implications for tumor progression [108].
Cell survival
The endurance of the metastatic process depends, in part, on the ability of tumor cells to survive and avoid apoptosis [100]. With respect to catecholamines, it has been reported that NE and DA trigger apoptosis via a G-protein-mediated signaling pathway in neuroblastoma cells. This catecholamine-induced cell death appears to be specific to neuronal cells, as demonstrated by the inability of catecholamines to trigger apoptosis in A549 lung carcinoma cells and Cos-7 kidney fibroblasts [151]. E can reduce the ability of breast and prostate tumor cell lines to undergo apoptosis by interacting with ADRB2 receptors, followed by PKA-dependent Bcl-2-associated death promoter phosphorylation [152]. Bcl-2-associated death promoter is involved in initiating apoptosis in its unphosphorylated form, but becomes inactive upon phosphorylation, thereby releasing Bcl-2 and Bcl-xl, which inhibit apoptosis.
Studies examining the effects of stress hormones on tumor cell survival have focused on GC hormones. As noted earlier, GCs regulate a wide variety of cellular processes through GCR-mediated activation or repression of target genes. Recent studies have demonstrated that while GC hormones induce apoptosis in lymphocytes [153], these hormones also activate survival genes that protect cancer cells from the effects of chemotherapy [154,155]. These hormones also activate survival genes that protect cancer cells from the effects of chemotherapy [154,155]. Clinically, GCs are frequently administered with cytotoxic agents to reduce the risk of emesis and other potential acute toxicities. However, concerns have been raised about this combination since some studies suggest that GCs may reduce the efficacy of chemotherapy [156]. These hormones are well-established inducers of apoptosis in lymphoblastic leukemia cells, which has been exploited in the therapy of malignant lymphoproliferative disorders. However, in solid tumors, their effects are quite different [157]. Herr and colleagues examined the effects of the GC dexamethasone on human cervical and lung carcinoma cells and found downregulation of proapoptotic elements of the death receptor and mitochondrial apoptosis pathways [154]. Similarly, Wu and associates found that dexamethasone pretreatment of breast cancer cell lines inhibits chemotherapy-induced apoptosis in a GCR-dependent manner and is associated with the transcriptional induction of serum and GC-inducible protein kinase-1 and mitogen-activated protein kinase phosphatase-1 [155]. Specific inhibition of these two proteins reversed the antiapoptotic effects of GC treatment [155]. In addition, it has been demonstrated that dexamethasone reduces sensitivity of hepatocellular and colorectal tumors toward cytotoxic therapy [158]. Dexamethasone therapy has also been demonstrated to promote the growth of cisplatin or 5-fluorouracil-treated breast cancer, cervical cancer, melanoma or neuroblastoma cell lines [159]. In 140 out of 157 analyzed tumors, dexamethasone inhibited 5-fluorouracil-induced apoptosis and promoted cell viability and cell cycle progression of most of the cancer cells examined. All these effects were reversible after suspension of dexamethasone therapy [160,161], but raised concerns regarding the application of steroids in nonhematological malignancies.
Glucocorticoids such as cortisol may also act in a synergistic fashion with catecholamines to facilitate cancer growth. For example, cortisol potentiated the isoproterenol-induced increase in cAMP accumulation, increased ADRB density and markedly increased the effects of IL-1α, IL-1β and TNF-α in lung carcinoma cells [72]. Thus, it is plausible that stressful situations characterized by both elevated catecholamine and cortisol levels (e.g., uncontrollable stress) may have the greatest impact on cancer-related processes.
Anoikis is a cell process by which normal cells enter apoptosis when separated from the extracellular matrix and neighboring cells. Recently, our group has demonstrated that catecholamines can protect ovarian cancer cells from anoikis [162]. These effects are mediated by focal adhesion kinase (FAK) phosphorylation through ADRB2-dependent activation of Src kinase. Parallel results were observed in ovarian carcinoma patients, linking increased levels of stress/depression to increased FAK activation and demonstrating accelerated cancer progression in patients with high levels of FAK activity.
Other stress mediators
Besides NE, E and cortisol, which are considered to be the major stress hormones, other hormones such as DA, prolactin, oxytocin and substance P are affected by stress [163-165].
Dopamine, the third member of the catecholamine family and precursor in the synthesis of NE and E, is one of the major neurotransmitters in the brain and also has important roles in the periphery [166-168]. DA has the opposite effect compared with NE and E with regard to the effects on tumor angiogenesis, growth and development of ascites [169,170]. In vivo and in vitro studies have shown that DA, via its speci fic DR2 receptors, inhibits tumor growth by suppressing the actions of VEGF on both tumor endothelial cells and bone marrow-derived endothelial progenitor cells (Figure 1) [171]. DA inhibits VEGF-induced angiogenesis by suppressing VEGFR-2 phosphorylation [172-174] and inhibits mitogen-activated protein kinase and FAK activation [174]. DA can also inhibit mobilization of endothelial progenitor cells from the bone marrow (Figure 1) [175]. It is known that DA levels are increased in the brain during acute stress [30]. By contrast, under chronic stress, DA levels are lower as a consequence of decreased release of DA [31]). However, it is not known whether DA levels are depleted in the tumor microenvironment in response to chronic stress. Moreno-Smith and colleagues have recently demonstrated that DA treatment can counteract the stimulatory effects of NE on tumor growth in two ovarian stress-cancer mice models [176]. These findings implicate DA as a potential therapeutic strategy for blocking the deleterious effects of chronic stress.
Prolactin plays a functional role in tumor cell growth and promotes survival of breast and other cancer cells [177,178]. A number of epidemiological studies have demonstrated a consistent correlation between prolactin levels and well-confirmed breast cancer risk factors such as parity and age at menarche [177]. Most breast cancer cell lines express the prolactin receptor, and exogenously added prolactin has modest trophic effects on human tumor tissues and cells in vitro [179]. Prolactin has been demonstrated to stimulate proliferation in prostate and endometrial cancer cells as well [178]. In addition to stimulation of proliferation, prolactin may also actively inhibit apoptosis of mammary tumor cells via stimulation of the Akt pathway [180,181]. Furthermore, prolactin can act as a chemoattractant to increase cell motility through activation of the Ras signaling cascade [182]. DA is the primary negative regulator of prolactin secretion [183], and the inter-relationships between these two hormones in the context of chronic stress remain to be determined.
Oxytocin is thought to play an important role in mediating social responses, and a positive correlation has been found between high social support and oxytocin levels [184]. It is also capable of ameliorating symptoms caused by stress, such as anxiety, by exerting anxiolytic effects in certain regions of the brain [185]. Similarly to DA, oxytocin levels increase in acute stress [186] and decrease during chronic stress [165]. Oxytocin inhibits the growth of some epithelial cell tumors (e.g., breast and endometrial tumors) and those of nervous or bone origin, but the hormone has a growth-stimulating effect in trophoblast and other tumors (e.g., small-cell lung tumors, Kaposi’s sarcoma and endothelial tumors) [187,188]. The presence of oxytocin receptors has been described in breast [189,190], ovarian [191] and prostate cancer cells [192].
Subtance P is a peptide in the neurokinin family, is found in both central and peripheral nervous systems and plays a role in stress reactions, anxiety, depression and pain [193,194]. Substance P promotes the migration of colon and breast carcinoma cell lines and is a chemoattractant for squamous cell lung cancer cells [195]. Recently, substance P has been shown to mediate the increase in macrophage cytokine production under stressful circumstances (acute stress) [196].
Stress effects on the immune response
It is known that the CNS, the endocrine system and the immune system interact with each other, and thus changes in any one system may have downstream effects in the other systems. The CNS modulates immunity both through release of GCs via the HPA axis as well as via the release of catecholamines through the autonomic nervous system. Several factors involved in the stress response are involved in activating or blunting the immune response, which may play a role in allowing tumor cells to escape detection and immune cell elimination. In contrast to chronic/long-term stress that suppresses/dysreg ulates immune function, an acute/short-term fight or fight stress can enhance innate immunity. A recent report has described that short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma [197]. Therefore, the physiological fight or fight response and its adjuvant-like immunoenhancing effects may provide a novel mechanism to promote or increase immune system-mediated tumor detection/elimination [197].
T helper (Th) cells are crucial for the development of an immune response by activating antigen-specific effector cells and recruiting cells of the innate immune system, such as macrophages and mast cells. There are two predominant Th-cell subtypes: Th1 and Th2. While Th1 cells directly kill tumor cells via release of cytokines that activate death receptors on the tumor cell surface [198], Th2 cells favor a predominantly humoral response. Th2 immunity may be enhanced by stress hormones (catecholamines and GCs) increasing a shift from predominantly Th1 to Th2 cells. This shift is thought to better enable tumor cells to evade immune surveillance [199,200].
T helper 17 cells play an active role in inflammatory and autoimmune diseases [201]; however, recent studies suggest a potential impact of Th17 on tumors. Kryczek and colleagues have shown that the levels of Th17 cells were significantly increased in peripheral blood, ascite fluid and tumor tissues in human ovarian, renal and pancreatic carcinomas [201]. Similarly, the proportion of Th17 cells in peripheral blood of gastric cancer patients was significantly higher than in healthy donors (6.7 ± 3.7 vs 1.8 ± 1.1%; p < 0.01). Moreover, patients with advanced disease had an even higher percentage of Th17 cells than patients with lower-stage disease or healthy controls [202]. It is known that CD4+, CD25+, FoxP3+ and other additional regulatory T cells (Tregs) are elevated in cancers [203]. Tregs play a crucial role in tumor immune pathogenesis and tumor immune therapeutic efficacy [201]. Recent human cancer trials suggest that depleting Tregs may be clinically beneficial [203].
Glucocorticoids & immune response
Glucocorticoids are essential for the regulation of immune and inflammatory responses. Physiological concentrations of GCs in the range of 350–950 nmol/l, as occur during physical or psychological stress, result in modulation of transcription of genes involved in the inflammatory response, whereas pharmacological doses (higher concentrations than are physio logical [>1 μmol/l]) result in a suppression of the inflammatory response [199]. Similarly, during chronic stress situations, elevated levels of GCs have been shown to be immunosuppressive, leading to an enhanced susceptibility to viral infection, prolonged wound healing or decreased antibody production after vaccination [204,205]. Breast cancer patients with higher mean diurnal cortisol concentrations also showed suppressed immunity against commonly encountered antigens, suggesting blunting of the cellular immune response. Within the immune system, T and B cells, neutrophils, monocytes and macrophages all carry GCRs, allowing for GC regulation of both the cellular and humoral immune responses [206,207]. In addition, GCs can induce apoptosis in monocytes, macrophages and T lymphocytes [208], providing further evidence of their ability to regulate normal immune function.
Catecholamines & immune response
Sympathetic nervous system innervation of primary and secondary lymphoid organs and the presence of ADRBs on cells of the immune system provide the means for noradrenergic signaling of lymphocytes and macrophages from sympathetic nerves [209]. Lymphocytes have been shown to express high-affinity ADRBs, mainly of the ADRB2 subclass [210-213]. ADRAs have not been identified in rodent lymphocytes, although ADRA expression has been characterized in human and guinea pig lymphocytes [214-216]. Activated rodent macrophages express ADRA2 and ADRBs [217,218]. Other hematopoietic cells involved in inflammation, including neutrophils, basophils and eosinophils, also express ADRBs [219,220].
Based on early in vitro studies, a simple functional distinction was made between ADRA - and ADRB-mediated effects on cells of the immune system. ADRB stimulation inhibited such activities as lymphocyte proliferation, antibody secretion and production of proinflammatory factors [221-223], whereas ADRA stimulation had the opposite effects [221]. Subsequently, in vitro studies have shown that the effect of ADR stimulation on lymphocyte activity cannot be categorized as a simple inhibition or enhancement. Rather, several factors must be taken into account, including involved cell type(s), subtype of stimulated ADR, the immune stimulus and the timing of agonist exposure. Thus, if a β-agonist is present during the activation phase of the response, then enhancement may occur, depending on the immune stimulus. If ADRB stimulation occurs late in a response, then inhibition of effector functions, such as antibody secretion or lytic activity, is possible. It is also apparent that catecholamines are most effective when cells are activated by antigens, mitogens or cytokines. This implies a synergistic, regulatory or modulatory role for catecholamines; they do not initiate or completely suppress a response on their own at any single step [209].
The study of SNS–immune system interactions in vivo adds a level of complexity that is not present in vitro. SNS modulation of immune reactivity may occur directly, via interactions of NE with ADRs on cells of the immune system, or indirectly, through interactions of catecholamines with other cell types, such as reticular cells, endothelial cells or smooth muscle cells associated with the vasculature. These interactions may alter a wide variety of functions, such as antigen presentation, lymphocyte proliferation, differentiation, expression of specific receptors, lymphokine production or cell trafficking. For example, peripheral infusion of NE and E in rodents results in the reduction of lymphocyte proliferation and lymphocyte migration [224-226]. Thus, changes in immune reactivity following administration of catecholamines in vivo may reflect changes in lymphocyte redistribution and trafficking, as well as changes in lymphocyte responsiveness to various stimuli [209].
The cellular immune response has been a central focus of much biobehavioral oncology research owing to of its role in immunosurveillance and lysis of tumor cells [227]. Experimental studies with animal models have demonstrated that tumor incidence and progression may be aggravated by chronic stress and surgical stress by suppressing Th1 cytokines and cytotoxic activities of T cells and NK cells, impairing antigen presentation and increasing Tregs [87,88,228,229]. Depression is also known to downregulate the cellular immune response [230-232], largely via adrenergic and GC signaling pathways. Stress has been associated with decrements in a broad range of markers of cellular immunity in breast cancer patients following surgery, including lower T-cell production of Th1 versus Th2 cytokines [233], decreases in T-cell responses to mitogen stimulation and impaired NK-cell cytotoxicity [234,235].
Immune, endocrine and behavioral parameters have been explored during the period of tumor growth that precedes the clinical detection of breast cancer recurrence [236]. Thornton and colleagues reported that breast cancer patients who ultimately recurred exhibited higher counts of white blood cells, neutrophils, lymphocytes and NK cells relative to disease-free patients in the 17 months prior to detection of recurrence. Patients with recurrence also showed higher cortisol and worse physical functioning, fatigue and quality of life [236]. The results from this study indicate that patients with eventual disease recurrence demonstrated reliable biobehavioral alterations more than a year prior to their diagnosis. This finding suggests the possibility of identifying metastatic disease months or even years earlier than is currently possible [236]. Among advanced breast cancer patients, depression has been related to a reduction in cellular immune response to a variety of specific antigens [237]. Distress among ovarian cancer patients at the time of surgery has been associated with poorer NK-cell activity in tumor-infiltrating lymphocytes (TILs) and lower T-cell production of Th1 versus Th2 cytokines in peripheral blood and TILs, whereas social support was related to greater NK-cell activity in both peripheral blood and TILs [238,239]. Taken together, these findings suggest psychosocial modulation of immune factors relevant to cancer detection and control.
Recent animal studies have demonstrated that paracrine and neuroendocrine stress responses may be considered risk factors for cancer recurrence. C57BL/6J mice inoculated in their footpad with syngeneic B16F10.9 melanoma or Lewis lung carcinoma were exposed to perioperative blockade of catecholamines and prostaglandins [240]. The combination of propranolol (β-adrenergic antagonist) and etodolac (COX-2 inhibitor) significantly and markedly improved survival rates in both tumor models. Monotherapy with these agents was not as effective. Surgery markedly reduced NK-cell cytotoxicity and NK-cell expression of Fas ligand and CD11a, reduced all circulating lymphocyte-subtype concentrations and increased corticosterone levels. Propranolol and etodolac administration counteracted these perturbations [240]. This study provides a rationale for clinical testing of a new approach for reducing long-term cancer recurrence in humans.
Dopamine can have a direct or indirect effect on the immune response. T cells express DA receptors [241], suggesting a possible role for DA in immune regulation. Moreover, in vivo studies using a dopaminergic neurotoxin have shown that DA depletion decreases T-cell responses and promotes tumor growth in mice [242].
Parkinson’s disease, which is associated with damaged dopaminergic neurons, results in decreased dopaminergic activity in the CNS; it has also been associated with decreased immune response [243-245]. By contrast, shizophrenia is associated with hyperdopaminergic activity, as well as increased immune function [246,247]. However, these findings remain contradictory owing to the immunosuppressive effects of the neuroleptics used to treat this disorder [248]. Interestingly, the incidence of cancer in patients with schizophrenia has been reported to be lower than in the general population [249-252]. Whether this reduced incidence is related to a hyperactive dopaminergic system is not known.
Conclusion
In modern lifestyle societies, chronic stress has been associated with the pathogenesis of many diseases, including cancer. Chronic stress results in the activation of specific signaling pathways in cancer cells and the tumor microenvironment, leading to tumor growth and progression. Elucidation of these pathways is essential for the development of novel approaches to block the deleterious effects of stress biology on cancer growth and metastasis.
Future perspective
Contemporary lifestyles and the environment of modern societies appear to be particularly inducive of stress-related disorders. With respect to cancer pathogenesis, there is growing and compelling mechanistic evidence for biological and clinical implications of psychosocial and biobehavioral influences [253]. However, despite significant progress in the past decade, further research is needed in order to understand how stress hormones modulate the interplay between tumor and stromal cells in the tumor microenvironment, resulting in regulation of signaling pathways with important implications for cancer progression. These studies may offer new alternatives for treatments based on behavioral and pharmacological approaches. β-blockers have been shown to block many of the deleterious effects of stress. While some clinical studies have demonstrated lower cancer incidence among patients treated with β-blockers [254,255], in others, the cancer risk was neutral [256-258]. Other potential targets in the tumor microenvironment are STAT-3 and VEGF, since these factors are differentially increased in tumors.
Since stress mediators not only affect tumor growth but also many related physiological processes, it will be important to identify additional pathways that could alter the efficacy of chemo- or immuno-modulatory therapy. The role of environmental factors on cancer progression remains poorly defined. Interestingly, a recent report described that an enriched environment in mice was able to decrease tumor growth and also to increase cancer remission. In this mouse model, hypothalamic brain-derived neurotrophic factor was selectively upregulated by the enriched environment, suggesting that genetic or environmental activation of this brain-derived neurotrophic factor–leptin axis may have therapeutic significance for cancer [259].
Similarly, the role of stress-related immuno-suppression in promoting tumor immune escape mechanisms has been poorly studied and represents an exciting new area of investigation [260,261]. Finally, the complex interplay between biobehavioral pathways and socioeconomic and cultural stressors merits further study in diverse populations. As cancer therapy moves towards being more patient specific, it will be crucial to define the behavioral and/or pharmacological interventions that are most likely to benefit individual patients.
Executive summary.
-
■
Collective evidence points to a prominent role for chronic stress in cancer growth and metastasis.
-
■
Sympathetic nervous system and hypothalamic–pituitary–adrenal axis activation, along with related hormones, have functionally and biologically significant impacts on the tumor microenvironment.
-
■
β-adrenergic receptor signaling pathways directly affect cancer cells. Stress hormones (e.g., norepinephrine and epinephrine) stimulate angiogenesis, cell migration and invasion, leading to increased tumor growth and progression.
-
■
Dopamine retards tumor growth by inhibiting angiogenesis.
-
■
Glucocorticoids inhibit chemotherapy-induced cancer cell apoptosis and promote cancer cell survival.
-
■
Integrated pharmacological and biobehavioral interventions are being developed to target neuroendocrine dynamics in the tumor microenvironment and create more successful cancer therapies.
Acknowledgments
Financial & competing interests disclosure
Portions of the work in this article were supported by National Cancer Institute (NCI) grants (CA110793, CA109298, CA140933, CA104825 and U54CA151668), the Ovarian Cancer Research Fund Program Project Development grant, the University of Texas MD Anderson Cancer Center Ovarian Cancer SPORE grant (P50 CA083639), the Gynecologic Cancer Foundation, the Zarrow Foundation, the Marcus Foundation and the Betty Anne Asche Murray Distinguished Professorship. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■ of considerable interest
- 1.Fidler IJ. The role of organ microenvironment in the biology and therapy of cancer metastasis. J. Cell. Biochem. 2007;101:927–936. doi: 10.1002/jcb.21148. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70(9–10):498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol. Psychiatry. 2003;54(3):269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 4.Bukberg J, Penman D, Holland JC. Depression in hospitalized cancer patients. Psychosom. Med. 1984;46:199–212. doi: 10.1097/00006842-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel D. Health caring: psychosocial support for patients with cancer. Cancer. 1994;74(4):1453–1457. doi: 10.1002/1097-0142(19940815)74:4+<1453::aid-cncr2820741609>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Chida Y, Hamer M, Wardle J, et al. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008;5(8):466–475. doi: 10.1038/ncponc1134. ■■ Provides an overview of the clinical studies addressing the impact of stress factors on cancer.
- 7.Duijts SF, Zeegers MP, Borne BV. The association between stressful life events and breast cancer risk: a meta-analysis. Int. J. Cancer. 2003;107(6):1023–1029. doi: 10.1002/ijc.11504. [DOI] [PubMed] [Google Scholar]
- 8.Lillberg K, Verkasalo P, Kaprio J, et al. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am. J. Epidemiol. 2003;157(5):415–423. doi: 10.1093/aje/kwg002. [DOI] [PubMed] [Google Scholar]
- 9.Geyer S. Life events prior to manifestation of breast cancer: a limited prospective study covering eight years before diagnosis. J. Psychosom. Res. 1991;35(2–3):355–363. doi: 10.1016/0022-3999(91)90090-b. [DOI] [PubMed] [Google Scholar]
- 10.Michael YL, Carlson NE, Chlebowski RT, et al. Influence of stressors on breast cancer incidence in the Women’s Health Initiative. Health Physiol. 2009;28:137–146. doi: 10.1037/a0012982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steel JL, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. J. Clin. Oncol. 2007;25:2397–2405. doi: 10.1200/JCO.2006.06.4592. GD. [DOI] [PubMed] [Google Scholar]
- 12.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;22:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 13.Everson SA, Goldberg DE, Kaplan GA, et al. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosom. Med. 1996;58(2):113–121. doi: 10.1097/00006842-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94(10):2719–2727. doi: 10.1002/cncr.10533. [DOI] [PubMed] [Google Scholar]
- 15.Watson M, Haviland JS, Greer S, et al. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet. 1999;354(9187):1331–1336. doi: 10.1016/s0140-6736(98)11392-2. [DOI] [PubMed] [Google Scholar]
- 16.Buccheri G. Depressive reactions to lung cancer are common and often followed by a poor outcome. Eur. Respir. J. 1998;11(1):173–178. doi: 10.1183/09031936.98.11010173. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Willis TA. Stress, social support, and the buffering hypothesis. Psychol. Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 18.Funch DP, Marshall J. The role of stress, social support and age in survival from breast cancer. J. Psychosom. Res. 1983;27:77–83. doi: 10.1016/0022-3999(83)90112-5. [DOI] [PubMed] [Google Scholar]
- 19.Marshall JR, Funch DP. Social environment and breast cancer. A cohort analysis of patient survival. Cancer. 1983;52(8):1546–1550. doi: 10.1002/1097-0142(19831015)52:8<1546::aid-cncr2820520835>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Maunsell E, Brisson J, Deschenes L. Social support and survival among women with breast cancer. Cancer. 1995;76(4):631–637. doi: 10.1002/1097-0142(19950815)76:4<631::aid-cncr2820760414>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Giraldi T, Rodani MG, Cartei G, et al. Psychosocial factors and breast cancer: a 6-year Italian follow-up study. Psychother. Psychosom. 1997;66(5):229–236. doi: 10.1159/000289140. [DOI] [PubMed] [Google Scholar]
- 22.Butow PN, Hiller JE, Price MA, et al. Epidemiological evidence for a relationship between life events, coping style, and personality factors in the development of breast cancer. J. Psychosom. Res. 2000;49(3):169–181. doi: 10.1016/s0022-3999(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke CH, Kubzansky LD, Schernhammer ES, et al. Social networks, social support, and survival after breast cancer diagnosis. J. Clin. Oncol. 2006;24(7):1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 24.Sapolsky RM. Why Zebras Don’t Get Ulcers: A Guide to Stress, Stress-Related Diseases, and Coping. WH Freeman and Co.; NY, USA: 1998. [Google Scholar]
- 25.Chrousos G. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 26.McEwen B. Stress and health: relevance to persian gulf veterans?; Presented at: International Society for Traumatic Stress Studies Annual Meeting 1998; Washington, DC, USA. 21–23 November 1998. [Google Scholar]
- 27.Schmidt C, Kraft K. β-endorphin and catecholamine concentrations during chronic and acute stress in intensive care patients. Eur. J. Med. Res. 1996;1(11):528–532. [PubMed] [Google Scholar]
- 28.Rupp H, Dhalla KS, Dhalla NS. Mechanisms of cardiac cell damage due to catecholamines: significance of drugs regulating central sympathetic outflow. J. Cardiovasc. Pharmacol. 1994;24(Suppl. 1):S16–S24. doi: 10.1097/00005344-199424001-00004. [DOI] [PubMed] [Google Scholar]
- 29.Rupp H, Jacob R. Excess catecholamines and the metabolic syndrome: should central imidazoline receptors be a therapeutic target? Med. Hypotheses. 1995;44(3):217–225. doi: 10.1016/0306-9877(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 30.Puglisi-Allegra S, Imperato A, Angelucci L, et al. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res. 1991;554(1–2):217–222. doi: 10.1016/0006-8993(91)90192-x. [DOI] [PubMed] [Google Scholar]
- 31.Imperato A, Angelucci L, Casolini P, et al. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577(2):194–199. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- 32.Seeman TE, Berkman LF, Blazer D, et al. Social ties and support and neuroendocrine function: the McArthur studies of successful aging. Ann. Behav. Med. 1994;16(95–106) [Google Scholar]
- 33.Seeman TE, McEwen BS. Impact of social environment characteristics on neuroendocrine regulation. Psychosom. Med. 1996;58(5):459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Seeman TE, Rowe JW, Horwitz RI, McEwen B. Price of adaptation-allostatic load and its health consequences. Arch. Intern. Med. 1997;157:2259–2268. SB. [PubMed] [Google Scholar]
- 35.Kiecolt-Glaser JK, Glaser R, Malarkey WB. Love, marriage, and divorce: newlyweds’ stress hormones foreshadow relationship changes. J. Consult. Clin. Psychol. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. BC. [DOI] [PubMed] [Google Scholar]
- 36.Tyrka AR, Wier L, Price LH, et al. Childhood parental loss and adult hypothalamic–pituitary–adrenal function. Biol. Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bevans K, Cerbone A, Overstreet S. Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Develop. Psychopathol. 2008;20:257–272. doi: 10.1017/S0954579408000126. [DOI] [PubMed] [Google Scholar]
- 38.Hughes J, Watkins L, Blumenthal JA, et al. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J. Psychosom. Res. 2004;57:353–358. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Grossman F, Potter WZ. Catecholamines in depression: a cumulative study of urinary norepinephrine and its major metabolites in unipolar and bipolar depressed patients versus healthy volunteers at the NIMH. Psychiatry Res. 1999;87:21–27. doi: 10.1016/s0165-1781(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 40.Lake C, Pickar D, Ziegler MG, et al. High plasma norepinephrine levels in patients with major affective disorder. Am. J. Psychiatry. 1982;139:1315–1318. doi: 10.1176/ajp.139.10.1315. [DOI] [PubMed] [Google Scholar]
- 41.McEwen B. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 42.Ebner K, Saria A, Singewald N. Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc. Natl Acad. Sci. USA. 2004;101:4280–4285. doi: 10.1073/pnas.0400794101. RN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakshmanan J. Nerve growth factor levels in mouse serum: variations due to stress. Neurochem. Res. 1987;12:393–397. doi: 10.1007/BF00993250. [DOI] [PubMed] [Google Scholar]
- 44.Lara HE, Porcile A, Espinoza J, et al. Release of norepinephrine from human ovary: coupling to steroidogenic response. Endocrine. 2001;15(2):187–192. doi: 10.1385/ENDO:15:2:187. [DOI] [PubMed] [Google Scholar]
- 45.Greenwald G, Roy S. Follicular development and its control. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press; NY, USA: 1994. pp. 629–724. [Google Scholar]
- 46.Nankova B, Kvetnansky R, Hiremagalur B, et al. Immobilization stress elevates gene expression for catecholamine biosynthetic enzymes and some neuropeptides in rat sympathetic ganglia: effects of adrenocorticotropin and glucocorticoids. Endocrinology. 1996;137(12):5597–5604. doi: 10.1210/endo.137.12.8940389. [DOI] [PubMed] [Google Scholar]
- 47.Paredes A, Galvez A, Leyton V, et al. Stress promotes development of ovarian cysts in rats: the possible role of sympathetic nerve activation. Endocrine. 1998;8(3):309–315. doi: 10.1385/ENDO:8:3:309. [DOI] [PubMed] [Google Scholar]
- 48.Lara HE, Dorfman M, Venegas M, et al. Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: studies on norepinephrine release. Microsc. Res. Tech. 2002;59(6):495–502. doi: 10.1002/jemt.10229. [DOI] [PubMed] [Google Scholar]
- 49.Maestroni GJ. Neurohormones and catecholamines as functional components of the bone marrow microenvironment. Ann. NY Acad. Sci. 2000;917:29–37. doi: 10.1111/j.1749-6632.2000.tb05370.x. [DOI] [PubMed] [Google Scholar]
- 50.Kobilka B. Adrenergic receptors as models for G protein-coupled receptor. Annu. Rev. Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- 51.Pullar CE, Isseroff RR. The β-2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J. Cell Sci. 2006;119(Pt 3):592–602. doi: 10.1242/jcs.02772. [DOI] [PubMed] [Google Scholar]
- 52.Lai LP, Mitchell J. β2-adrenergic receptors expressed on murine chondrocytes stimulate cellular growth and inhibit the expression of Indian hedgehog and collagen type X. J. Cell Biochem. 2008;104:545–553. doi: 10.1002/jcb.21646. [DOI] [PubMed] [Google Scholar]
- 53.Bylund D, Blaxall HS, Iversen LJ, Caron MG, Lefkowitz RJ, Lomasney JW. Pharmacological characteristics of α 2-adrenergic receptors: comparison of pharmacologically defined subtypes with subtypes identified by molecular cloning. Mol. Pharmacol. 1992;42:1–5. [PubMed] [Google Scholar]
- 54.Fernando M, Heaney AP. α1-adrenergic receptor antagonists: novel therapy for pituitary adenomas. Mol. Endocrinol. 2005;19(12):3085–3096. doi: 10.1210/me.2004-0471. [DOI] [PubMed] [Google Scholar]
- 55.Badino GR, Novelli A, Girardi C, et al. Evidence for functional β-adrenoceptor subtypes in CG-5 breast cancer cell. Pharmacol. Res. 1996;33(4–5):255–260. doi: 10.1006/phrs.1996.0036. [DOI] [PubMed] [Google Scholar]
- 56.Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin. Cancer Res. 2003;9(12):4514–4521. [PubMed] [Google Scholar]
- 57.McDonald PH, Lefkowitz RJ. β-arrestins: new roles in regulating heptahelical receptors functions. Cell Signal. 2001;13(10):683–689. doi: 10.1016/s0898-6568(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 58.Dixon RA, Kobilka BK, Strader DJ, et al. Cloning of the gene and cDNA for mammalian β-adrenergic receptor and homology with rhodopsin. Nature. 1986;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- 59.Emorine LJ, Marullo S, Briend-Sutren MM, et al. Molecular characterization of the human β-3-adrenergic receptor. Science. 1989;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- 60.Frielle T, Collins S, Daniel KW, et al. Cloning of the cDNA for the human β 1-adrenergic receptor. Proc. Natl Acad. Sci. USA. 1987;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006;12(8):939–944. doi: 10.1038/nm1447. ■■ First study to demonstrate the impact of stress hormones on cancer angiogenesis.
- 62.Rhen T, Cidlowski J. Anti-inflammatory action of glucocorticoids – new mechanisms for old drugs. N. Engl. J. Med. 2005;353(16):1711. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 63.Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55(7):603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pazirandeh A, Xue Y, Prestegaard T, et al. Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. FASEB J. 2002;16(7):727–729. doi: 10.1096/fj.01-0891fje. [DOI] [PubMed] [Google Scholar]
- 65.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 66.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 67.Antoni MH, Cruess S, Cruess DG, et al. Cognitive–behavioral stress management reduces distress and 24-hour urinary free cortisol output among symptomatic HIV-infected gay men. Ann. Behav. Med. 2000;22(1):29–37. doi: 10.1007/BF02895165. [DOI] [PubMed] [Google Scholar]
- 68.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine–immune pathway from stress to disease? Brain Behav. Immun. 2003;17(5):321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 69.Sephton SE, Sapolsky RM, Kraemer HC, et al. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl Cancer Inst. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. ■ Important clinical study addressing the role of cortisol in breast cancer patients.
- 70.Schernhammer ES, Laden F, Speizer FE, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J. Natl Cancer Inst. 2003;95(11):825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 71.Kawamura A, Tamaki N, Kokunai T. Effect of dexamethasone on cell proliferation of neuroepithelial tumor cell lines. Neurol. Med. Chir. (Tokyo) 1998;38(10):633–638. doi: 10.2176/nmc.38.633. discussion 638–640. [DOI] [PubMed] [Google Scholar]
- 72.Nakane T, Szentendrei T, Stern L, et al. Effects of IL-1 and cortisol on β-adrenergic receptors, cell proliferation, and differentiation in cultured human A549 lung tumor cells. J. Immunol. 1990;145(1):260–266. [PubMed] [Google Scholar]
- 73.Sheridan JF, Feng NG, Bonneau RH, et al. Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J. Neuroimmunol. 1991;31(3):245–255. doi: 10.1016/0165-5728(91)90046-a. [DOI] [PubMed] [Google Scholar]
- 74.Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav. Immun. 1998;12(1):64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- 75.Padgett DA, Sheridan JF, Dorne J, et al. Social stress and the reactivation of latent herpes simplex virus type 1. Proc. Natl Acad. Sci. USA. 1998;95(12):7231–7235. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iwakabe K, Shimada M, Ohta A, et al. The restraint stress drives a shift in Th1/Th2 balance toward Th2-dominant immunity in mice. Immunol. Lett. 1998;62(1):39–43. doi: 10.1016/s0165-2478(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 77.Fiserova A, Starec M, Kuldova M, et al. Effects of D2-dopamine and α-adrenoceptor antagonists in stress induced changes on immune responsiveness of mice. J. Neuroimmunol. 2002;130(1–2):55–65. doi: 10.1016/s0165-5728(02)00211-4. [DOI] [PubMed] [Google Scholar]
- 78.Nukina H, Sudo N, Aiba Y, et al. Restraint stress elevates the plasma interleukin-6 levels in germ-free mice. J. Neuroimmunol. 2001;115(1–2):46–52. doi: 10.1016/s0165-5728(01)00260-0. [DOI] [PubMed] [Google Scholar]
- 79.Zhou D, Kusnecov AW, Shurin MR, et al. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic–pituitary–adrenal axis. Endocrinology. 1993;133(6):2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- 80.Zorzet S, Perissin L, Rapozzi V, et al. Restraint stress reduces the antitumor efficacy of cyclophosphamide in tumor-bearing mice. Brain Behav. Immun. 1998;12(1):23–33. doi: 10.1006/brbi.1997.0504. [DOI] [PubMed] [Google Scholar]
- 81.Steplewski Z, Vogel WH, Ehya H, et al. Effects of restraint stress on inoculated tumor growth and immune response in rats. Cancer Res. 1985;45(10):5128–5133. [PubMed] [Google Scholar]
- 82.Cao L, Filipov NM, Lawrence DA. Sympathetic nervous system plays a major role in acute cold/restraint stress inhibition of host resistance to Listeria monocytogenes. J. Neuroimmunol. 2002;125(1–2):94–102. doi: 10.1016/s0165-5728(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 83.Steplewski Z, Goldman PR, Vogel WH. Effect of housing stress on the formation and development of tumors in rats. Cancer Lett. 1987;34(3):257–261. doi: 10.1016/0304-3835(87)90175-3. [DOI] [PubMed] [Google Scholar]
- 84.Tjurmina OA, Armando I, Saavedra JM, et al. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143(12):4520–4526. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- 85.Kvetnansky R, Fukuhara K, Pacak K, et al. Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology. 1993;133(3):1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- 86.Ghoshal K, Wang Y, Sheridan JF, Jacob ST. Metallothionein induction in response to restraint stress. Transcriptional control, adaptation to stress, and role of glucocorticoid. J. Biol. Chem. 1998;273:27904–27910. doi: 10.1074/jbc.273.43.27904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ben-Eliyahu S, Yirmiya R, Liebeskind JC, Taylor AN, Gale RP. Stress increases metastatic spread of a mammary tumor in rats: evidence for mediation by the immune system. Brain Behav. Immun. 1991;5(2):193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- 88.Ben-Eliyahu S, Page GG, Yirmiya R, et al. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer. 1999;80(6):880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 89.Page GG, Ben-Eliyahu S. A role for NK-cells in greater susceptibility of young rats to metastatic formation. Develop. Comp. Immunol. 1999;23(1):87–96. doi: 10.1016/s0145-305x(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 90.Page GG, Ben-Eliyahu S, Yirmiya R, et al. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54(1):21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 91.Ben-Eliyahu S, Shakhar G, Rosenne E, et al. Hypothermia in barbiturate-anesthetized rats suppresses natural killer cell activity and compromises resistance to tumor metastasis: a role for adrenergic mechanisms. Anesthesiology. 1999;91(3):732–740. doi: 10.1097/00000542-199909000-00026. [DOI] [PubMed] [Google Scholar]
- 92.Hermes GL, Delgado B, Tretiakova M, et al. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc. Natl Acad. Sci. USA. 2009;106(52):22393–22398. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hermes GL, Rosenthal L, Montag A, McClintock MK. Social isolation and the inflammatory response: sex differences in the enduring effects of a prior stressor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290(2):R273–R282. doi: 10.1152/ajpregu.00368.2005. [DOI] [PubMed] [Google Scholar]
- 94.Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. Contemp. Top. Lab. Anim. Sci. 2003;42:9–18. [PubMed] [Google Scholar]
- 95.Fisher ER, Fisher B. Recent observations on concepts of metastasis. Arch. Pathol. 1967;83(4):321–324. [PubMed] [Google Scholar]
- 96.Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? – G.H.A. Clowes Memorial Award Lecture. Cancer Res. 1986;46(2):467–473. [PubMed] [Google Scholar]
- 97.Liotta LA. Tumor invasion and metastases-role of the extracellular matrix: Rhoads Memorial Award Lecture. Cancer Res. 1986;46(1):1–7. [PubMed] [Google Scholar]
- 98.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. ■ Important review of the metastatic process.
- 99.Folkman J. Toward an understanding of angiogenesis: search and discovery. Perspect. Biol. Med. 1985;29(1):10–36. doi: 10.1353/pbm.1985.0049. [DOI] [PubMed] [Google Scholar]
- 100.Langley RR, Fidler IJ. Tumor cell–organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr. Rev. 2007;28(3):297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 101.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 102.Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat. Clin. Pract. Oncol. 2008;5(4):194–204. doi: 10.1038/ncponc1051. [DOI] [PubMed] [Google Scholar]
- 103.Ferrara N. Vascular endothelial growth factor. Eur. J. Cancer. 1996;32A(14):2413–2422. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- 104.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 105.Fredriksson JM, Lindquist JM, Bronnikov GE, et al. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a β-adrenoreceptor/cAMP/protein kinase-A pathway involving Src but independently of ERK1/2. J. Biol. Chem. 2000;275(18):13802–13811. doi: 10.1074/jbc.275.18.13802. [DOI] [PubMed] [Google Scholar]
- 106.Yang E, Donovan EL, Benson DM, Glaser R. VEGF is differentially regulated in multiple myeloma-derived cell lines by norepinephrine. Brain Behav. Immun. 2008;22:318–322. doi: 10.1016/j.bbi.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95(4):808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- 108.Lutgendorf SK, Lamkin DM, Jennings NB, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin. Cancer Res. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma A, Greenman J, Sharp DM, Walker LG, Monson JR. Vascular endothelial factor and psychosocial factors in colorectal cancer. Psychooncology. 2008;17(1):66–73. doi: 10.1002/pon.1191. [DOI] [PubMed] [Google Scholar]
- 110.Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 111.Van Snick J. Interleukin-6: an overview. Annu. Rev. Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 112.Obata NH, Tamakoshi K, Shibata K, et al. Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Res. 1997;17(1A):337–342. [PubMed] [Google Scholar]
- 113.Wu S, Rodabaugh K, Martinez-Maza O, et al. Stimulation of ovarian tumor cell proliferation with monocyte products including interleukin-1, interleukin-6, and tumor necrosis factor-α. Am. J. Obstet. Gynecol. 1992;166(3):997–1007. doi: 10.1016/0002-9378(92)91379-o. [DOI] [PubMed] [Google Scholar]
- 114.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65(23):10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J. Biol. Chem. 2007;282(41):29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 116.Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66(21):10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 117.Landen CN, Lin YG, Armaiz Pena GN, et al. Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res. 2007;67(21):10389–10396. doi: 10.1158/0008-5472.CAN-07-0858. [DOI] [PubMed] [Google Scholar]
- 118.Machein MR, Kullmer J, Ronicke V, et al. Differential downregulation of vascular endothelial growth factor by dexamethasone in normoxic and hypoxic rat glioma cells. Neuropathol. Appl. Neurobiol. 1999;25(2):104–112. doi: 10.1046/j.1365-2990.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 119.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth GHA Clowes Memorial Award Lecture. Cancer Res. 1990;50(19):6130–6138. [PubMed] [Google Scholar]
- 120.Flaxman BA, Harper RA. In vitro analysis of the control of keratinocyte proliferation in human epidermis by physiologic and pharmacologic agents. J. Invest. Dermatol. 1975;65(1):52–59. doi: 10.1111/1523-1747.ep12598043. [DOI] [PubMed] [Google Scholar]
- 121.Vandewalle B, Revillion F, Lefebvre J. Functional β-adrenergic receptors in breast cancer cells. J. Cancer Res. Clin. Oncol. 1990;116(3):303–306. doi: 10.1007/BF01612908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marchetti B, Spinola PG, Pelletier G, et al. A potential role for catecholamines in the development and progression of carcinogen- induced mammary tumors: hormonal control of β-adrenergic receptors and correlation with tumor growth. J. Steroid Biochem. Mol. Biol. 1991;38(3):307–320. doi: 10.1016/0960-0760(91)90102-b. [DOI] [PubMed] [Google Scholar]
- 123.Abramovitch R, Tavor E, Jacob-Hirsch J, et al. A pivotal role of cyclic AMP-responsive element binding protein in tumor progression. Cancer Res. 2004;64(4):1338–1346. doi: 10.1158/0008-5472.can-03-2089. [DOI] [PubMed] [Google Scholar]
- 124.Lang K, Drell TL, 4th, Lindecke A, et al. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int. J. Cancer. 2004;112(2):231–238. doi: 10.1002/ijc.20410. [DOI] [PubMed] [Google Scholar]
- 125.Jean D, Bar-Eli M. Regulation of tumor growth and metastasis of human melanoma by the CREB transcription factor family. Mol. Cell. Biochem. 2000;212(1–2):19–28. [PubMed] [Google Scholar]
- 126.Scarparo AC, Sumida D, Patrao MT, et al. Catecholamine effects on human melanoma cells evoked by α1-adrenoceptors. Arch. Dermatol. Res. 2004;296(3):112–119. doi: 10.1007/s00403-004-0488-x. [DOI] [PubMed] [Google Scholar]
- 127.Pifl C, Zezula J, Spittler A, et al. Antiproliferative action of dopamine and norepinephrine in neuroblastoma cells expressing the human dopamine transporter. FASEB J. 2001;15(9):1607–1609. doi: 10.1096/fj.00-0738fje. [DOI] [PubMed] [Google Scholar]
- 128.Cox ME, Deeble PD, Lakhani S, et al. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59(15):3821–3830. [PubMed] [Google Scholar]
- 129.Cohen RJ, Glezerson G, Haffejee Z. Neuroendocrine cells – a new prognostic parameter in prostate cancer. Br. J. Urol. 1991;68(3):258–262. doi: 10.1111/j.1464-410x.1991.tb15318.x. [DOI] [PubMed] [Google Scholar]
- 130.Theodorescu D, Broder SR, Boyd JC, et al. Cathepsin D and chromogranin A as predictors of long term disease specific survival after radical prostatectomy for localized carcinoma of the prostate. Cancer. 1997;80(11):2109–2119. [PubMed] [Google Scholar]
- 131.di Sant’Agnese PA. Neuroendocrine differentiation in human prostatic carcinoma. Hum. Pathol. 1992;23(3):287–296. doi: 10.1016/0046-8177(92)90110-o. [DOI] [PubMed] [Google Scholar]
- 132.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat. Med. 2000;6(6):703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 133.Simon WE, Albrecht M, Trams G, et al. In vitro growth promotion of human mammary carcinoma cells by steroid hormones, tamoxifen, and prolactin. J. Natl Cancer Inst. 1984;73(2):313–321. doi: 10.1093/jnci/73.2.313. [DOI] [PubMed] [Google Scholar]
- 134.Boudreau N, Bissell MJ. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr. Opin. Cell. Biol. 1998;10(5):640–646. doi: 10.1016/s0955-0674(98)80040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hay ED, editor. Biology of the Extracellular Matrix. 2nd edition Plenum Press; NY, USA: 1991. [Google Scholar]
- 136.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 137.Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion – lessons from the α6β-4 integrin. Semin. Cancer Biol. 2001;11(2):129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 138.Kawasaki H, Springett GM, Mochizuki N, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282(5397):2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 139.de Rooij J, Zwartkruis FJ, Verheijen MH, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396(6710):474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 140.Rangarajan S, Enserink JM, Kuiperij HB, et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the β2-adrenergic receptor. J. Cell Biol. 2003;160(4):487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Enserink JM, Price LS, Methi T, et al. The cAMP–Epac–Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the α3β1 integrin but not the α6β4 integrin. J. Biol. Chem. 2004;279(43):44889–44896. doi: 10.1074/jbc.M404599200. [DOI] [PubMed] [Google Scholar]
- 142.Yang EV, Bane CM, MacCallum RC, et al. Stress-related modulation of matrix metalloproteinase expression. J. Neuroimmunol. 2002;133(1–2):144–150. doi: 10.1016/s0165-5728(02)00270-9. [DOI] [PubMed] [Google Scholar]
- 143.Wu W, Yamaura T, Murakami K, et al. Involvement of TNF-α in enhancement of invasion and metastasis of colon 26-L5 carcinoma cells in mice by social isolation stress. Oncol. Res. 1999;11(10):461–469. [PubMed] [Google Scholar]
- 144.Entschladen F, Lang K, Drell TL, et al. Neurotransmitters are regulators for the migration of tumor cells and leukocytes. Cancer Immunol. Immunother. 2002;51(9):467–482. doi: 10.1007/s00262-002-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Masur K, Niggemann B, Zanker KS, et al. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 2001;61(7):2866–2869. [PubMed] [Google Scholar]
- 146.Joseph J, Niggemann B, Zaenker KS, et al. The neurotransmitter γ-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 2002;62(22):6467–6469. [PubMed] [Google Scholar]
- 147.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 2006;12(2):369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Drell T, Joseph J, Lang K, et al. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res. Treat. 2003;80(1):63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 149.Pollard J. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 150.Coussens LM, Werb Z. Inflammation and cancer. Nat. Rev. Cancer. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chan AS, Ng LW, Poon LS, Chan WW, Wong YH. Dopaminergic and adrenergic toxicities on SK-N-MC human neuroblastoma cells are mediated through G protein signaling and oxidative stress. Apoptosis. 2007;12:167–179. doi: 10.1007/s10495-006-0524-8. [DOI] [PubMed] [Google Scholar]
- 152.Sastry K. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J. Biol. Chem. 2007;282:14094–14100. doi: 10.1074/jbc.M611370200. [DOI] [PubMed] [Google Scholar]
- 153.Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ. 2002;9(1):6–19. doi: 10.1038/sj.cdd.4400969. [DOI] [PubMed] [Google Scholar]
- 154.Herr I, Ucur E, Herzer K, et al. Glucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res. 2003;63(12):3112–3120. [PubMed] [Google Scholar]
- 155.Wu W, Chaudhuri S, Brickley DR, et al. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64(5):1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 156.Zhang C, Kolb A, Buchler P, et al. Corticosteroid co-treatment induces resistance to chemotherapy in surgical resections, xenografts and established cell lines of pancreatic cancer. BMC Cancer. 2006;6:61. doi: 10.1186/1471-2407-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bekasi S, Zalatnai A. Overexpression of glucocorticoid receptor in human pancreatic cancer and in xenografts. An immunohistochemical study. Pathol. Oncol. Res. 2009;15(4):561–566. doi: 10.1007/s12253-009-9154-0. [DOI] [PubMed] [Google Scholar]
- 158.Zhang C, Kolb A, Mattern J, et al. Dexamethasone desensitizes hepatocellular and colorectal tumours toward cytotoxic therapy. Cancer Lett. 2006;242(1):104–111. doi: 10.1016/j.canlet.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 159.Zhang C, Beckermann B, Kallifatidis G, et al. Corticosteroids induce chemotherapy resistance in the majority of tumour cells from bone, brain, breast, cervix, melanoma and neuroblastoma. Int. J. Oncol. 2006;29(5):1295–1301. [PubMed] [Google Scholar]
- 160.Zhang C, Marme A, Wenger T, et al. Glucocorticoid-mediated inhibition of chemotherapy in ovarian carcinomas. Int. J. Oncol. 2006;28(2):551–558. [PubMed] [Google Scholar]
- 161.Zhang C, Wenger T, Mattern J, et al. Clinical and mechanistic aspects of glucocorticoid-induced chemotherapy resistance in the majority of solid tumors. Cancer Biol. Ther. 2007;6(2):278–287. doi: 10.4161/cbt.6.2.3652. [DOI] [PubMed] [Google Scholar]
- 162.Sood AK, Armaiz-Pena GN, Halder J, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J. Clin. Invest. 2010;120(5):1515–1523. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dave JR, Anderson SM, Saviolakis GA, et al. Chronic sustained stress increases levels of anterior pituitary prolactin mRNA. Pharmacol. Biochem. Behav. 2000;67(3):423–431. doi: 10.1016/s0091-3057(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 164.Almeida SA, Petenusci SO, Franci JA, et al. Chronic immobilization-induced stress increases plasma testosterone and delays testicular maturation in pubertal rats. Andrologia. 2000;32(1):7–11. [PubMed] [Google Scholar]
- 165.Young WS, 3rd, Lightman SL. Chronic stress elevates enkephalin expression in the rat paraventricular and supraoptic nuclei. Brain Res. Mol. Brain Res. 1992;13(1–2):111–117. doi: 10.1016/0169-328x(92)90050-l. [DOI] [PubMed] [Google Scholar]
- 166.Glavin GB, Szabo S. Dopamine in gastrointestinal disease. Dig. Dis. Sci. 1990;35(9):1153–1161. doi: 10.1007/BF01537589. [DOI] [PubMed] [Google Scholar]
- 167.Mezey E, Eisenhofer G, Hansson S, et al. Dopamine produced by the stomach may act as a paracrine/autocrine hormone in the rat. Neuroendocrinology. 1998;67(5):336–348. doi: 10.1159/000054332. [DOI] [PubMed] [Google Scholar]
- 168.Thaker PH, Sood AK. Neuroendocrine influences on cancer biology. Semin. Cancer Biol. 2007;18:164–170. doi: 10.1016/j.semcancer.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Basu S, Nagy JA, Pal S, et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 2001;7(5):569–574. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- 170.Teunis MA, Kavelaars A, Voest E, et al. Reduced tumor growth, experimental metastasis formation, and angiogenesis in rats with a hyperreactive dopaminergic system. FASEB J. 2002;16(11):1465–1467. doi: 10.1096/fj.02-0145fje. [DOI] [PubMed] [Google Scholar]
- 171.Chakroborty D, Sarkar C, Basu B, et al. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009;69(9):3727–3730. doi: 10.1158/0008-5472.CAN-08-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Basu S, Sarkar C, Chakroborty D, et al. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 2004;64:5551–5555. doi: 10.1158/0008-5472.CAN-04-1600. [DOI] [PubMed] [Google Scholar]
- 173.Chakroborty D, Sarkar C, Mitra RB, et al. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin. Cancer Res. 2004;10(13):4349–4356. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- 174.Sarkar C, Chakroborty D, Chowdhury UR, et al. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin. Cancer Res. 2008;14(8):2502–2510. doi: 10.1158/1078-0432.CCR-07-1778. [DOI] [PubMed] [Google Scholar]
- 175.Chakroborty D, Chowdhury UR, Sarkar C, et al. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J. Clin. Invest. 2008;118(4):1380–1389. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moreno-Smith M, Armaiz-Pena GN, Allen JK, et al. Dopamine blocks stress-mediated tumor growth in ovarian carcinoma; Presented at: 100th Annual Meeting of American Association for Cancer Resesarch; Denver, CL, USA. 18–22 April 2009. [Google Scholar]
- 177.Clevenger CV, Furth PA, Hankinson SE, et al. The role of prolactin in mammary carcinoma. Endocr. Rev. 2003;24(1):1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ben-Jonathan N, Liby K, McFarland M, et al. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol. Metab. 2002;13(6):245–250. doi: 10.1016/s1043-2760(02)00603-3. [DOI] [PubMed] [Google Scholar]
- 179.Vonderhaar BK. Prolactin in human breast cancer development. In: Stephen PE, editor. Endocrine Oncology. Humana Press; NJ, USA: 2000. pp. 101–120. [Google Scholar]
- 180.Chen WY, Ramamoorthy P, Chen N, et al. A human prolactin antagonist, HPRL-G129R, inhibits breast cancer cell proliferation through induction of apoptosis. Clin. Cancer Res. 1999;5(11):3583–3593. [PubMed] [Google Scholar]
- 181.Richert MM, Decker K, Anderson SM. Mechanisms underlying consitutive activation of Akt in breast cancer cell lines; Presented at: 83rd Annual Meeting of the Endocrine Society; Denver, CO, USA. 20–23 June 2001. [Google Scholar]
- 182.Maus MV, Reilly SC, Clevenger CV. Prolactin as a chemoattractant for human breast carcinoma. Endocrinology. 1999;140(11):5447–5450. doi: 10.1210/endo.140.11.7245. [DOI] [PubMed] [Google Scholar]
- 183.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin inhibitor. Endocr. Rev. 2001;22(6):724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 184.Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom. Med. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- 185.McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav. 1996;60(5):1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 186.Jezova D, Skultetyova I, Vedhara K, et al. Vasopressin and oxytocin in stress. Ann. NY Acad. Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- 187.Pequeux C, Keegan BP, Hagelstein MT, et al. Oxytocin- and vasopressin-induced growth of human small-cell lung cancer is mediated by the mitogen-activated protein kinase pathway. Endocr. Relat. Cancer. 2004;11(4):871–885. doi: 10.1677/erc.1.00803. [DOI] [PubMed] [Google Scholar]
- 188.Cassoni P, Marrocco T, Deaglio S, et al. Biological relevance of oxytocin and oxytocin receptors in cancer cells and primary tumors. Ann. Oncol. 2001;12(Suppl. 2):S37–S39. doi: 10.1093/annonc/12.suppl_2.s37. [DOI] [PubMed] [Google Scholar]
- 189.Taylor AH, Ang VT, Jenkins JS, et al. Interaction of vasopressin and oxytocin with human breast carcinoma cells. Cancer Res. 1990;50(24):7882–7886. [PubMed] [Google Scholar]
- 190.Bussolati GC. The oxytocin/oxytocin receptor system – expect the unexpected. Endocrinology. 2001;142(4):1377–1379. doi: 10.1210/endo.142.4.8147. [DOI] [PubMed] [Google Scholar]
- 191.Reversi A, Cassoni P, Chini B. Oxytocin receptor signaling in myoepithelial and cancer cells. J. Mammary Gland Biol. Neoplasia. 2006;10(3):221–229. doi: 10.1007/s10911-005-9583-7. [DOI] [PubMed] [Google Scholar]
- 192.Péqueux C, Breton C, Hendrick JC, et al. Oxytocin synthesis and oxytocin receptor expression by cell lines of human small cell carcinoma of the lung stimulate tumor growth through autocrine/paracrine signaling. Cancer Res. 2002:4623–4629. [PubMed] [Google Scholar]
- 193.Mantyh P. Neurobiology of substance P and the NK1 receptor. J. Clin. Psychiatry. 2002;63:6–10. [PubMed] [Google Scholar]
- 194.Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 195.Ruff M, Schiffmann E, Terranova V, et al. Neuropeptides are chemoattractants for human tumor cells and monocytes: a possible mechanism for metastasis. Clin. Immunol. Immunopathol. 1985;37(3):387–396. doi: 10.1016/0090-1229(85)90108-4. [DOI] [PubMed] [Google Scholar]
- 196.Dragos D, Tanasescu MD. The effect of stress on the defense systems. J. Med. Life. 2010;3(1):10–18. [PMC free article] [PubMed] [Google Scholar]
- 197.Dhabhar FS, Saul AN, Daugherty C, et al. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav. Immun. 2010;24(1):127–137. doi: 10.1016/j.bbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 200.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann. NY Acad. Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 201.Kryczek I, Wei S, Zou L, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol. 2007;178(11):6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 202.Zhang B, Rong R, Wei H, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem. Biophys. Res. Commun. 2008;374(3):533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 203.Curiel TJ. Regulatory T cells and treatment of cancer. Curr. Opin. Immunol. 2008;20(2):241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vedhara K, Cox NK, Wilcock GK, et al. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999;353(9153):627–631. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- 205.Kiecolt-Glaser JK, Ricker D, George J, et al. Urinary cortisol levels, cellular immunocompetency, and loneliness in psychiatric inpatients. Psychosom. Med. 1984;46(1):15–23. doi: 10.1097/00006842-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 206.Aloyz RS, Bamji SX, Pozniak CD, et al. p53 is essential for developmental neuron death as regulated by the TRKA and p75 neurotrophin receptors. J. Cell Biol. 1998;143(6):1691–1703. doi: 10.1083/jcb.143.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Elenkov I. Systemic stress-induced Th2 shift and its clinical implications. J. Int. Rev. Neurobiol. 2002;52:163–186. doi: 10.1016/s0074-7742(02)52009-2. [DOI] [PubMed] [Google Scholar]
- 208.Almog B, Fainaru O, Gamzu R, et al. Placental apoptosis in discordant twins. Placenta. 2002;23(4):331–336. doi: 10.1053/plac.2002.0788. [DOI] [PubMed] [Google Scholar]
- 209.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu. Rev. Pharmacol. Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 210.Brodde OE, Engel G, Hoyer D, et al. The β-adrenergic receptor in human lymphocytes: subclassification by the use of a new radio-ligand ± 125 iodocyanopindolol. Life Sci. 1981;29(21):2189–2198. doi: 10.1016/0024-3205(81)90490-2. [DOI] [PubMed] [Google Scholar]
- 211.Fuchs BA, Campbell KS, Munson AE. Norepinephrine and serotonin content of the murine spleen: its relationship to lymphocyte β-adrenergic receptor density and the humoral immune response in vivo and in vitro. Cell Immunol. 1988;117(2):339–351. doi: 10.1016/0008-8749(88)90123-2. [DOI] [PubMed] [Google Scholar]
- 212.Landmann R, Bittiger H, Buhler FR. High affinity β-2-adrenergic receptors in mononuclear leucocytes: similar density in young and old normal subjects. Life Sci. 1981;29(17):1761–1771. doi: 10.1016/0024-3205(81)90186-7. [DOI] [PubMed] [Google Scholar]
- 213.Loveland BE, Jarrott B, McKenzie IF. The detection of β-adrenoceptors on murine lymphocytes. Int. J. Immunopharmacol. 1981;3(1):45–55. doi: 10.1016/0192-0561(81)90044-8. [DOI] [PubMed] [Google Scholar]
- 214.Titinchi S, Clark B. α2-adrenoceptors in human lymphocytes: direct characterisation by [3H]yohimbine binding. Biochem. Biophys. Res. Commun. 1984;121(1):1–7. doi: 10.1016/0006-291x(84)90679-x. [DOI] [PubMed] [Google Scholar]
- 215.McPherson GA, Summers RJ. Characterization and localization of 3H-clonidine binding in membranes prepared from guinea-pig spleen. Clin. Exp. Pharmacol. Physiol. 1982;9(1):77–87. doi: 10.1111/j.1440-1681.1982.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 216.Goin JC, Sterin-Borda L, Borda ES, et al. Active α-2 and β-adrenoceptors in lymphocytes from patients with chronic lymphocytic leukemia. Int. J. Cancer. 1991;49(2):178–181. doi: 10.1002/ijc.2910490205. [DOI] [PubMed] [Google Scholar]
- 217.Abrass CK, O’Connor SW, Scarpace PJ, et al. Characterization of the β-adrenergic receptor of the rat peritoneal macrophage. J. Immunol. 1985;135(2):1338–1341. [PubMed] [Google Scholar]
- 218.Spengler RN, Allen RM, Remick DG, et al. Stimulation of α-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J. Immunol. 1990;145(5):1430–1434. [PubMed] [Google Scholar]
- 219.Plaut M. Lymphocyte hormone receptors. Annu. Rev. Immunol. 1987;5:621–669. doi: 10.1146/annurev.iy.05.040187.003201. [DOI] [PubMed] [Google Scholar]
- 220.Yukawa T, Ukena D, Kroegel C, et al. β-2-adrenergic receptors on eosinophils. Binding and functional studies. Am. Rev. Respir. Dis. 1990;141(6):1446–1452. doi: 10.1164/ajrccm/141.6.1446. [DOI] [PubMed] [Google Scholar]
- 221.Hadden JW, Hadden EM, Middleton E. Lymphocyte blast transformation. I. Demonstration of adrenergic receptors in human peripheral lymphocytes. Cell Immunol. 1970;1(6):583–595. doi: 10.1016/0008-8749(70)90024-9. [DOI] [PubMed] [Google Scholar]
- 222.Melmon KL, Bourne HR, Weinstein Y, et al. Hemolytic plaque formation by leukocytes in vitro. Control by vasoactive hormones. J. Clin. Invest. 1974;53(1):13–21. doi: 10.1172/JCI107530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bourne HR, Lichtenstein LM, Melmon KL, et al. Modulation of inflammation and immunity by cyclic AMP. Science. 1974;184(132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- 224.Depelchin A, Letesson JJ. Adrenaline influence on the immune response. I. Accelerating or suppressor effects according to the time of application. Immunol. Lett. 1981;3(4):199–205. doi: 10.1016/0165-2478(81)90075-4. [DOI] [PubMed] [Google Scholar]
- 225.Gader AM. The effects of β-adrenergic blockade on the responses of leucocyte counts to intravenous epinephrine in man. Scand. J. Haematol. 1974;13(1):11–16. doi: 10.1111/j.1600-0609.1974.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 226.Crary B, Borysenko M, Sutherland DC, et al. Decrease in mitogen responsiveness of mononuclear cells from peripheral blood after epinephrine administration in humans. J. Immunol. 1983;130(2):694–697. [PubMed] [Google Scholar]
- 227.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Immunol. Nat. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 228.Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J. Natl Cancer Inst. 2005;97(23):1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Greenfeld K, Avraham R, Benish M, et al. Immune suppression while awaiting surgery and following dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav. Immun. 2007;21:503–513. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 230.Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, Glaser R. Marital quality, marital disruption, and immune function. Psychosom. Med. 1987;49:13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 231.Zorilla EP, Luborsky L, McKay JR, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav. Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 232.Irwin M. Psychoneuroimmunology of depression: clinical implications. Brain Behav. Immun. 2002;16(1):1–16. doi: 10.1006/brbi.2001.0654. ■ Important review describing the impact of depression on mortality risk and its association with neuroimmune mechanisms.
- 233.Blomberg BB, Alvarez JP, Diaz A, et al. Psychosocial adaptation and cellular immunity in breast cancer patients in the weeks after surgery: an exploratory study. J. Psychosom. Res. 2009;67:369–376. doi: 10.1016/j.jpsychores.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J. Natl Cancer Inst. 1998;90(1):30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Thornton LM, Andersen BL, Crespin TR, Carson WE. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav. Immun. 2007;21:185–194. doi: 10.1016/j.bbi.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Thornton LM, Andersen BL, Carson WE., 3rd Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case–control analysis. Cancer Immunol. Immunother. 2008;57(10):1471–1481. doi: 10.1007/s00262-008-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav. Immun. 2009;23(8):1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 238.Lutgendorf SK, Sood AK, Anderson B, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005;23(28):7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 239.Lutgendorf SK, Lamkin DM, DeGeest K, et al. Depressed and anxious mood and T-cell cytokine producing populations in ovarian cancer patients. Brain Behav. Immun. 2008;22:890–900. doi: 10.1016/j.bbi.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Glasner A, Avraham R, Rosenne E, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a β-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J. Immunol. 2010;184(5):2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 241.Basu S, Dasgupta PS, Lahiri T, et al. Uptake and biodistribution of dopamine in bone marrow, spleen and lymph nodes of normal and tumor bearing mice. Life Sci. 1993;53(5):415–424. doi: 10.1016/0024-3205(93)90645-j. [DOI] [PubMed] [Google Scholar]
- 242.Basu S, Dasgupta PS, Chowdhury JR. Enhanced tumor growth in brain dopamine-depleted mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment. J. Neuroimmunol. 1995;60(1–2):1–8. doi: 10.1016/0165-5728(95)00044-3. [DOI] [PubMed] [Google Scholar]
- 243.Marttila RJ, Eskola J, Päivärinta M, Rinne UK. Immune functions in Parkinson’s disease. Adv. Neurol. 1984;40:315–323. [PubMed] [Google Scholar]
- 244.Marttila RJ, Eskola J, Soppi E, Rinne UK. Immune functions in Parkinson’s disease lymphocyte subsets, concanavalin A-induced suppressor cell activity and in vitro immunoglobulin production. J. Neurol. Sci. 1985;69(3):121–131. doi: 10.1016/0022-510x(85)90127-3. [DOI] [PubMed] [Google Scholar]
- 245.Fiszer U, Piotrowska K, Korlak J, et al. The immunological status in Parkinson’s disease. Med. Lab. Sci. 1991;48(3):196–200. [PubMed] [Google Scholar]
- 246.Villemain F, Chatenoud L, Galinowski A, et al. Aberrant T cell-mediated immunity in untreated schizophrenic patients: deficient interleukin-2 production. Am. J. Psychiatry. 1989;146(5):609–616. doi: 10.1176/ajp.146.5.609. [DOI] [PubMed] [Google Scholar]
- 247.Ganguli R, Brar JS, Chengappa KR, et al. Mitogen-stimulated interleukin-2 production in never-medicated, first-episode schizophrenic patients. The influence of age at onset and negative symptoms. Arch. Gen. Psychiatry. 1995;52(8):668–672. doi: 10.1001/archpsyc.1995.03950200058014. [DOI] [PubMed] [Google Scholar]
- 248.Maes M, Bosmans E, Calabrese J, et al. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J. Psychiatr. Res. 1995;29(2):141–152. doi: 10.1016/0022-3956(94)00049-w. [DOI] [PubMed] [Google Scholar]
- 249.Mortensen PB. The incidence of cancer in schizophrenic patients. J. Epidemiol. Commun. Health. 1989;43(1):43–47. doi: 10.1136/jech.43.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Goldacre M, Kurina L, Wotton C, et al. Schizophrenia and cancer: an epidemiological study. Br. J. Psychiatry. 2005;187:334–338. doi: 10.1192/bjp.187.4.334. [DOI] [PubMed] [Google Scholar]
- 251.Asada M, Ebihara S, Numachi Y, et al. Reduced tumor growth in a mouse model of schizophrenia lacking the dopamine transporter. Int. J. Cancer. 2008;123:511–518. doi: 10.1002/ijc.23562. [DOI] [PubMed] [Google Scholar]
- 252.Barak Y, Achiron A, Mandel M, et al. Reduced cancer incidence among patients with schizophrenia. Cancer. 2005;104(12):2817–2821. doi: 10.1002/cncr.21574. [DOI] [PubMed] [Google Scholar]
- 253.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer. 2006;6(3):240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Perron L, Bairati I, Harel F, et al. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15(6):535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 255.Algazi M, Plu-Bureau G, Flahault A, et al. Could treatments with β-blockers be associated with a reduction in cancer risk? Rev. Epidemiol. Sante Publique. 2004;52(1):53–65. doi: 10.1016/s0398-7620(04)99022-0. [DOI] [PubMed] [Google Scholar]
- 256.Li CI, Malone KE, Weiss N, et al. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65–79 years. Cancer. 2003;98(7):1504–1513. doi: 10.1002/cncr.11663. [DOI] [PubMed] [Google Scholar]
- 257.Meier CR, Derby LE, Jick SS, et al. Angiotensin-converting enzyme inhibitors, calcium channel blockers, and breast cancer. Arch. Intern. Med. 2000;160(3):349–353. doi: 10.1001/archinte.160.3.349. [DOI] [PubMed] [Google Scholar]
- 258.Rosenberg L, Rao RS, Palmer JR, et al. Calcium channel blockers and the risk of cancer. JAMA. 1998;279(13):1000–1004. doi: 10.1001/jama.279.13.1000. [DOI] [PubMed] [Google Scholar]
- 259.Cao L, Liu X, Lin EJ, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142(1):52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat. Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Benish M, Bartal I, Goldfarb Y, et al. Perioperative use of β-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann. Surg. Oncol. 2008;15:2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]