Abstract
Platinum-based chemotherapy, with cytoreductive surgery, is the cornerstone of treatment of advanced ovarian cancer, however acquired drug resistance is a major clinical obstacle. It has been proposed that subpopulations of tumour cells with stem-cell like properties, such as so-called side populations (SP) which over-express ABC drug-transporters, can sustain the growth of drug resistant tumour cells, leading to tumour recurrence following chemotherapy. The histone methyltransferase EZH2 is a key component of the Polycomb Repressive Complex 2 (PRC2) required for maintenance of a stem cell state and overexpression has been implicated in drug resistance and shorter survival of ovarian cancer patients. We observe higher percentage SP in ascites from patients that have relapsed following chemotherapy compared to chemonaive patients, consistent with selection for this subpopulation during platinum-based chemotherapy. Furthermore, ABCB1 (P-glycoprotein) and EZH2 are consistently over-expressed in SP compared to non-SP from patients’ tumour cells. SiRNA knockdown of EZH2 leads to loss of SP in ovarian tumour models, reduced anchorage-independent growth and reduced tumour growth in vivo. Together these data support a key role for EZH2 in the maintenance of a drug-resistant tumour-sustaining subpopulation of cells in ovarian cancers undergoing chemotherapy. As such, EZH2 is an important target for anticancer drug development.
Keywords: Ovarian cancer, side population, polycomb repressive complex 2, drug resistance, EZH2, EZH1
Introduction
The majority of ovarian cancer patients present with advanced disease. Although up to 80% respond well to surgery and platinum based chemotherapy, tumour recurrence is a common event (1). Following relapse, treatment with platinum can elicit a further response, however the duration of response tends to decrease with each round of therapy, as patients develop disease with increased chemoresistance. Subpopulations of tumour cells with stem-cell like properties have been proposed to sustain the growth of tumour cells and the inherent drug resistance of these stem-cell like cells could lead to tumour recurrence following chemotherapy (1, 2). Support for the existence of ovarian tumour stem cells comes from studies examining both tumour biopsies and ascites (3-8). Thus, ovarian tumours contain cells which are capable of prolonged growth in an anchorage-independent manner as spheroids and which are tumorigenic when injected at low cell numbers into mice (7). Subpopulations of cells capable of forming tumours in xenogeneic mice have been isolated from ascites of ovarian cancer patients (8).
One widely used method for isolating putative cancer stem cells is based on ABC transporter mediated efflux of the Hoechst 33342 dye to isolate a “side population” (SP). This dye-excluding side-population (SP) phenotype has been shown to be enriched with cancer stem-like cells in a variety of tumours (8-14). Furthermore, SP cells from ovarian cancer ascites can form tumours more readily in mice than non-SP cells following 5 days in culture (8). The presence of SP cells with increased in vitro drug resistance has been shown in mouse and human ovarian cancer cell lines (5, 8), although the relevance to clinical acquired drug resistance is still to be established.
Polycomb Group (PcG) proteins have been shown to be required for the maintenance of embryonic stem cells and could therefore also have a role in maintaining tumour stem/sustaining cells (15-17). Indeed, the key component of Polycomb Repressive Complex (PRC2) EZH2, a specific histone 3 lysine 27 (H3K27) methyltransferase, is essential for stem cell maintenance in glioblastoma (18). EZH2 plays a critical role in tumorigenesis and cancer progression through epigenetic gene silencing and chromatin remodeling (19, 20). There is increasing evidence that overexpression of the EZH2 gene occurs in a variety of human malignancies, including oral, esophageal, gastric, colon, hepatocellular, bladder, breast, prostate, and endometrial cancers (21-23). Putative oncogenic and tumour suppressive roles for EZH2 have been suggested (24, 25). Elevated expression of EZH2 has been shown to be associated with advanced stages of ovarian cancer and independently associated with short overall survival of ovarian cancer patients (26). Furthermore EZH2 knockdown in ovarian cell lines leads to reduced cell growth/proliferation and inhibited cell migration and/or invasion in vitro (26), as well as resensitisation of drug-resistant ovarian cancer cells to cisplatin (27). However, these studies have not examined EZH2 in an ovarian tumour stem cell population derived from patients during clinical acquired resistance.
We have address whether SP cells can be isolated from ascites from ovarian cancer patients and whether the size of the SP subpopulation changes during chemotherapy. Given the observed role of EZH2 in platinum resistance of ovarian cell lines (27), we have examined whether EZH2 is over-expressed in these tumour derived SP cells and whether inhibition of EZH2 may have the potential to inhibit growth of drug resistant ovarian tumour stem cells.
Methods
Patient material preparation and cell line culture
Ascitic fluid was obtained from ovarian cancer patients requiring therapeutic paracentesis following informed consent for this study, approved by the Royal Marsden Hospital Ethics Review Committee and the Hammersmith Hospital Ethics Review Committee. Within 24h of receipt, ascites were centrifuged at 400g for 15min to concentrate the cellular content. The concentrated fraction was over-laid onto lymphocyte preparation medium (PAA Laboratories, Yeovil, UK) and centrifuged for 30min at 400g without braking. Cells at the interface, enriched for tumour cells and lymphocytes, were collected and washed in RPMI1640 (Invitrogen, Paisley, UK) plus 10% fetal bovine serum (FBS, PAA Laboratories) then centrifuged for 10min at 400g. Contaminating red blood cells were lysed using a RBCLysis kit (Miltenyi Biotec Ltd, Bisley, Surrey, UK) according to the manufacturer’s instructions.
IGROV1 (28), PEO1, PEO4, PEA1, PEA2, PEO14, PEO23 (29) and OSEC2 (30) were obtained from Ovarian Cancer Action, Imperial College, London, UK. All cell lines were maintained in RPMI1640 + 10% FBS. IOSE21 were obtained from Prof Frances Balkwill, Institute of Cancer, Centre for Cancer and Inflammation, Barts and The London School of Medicine and Dentistry, London. Cell lines were shown to be identical to those received based on DNA methylation pattern analysed within one month of use and the methylation patterns showed close similarity between samples from the same patient, otherwise no further authentication was carried out.
Chemotherapeutic treatment of cell lines
Purified cell populations were plated and treated 24h later with cisplatin (Sigma-Aldrich, Dorset, UK), carboplatin (Sigma-Aldrich) and paclitaxel (Sigma-Aldrich) for 24h. The medium was replaced and the cells were allowed to recover for 48h then survival was assessed by MTT assay or the change in SP was measured by flow cytometry following cell enumeration by haemocytometer.
Flow Cytometry
Hoechst 33342 staining of cells (22) in combination with immunophenotyping. Cells were resuspended at 106/ml RPMI1640 + 2% FBS, 10mM HEPES (Sigma-Aldrich) and 5μg/ml Hoechst 33342 then incubated at 37°C with rotation, optimal incubation times were determined for each cell line. Cells were washed in 4 volumes of analysis buffer consisting of ice-cold HBSS containing 2% FBS and 10mM HEPES. Cells were analysed and sorted using a BD FACS VantageSE DiVa (San Jose, CA, USA) equipped with two Coherent Innova 90 lasers (Santa Clara, CA, USA), one with visible optics tuned to 488nm and set to 200mW and one with UV optics emitting multi-line UV 333.6-363.8nm set to 50mW. Sheath fluid was sterile PBS pH7.2 (in house) and cells were sorted with sheath pressure set at 12psi (0.82 bar). On reanalysis of sorted populations the purity generally exceeded 90%. To confirm the presence of a side population and define the gate for cell sorting, verapamil was added to control samples at a final concentration of 20μM.
For primary ascites cells CD45-FITC (Clone HI30, BD Biosciences) and/or CD326-APC (EpCAM, Clone HEA-125, Miltenyi Biotech) was added to the cells for 30min on ice and the excess removed by washing, appropriate matched isotype controls were also used to identify non-specific labelling. Cells were resuspended in Hanks’ balanced salt solution (Invitrogen) containing 2%FBS and 2μg/ml propidium iodide (Sigma-Aldrich) then analysed and sorted as described above. Data was analysed using FCS Express Professional version 3 (De Novo Software, Los Angeles, CA, USA).
In vivo grafts
All procedures were approved by the Institute of Cancer Research Ethics committee and all work performed in accordance with UK Home Office regulations under the Animals (Scientific Procedures) Act 1986 and UKCCR guidelines for animal experimentation (30). Side and non-side populations from IGROV1 cells were sorted as described above and resuspended in ice cold 50% Matrigel™ (BD biosciences, Oxford, UK) in RPMI1640 at 50μl per xenograft. Between 50 and 250 cells were injected into the mammary fat pad of 10 week-old NOD/SCID mice (Charles River, France) and allowed to grow for up to 12 weeks. Unsorted cells were grafted as a positive control for cell viability and graft take. Similarly, 48h post-siRNA treatment, IGROV1 cells were harvested using TrypLE and counted, 50μl grafts were prepared and transplanted as described above, using 250 cells per graft. Tumour size was monitored by calliper measurements across 2 diameters (d) at regular intervals to calculate tumour volume (cm3) = 4/3π[(d1+d2)/4]3.
Spheroid forming assays
Cells were plated at a range of densities in 6-well ultra-low adhesion tissue culture plates in 2ml RPMI1640 (Invitrogen, Paisley, UK) plus 10% fetal bovine serum (FBS, PAA Laboratories) and transferred to an incubator with supplementary feeding with same media and serum every 3-4 days. Spheroids were allowed to form for 18 days and then counted. In some cases, spheroids were transferred to adherent plates. The adhered spheroids were fixed in 3.7% formalin in PBS then stained with 1% rhodamine B in water. Images of each well were captured then analysed by Image-Pro Analyser 6.3 to quantitate colony size and frequency.
siRNA treatment
Optimal plating densities were determined to allow proliferation for up to 96h for each cell line. Protocols were optimised as recommended by the manufacturer’s instructions. siRNAs were delivered using HiPerfect (Qiagen, Crawley, UK) within 16hrs of plating cells. All experiments consisted of untreated controls, mock controls, control siRNA (Allstars scrambled siRNA, Qiagen) and positive control siRNA (MAPK, Qiagen).
RNA preparation, reverse transcription and quantitative real-time-PCR
Total cellular ribonucleic acid (TRNA) was extracted (RNeasy Minikit Plus, Qiagen) and quantified by measurement of optical density at OD λ260. Up to 2μg of RNA was reverse transcribed and first strand cDNA synthesised in 40μl using the Superscript II reverse transcriptase kit (Invitrogen) according to manufacturer’s instructions. Samples were amplified using TaqMan gene expression assays (Applied Biosystems, Warrington, UK) and a Step One PCR machine (Applied Biosystems). TaqMan probe and primers (see manufacturer’s website) were used: ABCB1 (assay ID Hs01067802_m1), ABCG2 (assay ID Hs01053790_m1), GAPDH (part no 4326317E), EZH1 (assay ID Hs00157470_ml) and EZH2 (assay ID Hs01016789_ml).
Histone extraction and immunoblotting
Cells were re-suspended in lysis buffer (PBS containing 0.5% Triton X 100 (v/v), 1X Complete protease inhibitor cocktail – Roche) and left to lyse for 10 min. at 4°C. Nuclei were then precipitated and re-suspended in 0.2N HCl overnight at 4°C. Nuclear debris were pelleted and the supernatant recovered. Protein concentration was calculated by Bradford assay (Bio-Rad). The lysate was separated by 8-16% SDS-PAGE and transferred to nitrocellulose membranes. Rabbit polyclonal antibodies anti-trimethyl-Histone-H3 (Lys27) (07-449, Millipore) was used for immunoblotting.
Microarray Hybridisation and Gene Set Analysis (GSA)
Gene expression in 3 independently sorted SP and non-SP from IGROV1 cells exponentially growing under standard tissue culture conditions was determined by affymetrix array using the HG-U133plus2 GeneChip array following amplification labelling. Labelling and hybridizations were carried out at the Paterson Institute microarray facility (Manchester, UK) following standard protocols. Full methods are available at http://bioinformatics.picr.man.ac.uk/mbcf.
The raw expression data of IGROV1 SP and non-SP populations in Affymetrix HG-U133plus2 microarrays were normalised as previously described (31) excluding the probes with low signal intensities (average expression across all the samples less than 25th quantile of the whole dataset). The data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE25191 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc= GSE25191). Expression values of multiple probes targeting the same gene were averaged, resulting in a total of 20323 unique genes in Gene Set Analysis (GSA) (32) to assess the differential expression between 3 pairs of SP and non-SP cells in 15 pre-defined characteristic signatures that embryonic stem cells have. We computed the gene scores (t-statistic zi) for all the genes in one gene set S. The larger absolute value of the average of the positive and of the negative parts of each zi in S is called ‘maxmean statistic’. The significance of the ‘maxmean statistic’ in each gene set was determined by False Discovery Rate (FDR), estimated based on row randomisation and label permutation. FDR < 5% was used to determine the enrichment of up-regulation/down-regulation of those signatures in SP cells. This analysis was done in R (version 2.8.1) using GSA package (33).
Statistical analysis
All further analyses were performed using the statistical analysis package GraphPad Prism v5 (GraphPad Software Inc., La Jolla, CA, USA). The significant level was set at two sided p<0.05. All figures are shown with means and standard errors unless otherwise stated.
Results
SP cells from IGROV1 ovarian tumour cell line have tumour stem cell like properties
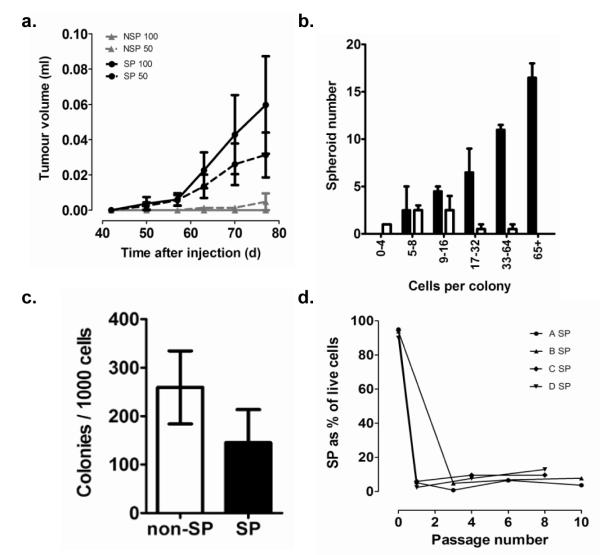
ABC transporter mediated efflux of the Hoechst 33342 dye to isolate a dye-excluding side-population (SP) has been shown to be enriched for cells with cancer stem-like properties in a variety of tumours (8-14). SP cells from ascites of ovarian cancer patients have been shown to form tumours more readily in mice than non-SP cells (8). Using differential Hoechst 33342 staining of cells to identify side-populations (SP) of the IGROV1 ovarian tumour cell line, we observe significantly increased tumorigenicity (Repeated Measures ANOVA: p<0.05) of SP compared to non-SP cells when injecting 50 or 100 cells into the mammary fat pad of NOD/SCID mice (Figure 1a). In addition to the differences in average tumour growth, SP cells initiated tumours more frequently (8/14 injections) than non-SP (1/12 injections (Fisher’s exact test: p=0.0145). Furthermore, SP cells have a greater ability to grow in an anchorage-independent manner as spheroids compared to non-SP (Figure 1b). The differences in growth properties of SP and non-SP cells could be due to toxicity of Hoechst 33342 and its increase accumulation in non-SP cells. Arguing against this interpretation, we observe non-SP IGROV1 cells to have in fact significantly increased plating efficiency compared to SP cells in standard adherent 2D tissue culture assays (Figure 1c) and the enhanced growth of SP was only observed in anchorage-independent spheroid growth conditions (Figure 1b). Asymmetric division is a key feature of stem cells (34). To examine whether isolated SP cells could repopulate SP and non-SP cell populations, multiple SP IGROV1 populations were isolated by FACS and continuously cultured for up to 10 passages. The number of SP cells in the SP selected cultures declined rapidly on passaging with an increase in non-SP cells (Figure 1d). The mean purity of SP cells following initial separation was 93.3%. It could be argues that a higher proliferative capacity of the contaminating non-SP could lead to the rapid re-emergence of the non-SP. However, the SP population became stable as a minority of the total population and the cell culture retained SP and non-SP populations equivalent to the proportions present in the original cell line. Furthermore, while the growth potential of the SP is slightly reduced compared to the non-SP (figure 1c), this is unlikely to explain the rapid emergence of the non-SP cells.
Figure 1. Tumour formation and anchorage independent growth of SP cells.
(a) When injected at low cell doses (50 or 100 cells, n=6-8 per group) in Matrigel™ into the NOD/SCID mammary fat pad, IGROV1 SP cells formed tumours more frequently than non-SP cell and produced significantly larger tumours over the duration of the experiment (Repeated measures ANOVA: p<0.05, error bars, standard errors). (b) Following FACS selection, SP (solid bars) and non-SP cells (open bars) were allowed to form spheroids in non-adherent culture for 18 days and were transferred to adherent plates to determine colony size. SP cells produced spheroids with a greater frequency and larger size than non-SP cells (c) SP and non-SP were selected by FACS and plated at low density in adherent culture, colonies were counted after ten days. Colony formation by non-SP was significantly greater than by SP (P=0.0092, paired Student’s t test). (d) IGROV1 SP and non-SP were sorted (biological replicates: n=4, mean purity 93.3%) and subsequently maintained in adherent culture through 8-10 passages. The cells were harvested at intervals and the size of the SP was measured by FACS. The SP fell rapidly and stabilized to levels similar to the original unsorted levels in all cases.
Gene set analysis (GSA) (32) was used to interrogate gene expression profiling of SP compared to non-SP from IGROV1 cells for 15 published embryonic stem cell related signatures (Supplementary Table 1). Four signatures with p<10−6 and FDR<10−4 were significantly upregulated in SP compared to non-SP: Oct4 regulated genes, ‘NOS’ signature (Nanog/Oct4/Sox2 regulated genes), E2F6 associated expression pattern and polycomb repressive complex 2 (PRC2). Consistent with upregulation of PRC2 genes, the PRC2 target genes were significantly repressed in the SP population (p<10−6 and FDR<10−4). The individual GSA scores for the PRC2 target genes are shown in Supplementary Table 2. Thus, based on cell phenotype and over-representation of stem cell gene expression patterns, SP cells isolated from the IGROV1 human ovarian tumour cell line have tumour stem cell like properties.
The proportion of SP in ovarian cancer ascites increases following chemotherapy
We assessed the relative proportion of SP cells present in tumours from ovarian cancer patients. We measured the proportion of SP cells present in EpCAM positive, CD45 negative tumour cells in patient ascites collected either at initial presentation or at relapse after platinum-based chemotherapy (Table 1). Ascites samples collected from relapsed patients show a significant (P=0.013) increase in proportion of SP cells compared to chemonaive patients (Figure 2a). Patients were categorised, post-ascitic drainage, as having either disease control (partial response or stable disease) or progressive disease to subsequent chemotherapy (Table 1). Those with progressive disease tended to have increased SP (mean = 1.8 ±0.9) compared to those with disease control (mean = 0.42 ±0.24, P=0.055, unpaired student’s t test with Welch’s correction).
Table 1.
Ascites collected from ovarian cancer patients: side population and clinical details at time of ascites collection and response to subsequent chemotherapy.
| Sample Number |
Age | Stage | Grade | Histology | Most recent chemo- therapy |
% SP (EpCAM+/ CD45neg) |
At time of ascites collection | Treatment subsequent to ascites collection | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lines of chemo- therapy |
Response to most recent chemotherapy |
Chemo- therapy |
Response to subsequent chemotherapy |
Duration of response (months) |
|||||||
| 1 | 68 | 4 | 3 | Serous Adeno- carcinoma |
Nil | ≤0.0001 | 0 | N/A | Carboplatin | Partial Response | 3.5 |
| 2 | 64 | 3c | 2 | Serous Adeno- carcinoma |
Nil | ≤0.0001 | 0 | N/A | Carboplatin/ Paclitaxel |
Partial Response | 5.0 |
| 3 | 62 | 3c | 3 | Serous Adeno- carcinoma |
Nil | 0.1746 | 0 | N/A | Carboplatin/ Paclitaxel |
Partial Response | 5.0 |
| 4 | 57 | 4 | 3 | Serous Adeno- carcinoma |
Nil | 0.1000 | 0 | N/A | Nil | - | - |
| 5 | 66 | 3 | Not Known |
Not Known |
Nil | 0.0007 | 0 | N/A | Carboplatin/ Paclitaxel |
Partial Response | 2 ongoing |
| 6 | 74 | 3 | Not Known |
Not Known |
Paclitaxel | 2.7392 | 1 | Progressive Disease |
Carboplatin/ Paclitaxel |
Progressive Disease |
Progressive Disease |
| 7 | 75 | 3 | 3 | Serous Adeno- carcinoma |
Carboplatin | - | 1 | Progressive Disease |
Paclitaxel | Progressive Disease |
Progressive Disease |
| 8 | 59 | 3c | 2 | Serous Adeno- carcinoma |
Carboplatin / Paclitaxel |
0.1874 | 2 | Stable Disease | Liposomal doxorubicin |
Progressive Disease |
Progressive Disease |
| 9 | 62 | 3 | 3 | Serous Adeno- carcinoma |
Carboplatin / Paclitaxel |
0.0650 | 2 | Stable Disease | Carboplatin/ Paclitaxel |
Progressive Disease |
Progressive Disease |
| 10 | 58 | 3c | 2 | Adeno- carcinoma |
Liposomal doxorubici n |
6.7880 | 3 | Progressive Disease |
Nil | - | - |
| 11 A | 62 | 3c | 1 | Papillary Serous Adeno- carcinoma |
Carboplatin / Paclitaxel |
0.0093 | 3 | Stable Disease | Nil | - | - |
| 12 A | 62 | 3c | 1 | Papillary Serous Adeno- carcinoma |
Carboplatin / Paclitaxel |
0.1902 | 3 | Stable Disease | Nil | - | - |
| 13 | 61 | 4 | 3 | Serous Adeno- carcinoma |
Paclitaxel | 0.0036 | 3 | Progressive Disease |
Etoposide | Progressive Disease |
Progressive Disease |
| 14 | 77 | 3 | Not Known |
Serous Adeno- carcinoma |
Carboplatin | 2.5647* | 3 | Partial Response | Phase I trial | Progressive Disease |
Progressive Disease |
| 15 B | 54 | 3 | 2 | Mullerian Serous Adeno- carcinoma |
Paclitaxel | 0.0044 | 4 | Progressive Disease |
Nil | - | - |
| 16 C | 56 | 3/4 | 3 | Serous Adeno- carcinoma |
Cisplatin | 1.7200 | 5 | Stable Disease | Phase I trial | Progressive Disease |
Progressive Disease |
| 17 | 75 | 3 | Not Known |
Adeno- carcinoma |
Paclitaxel | 0.0242 | 6 | Progressive Disease |
Carboplatin | Progressive Disease |
Progressive Disease |
| 18 C | 56 | 3/4 | 3 | Serous Adeno- carcinoma |
Liposomal doxorubici n |
3.3400 | 7 | Progressive Disease |
Nil | - | - |
| 19 B | 54 | 3 | 2 | Mullerian Serous Adeno- carcinoma |
Paclitaxel | 0.6536 | 7 | Progressive Disease |
Nil | - | - |
| 20 | 73 | 3c | Not Known |
Adeno- carcinoma |
Carboplatin | 0.0586 | 9 | Stable Disease | Megesterol | Stable Disease | 5 (ongoing) |
Patient matched pairs of ascites taken from 3 patients with 0.25-2months between collection. Not Known - Information not available from patient notes of histology records. Partial response was assessed radiologically using RECIST 1.1 criteria by repeat imaging (generally CT scan).
Patient matched pairs of ascites taken from 3 patients with 0.25-2months between collection. Not Known - Information not available from patient notes of histology records. Partial response was assessed radiologically using RECIST 1.1 criteria by repeat imaging (generally CT scan).
Patient matched pairs of ascites taken from 3 patients with 0.25-2months between collection. Not Known - Information not available from patient notes of histology records. Partial response was assessed radiologically using RECIST 1.1 criteria by repeat imaging (generally CT scan).
Figure 2. Percent SP in ovarian tumour samples.
(a) Percent SP was significantly (p=0.0126, unpaired Student’s t test with Welch’s correction) lower in freshly isolated patient ascites (solid symbols) from untreated patients (n=5) compared to post-chemotherapy ascites (n=15). Open circles are mean percent SP in isogenic matched prechemotherapy and post-chemotherapy cell lines. (b) Percent SP in 3 pairs of isogenic cell lines derived from patients tumours with chemoresponsive or chemoresistant disease. Immortalised normal ovarian surface epithelium had undetectable SP.
Sequential ascites samples taken from 3 patients during treatment show progressive increase in the SP (Table 1). We have also examined the proportion of SP present in matched cell lines derived from patients’ tumours at diagnosis and at platinum resistant relapse (Figure 2b). These lines have previously been shown to acquire in vivo increased resistance to carboplatin during chemotherapy (29) and represent isogenic models of clinical drug resistance. We observe an increase in the percent SP in all three of the lines that have acquired platinum resistance compared to the matched chemosensitive tumour line. Two immortalised cell lines generated from normal surface ovarian epithelial cells did not contain a detectable SP.
Carboplatin selects for increased SP in vitro
The increase in SP observed with development of chemo-resistance following carboplatin based chemotherapy argues that SP cells may have a selective survival or growth advantage during chemotherapy. Resistance of tumour stem cells to chemotherapeutic drugs has previously been described in a variety of tumour types including ovarian cancer SP (8, 35). Indeed, we observe increased resistance of IGROV1 SP compared to non-SP to platinum (cisplatin and carboplatin) and to paclitaxel (Figure 3a). However, it could be argued that this differential drug sensitivity is influenced by differential levels of Hoechst dye remaining in the SP and non-SP cells. Therefore we examined whether exposure of IGROV1 cells to physiologically relevant levels of carboplatin (in the absence of Hoechst dye) could select for an increased proportion of SP cells. As shown in Figure 3b, SP cells remain relatively unaffected by the carboplatin treatment, while there is a significant decrease in viability of the non-SP, consistent with the effects of carboplatin on the viability of the bulk population of cells. The net effect will be an enrichment or selection for the SP cell population during platinum exposure.
Figure 3. Chemosensitivity of SP.
(a) Chemosensitivity of isolated SP and non-SP from IGROV1 at IC80 of unseparated cells to cisplatin, carboplatin and paclitaxel, expressed as a surviving fraction of the control (SP vs non-SP, cisplatin P=0.059, carboplatin P=0.01, paclitaxel P=0.12, respectively, one way paired Student’s t test). (b) Unselected IGROV1 cells were treated with carboplatin at 60μM. Following 48h recovery, SP and non-SP were measured by FACS to calculate the surviving fractions relative to untreated controls. The surviving fraction of SP was significantly greater than the non-SP surviving fraction (P= 0.011, one way paired Student’s t test) (c) SP and non-SP cells were selected to >90% purity by FACS. Gene expression of ABCB1 and ABCG2 was quantified by real-time PCR from 5 ovarian cancer cell lines and 10 patient ascites. All samples showed enrichment of ABCB1 mRNA expression SP compared to non-SP, with the exception of PEA2 where ABCG2 was overexpressed in the SP (Below Quantification: mRNA below minimum level for reliable quantification by real-time PCR. All values were normalized to GAPDH).
Differential expression of ABC transporters has been widely observed in SP from a variety of tumour types, although in the majority of studies over-expression of ABCG2 is observed (8, 35). Real time-PCR quantification of ABCB1 and ABCG2 mRNA showed that ABCB1 was over-expressed in SP cells in comparison to non-SP cells from 4 of 5 ovarian cancer cell lines and in primary patient ascites (n=10), whereas ABCG2 was below the level of quantification by RT-PCR, except for PEA2, from which ABCB1 was absent (Figure 3c). Carboplatin is not a substrate for P-glycoprotein (36) and therefore over-expression of ABCB1 is unlikely to be the explanation of the increased resistance to carboplatin or selection of SP cells observed. Since ABCC5 and ABCC6 have been implicated in cisplatin resistance (37, 38) we specifically examined by qRT-PCR their levels in SP and non-SP from patient ascites, but in the case of ABCC5 no significant difference was observed (1.22 SP to non-SP ratio, n=10) and for ABCC6 the levels were undetectable in SP and non-SP (n=10). Paclitaxel is a substrate for ABCB1 and therefore over-expression of ABCB1 in this subpopulation could affect the likelihood of relapse following taxane containing chemotherapy, however we do not observe increased resistance of the SP cells compared to non-SP for paclitaxel sensitivity (Figure 3A).
Increased expression of EZH2 in SP from patient ascites compared to non-SP
PRC2 contains the histone methyltransferase EZH2, which, together with EED and SUZ12, trimethylates histone H3 on Lysine 27 (H3K27me3) and is associated with a repressive chromatin state (20). EZH2 is over-expressed in many cancers and levels of EZH2 correlate with poor prognosis, including ovarian cancer (21-23, 26). We observe increased expression of EZH2 in 7/10 SP compared with non-SP isolated from ascites in patients with recurrent ovarian cancer (Table 2), with up to 14-fold increase expression in SP compared to non-SP in patient samples. The increased EZH2 expression observed in SP from patient ascites is variable and may represent differences in purity of the FACS sorted SP, although there is no obvious correlation with level of ABCB1 (Table 2). Previous studies have suggested that differential levels of Hoechst dye can affect gene expression in SP cells (39). We observed no effect of Hoechst dye on EZH2 levels or ABCB1 levels (data not shown).
Table 2.
EZH2 mRNA levels in SP and non-SP from ovarian tumour ascites samples.
| Patient Ascites sample number |
Ratio of expression of ABCB1 mRNA SP:non-SP |
Ratio of expression of EZH2 mRNA SP:non-SP |
|---|---|---|
| 6 | 12.9 | 14.5* |
| 7 | 3.1 | 4.2* |
| 9 | 10.3 | 1.3 |
| 10 | 2.9 | 1.3 |
| 14 | 57.6 | 3.4* |
| 16 | 8.4 | 5.9* |
| 17 | 16.9 | 8.6* |
| 18 | 3.7 | 1.6* |
| 19 | 36.8 | 1.1 |
| 21 | 51.8 | 2.1* |
significant (p<0.05) difference in EZH2 expression in SP compared to non-SP
Knock-down of EZH2 and EZH1 in ovarian tumour cell lines reduces SP and tumour stem cell like phenotype
Propagation of the H3K27me3 mark during cell division accounts for the maintenance and somatic inheritance of repressive chromatin domains (40). Therefore inhibition of EZH2 should reduce H3K27 methylation levels, leading to gene reactivation. In order to examine whether EZH2 has a direct role in maintaining an SP, we performed siRNA knock-down of EZH2 in three independent ovarian tumour cell lines. EZH2 knockdown consistently reduced EZH2 mRNA by >70% and produces a dramatic and prolonged reduction in the levels of H3K27 tri-methylation in cells (Figure 4a).
Figure 4. EZH2 knock-down in ovarian cell lines.
For all experiments, we confirmed the activity of siRNA used by their ability to knockdown expression of EZH1 and EZH2 using real-time qPCR, achieving 78%-85% reduction in EZH2 mRNA by 48-72h for IGROV1 and 72-79% for the other lines, while EZH1 mRNA was reduced by 61-72% in IGROV1 and 57-62% for the other lines. Inhibition of EZH2 mRNA also resulted in reduced protein expression as confirmed by western blot analysis and by immunofluoresence which also demonstrated nuclear localisation of EZH2 protein. Finally, reduced histone methyltransferase activity was demonstrated by time-dependent reduction of H3K27 methylation levels, as shown by immunoblotting analysis (a, upper panel). No effect on the total levels of histone H3 were detected (a, lower panel). SP was measured by FACS following (b) EZH2 alone siRNA knockdowns or (c) EZH1 and EZH2 siRNA knockdowns expressed as a percentage of scrambled siRNA controls (mean±s.e.m, n=3 for each cell line): (Pooled data for all cell lines: paired Student’s t test P = 0.0044, P = 0.028, respectively). (d) IGROV1 cells were treated with siRNA and allowed to form spheroids for 18 days and then counted (n=3). EZH1 and EZH2 reduced spheroids formation, alone and in combination, EZH2 inhibiting formation to a greater extent than EZH1 alone. (e) IGROV1 cells or (f) PEO23 cells were treated with scrambled control siRNA or EZH1+EZH2 siRNA in vitro then grafted into the mammary fat pad of NOD/SCID mice at 250 cells per graft. (n=6 per group). Mean tumour volume was determined at regular intervals for up to 10 weeks: mean graft volume was persistently smaller following EZH1+EZH2 over the duration of the experiment (for IGROV1, P=0.072 and for PEO23 P=0.032, one way Students t test).
Since EZH1 also mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency (41), we performed double knock-downs of EZH2 and EZH1, as well as EZH2 alone, in case of potential redundancy of functional effects on tumour stem cells. EZH2 knock-down alone, or when combined with EZH1 knock-down, consistently reduced the SP in independent ovarian tumour cell lines (Figure 4b and 4c). SP compared to non-SP cells have a greater ability to grow in anchorage-independent culture and form large (>32 cell) spheroids (Figure 1b). Loss of anchorage-independent growth following knockdown of EZH2 alone or combined to EZH1 in IGROV1, PEO14 and PEO23 is observed (data for IGROV1 shown in Figure 4d). As well as for the pool of four SiRNA for EZH2 shown in Figure 4d, similar reduction in SP and spheroid growth was observed for all four SiRNA when tested individually. Following siRNA knockdown of EZH1+EZH2, IGROV1 or PEO23 cells were injected into a NOD/SCID mouse model and tumour growth measured. Although knockdown will be transitory and not maintained throughout the xenograft experiment, reduced expression of EZH2 and reduced H3K27me is observed 48-96h following SiRNA treatment, the important time-frame for tumour take. Despite the transitory nature of the SiRNA knockdown, a persistent reduction in tumour volume in the EZH2+EZH1 knock-downs compared to the controls was observed (Figure 4e and 4f).
Discussion
In support of the existence of tumour stem cell-like cells in ovarian tumours, subpopulations of tumour initiating or sustaining cells have been identified which grow more readily in an anchorage independent manner and as tumours in xenogeneic mice (3-8). These subpopulations can be shown to over-express stem cell associated markers and to be more resistant to drugs used in the treatment of ovarian cancer (7, 8). One of the methods that has been successfully used to isolate such putative tumour stem cells is differential Hoechst dye uptake (8-13). Using this approach we have identified a subpopulation (SP) of cells from ovarian cell lines that have a more aggressive phenotype, as defined by anchorage-independent growth and tumour formation in NOD/SCID mice, and are resistant to carboplatin and paclitaxel. Consistent with the in vitro observation of a survival advantage of SP following exposure to carboplatin and hence potential drug selection, we observe increased SP in tumour cells isolated from patient ascites following chemotherapy and at relapse compared to chemo-naïve patients.
SP isolated from ovarian tumour cell lines over-express a NOS cell signature, frequently associated with Embryonic Stem Cells (17). Furthermore, we show that SP isolated from IGROV1 SP over-express PRC2 genes, while consistent with this PRC2 repressed targets are under-expressed. PRC2 contains the histone methyltransferase EZH2, which, together with EED and SUZ12, trimethylates histone H3 on Lysine 27 (H3K27me3) and is associated with a repressive chromatin state (20). Many genes in cancer, including tumour suppressor genes, are epigentically silenced by mechanisms associated with H3K27me3 which can be independent of DNA methylation (42). Since H3K27me3 is somatically inherited during cell division (40), this argues that it truly is an epigenetic mark that is maintained and as such is a key target for reversing aberrant epigenetic silencing. Consistent with the data presented in our current study in ovarian cancer, EZH2 has been shown to be essential for Glioblastoma cancer stem cell maintenance (18). EZH2 is over-expressed in many cancers and levels of EZH2 correlate with poor prognosis in various cancers, including ovarian cancer (21-23, 26).
Our data suggest that EZH2 has a key role in maintenance of the drug resistant SP subpopulation in ovarian tumour cells. We have shown that EZH2 is over-expressed in SP derived from ovarian cancer ascites at relapse. EZH2 knockdown in ovarian cell lines has been shown to lead to reduced cell growth/proliferation, as well as cell migration and/or invasion in vitro (26), while overexpression of EZH2 has been associated with acquired cisplatin resistance (27). Furthermore, loss of H3K27 trimethylation has been shown to result in resensitization of ovarian cancer cells to cisplatin (43), although lower H3K27 trimethylation levels were associated with shorter overall survival. (44). However, these previous studies have not examined EZH2 in an ovarian tumour stem cell population derived from patients during clinical acquired resistance, and our present data strongly support the clinical relevance of EZH2 in this important subpopulation of cells selected for during chemotherapy. This drug resistant subpopulation will be important to eradicate if we aim to improve the treatment of ovarian cancer. Catalytic site inhibitors of histone methyltransferase (45), have been reported, as has indirect pharmacological inhibition of PRC2 (46), but so far no specific EZH2 inhibitor has been described. Development of specific inhibitors of EZH2 will have potential as drugs that target this key subpopulation of drug resistant tumour sustaining cells.
Supplementary Material
Acknowledgements
With thanks to all those involved in tissue and patient data collection, including Debbie Tandy, Nicole Martin, Nona Rama, Amy Ford, Michelle Everard, Claudia Hayford, Tim Crook and Matthew Ng. This work was supported by Ovarian Cancer Action (SR, PM) and Cancer Research UK (WD, JQ, AS-S) grants to RB (C536/A6689), Cancer Research UK Experimental Cancer Medicine Centre, The Biomedical Research Council of the Agency for Science, Technology and Research, Singapore (LL), and Leukaemia Research Fund (IT) and Institute of Cancer Research (IT, DH, RB, SBK).
Abbreviations
- CT
computed tomography
- N/A
not applicable
- PR
partial response
- PD
progressive disease
- SD
stable disease
- ND
not done
- PRC2
polycomb repressive complex 2
- SP
side population
- PcG
polycomb group
- H3K27
Histone 3 lysine 27.
References
- 1.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–6. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 2.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 3.Ferrandina G, Bonanno G, Pierelli L, et al. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18:506–14. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 4.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–29. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 5.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–59. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moserle L, Indraccolo S, Ghisi M, et al. The side population of ovarian cancer cells is a primary target of IFN-alpha antitumor effects. Cancer Res. 2008;68:5658–68. doi: 10.1158/0008-5472.CAN-07-6341. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer. 2010;102:1276–83. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–24. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101:781–6. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirschmann-Jax C, Foster AE, Wulf GG, Goodell MA, Brenner MKA. distinct “side population” of cells in human tumor cells: implications for tumor biology and therapy. Cell Cycle. 2005;4:203–5. [PubMed] [Google Scholar]
- 12.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–13. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 13.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 14.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 15.Sauvageau M, Sauvageau G. Polycomb group genes: keeping stem cell activity in balance. PLoS Biol. 2008;6:e113. doi: 10.1371/journal.pbio.0060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TI, Jenner RG, Boyer LA, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–13. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suva ML, Riggi N, Janiszewska M, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–18. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- 19.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–64. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Matsukawa Y, Semba S, Kato H, Ito A, Yanagihara K, Yokozaki H. Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci. 2006;97:484–91. doi: 10.1111/j.1349-7006.2006.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryant RJ, Cross NA, Eaton CL, Hamdy FC, Cunliffe VT. EZH2 promotes proliferation and invasiveness of prostate cancer cells. Prostate. 2007;67:547–56. doi: 10.1002/pros.20550. [DOI] [PubMed] [Google Scholar]
- 23.Collett K, Eide GE, Arnes J, et al. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12:1168–74. doi: 10.1158/1078-0432.CCR-05-1533. [DOI] [PubMed] [Google Scholar]
- 24.Karanikolas BD, Figueiredo ML, Wu L. Polycomb group protein enhancer of zeste 2 is an oncogene that promotes the neoplastic transformation of a benign prostatic epithelial cell line. Mol Cancer Res. 2009;7:1456–65. doi: 10.1158/1541-7786.MCR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikoloski G, Langemeijer SM, Kuiper RP, et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42:665–7. doi: 10.1038/ng.620. [DOI] [PubMed] [Google Scholar]
- 26.Rao ZY, Cai MY, Yang GF, et al. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-{beta}1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis. 2010;31:1576–83. doi: 10.1093/carcin/bgq150. [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Yu L, Li Z, et al. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo. Cancer Biol Ther. 2010;10 doi: 10.4161/cbt.10.8.12913. in press. [DOI] [PubMed] [Google Scholar]
- 28.Benard J, Da Silva J, De Blois MC, et al. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res. 1985;45:4970–79. [PubMed] [Google Scholar]
- 29.Langdon SP, Lawrie SS, Hay FG, et al. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988;48:6166–72. [PubMed] [Google Scholar]
- 30.Workman P, Balmain A, Hickman JA, et al. UKCCCR guidelines for the welfare of animals in experimental neoplasia. Lab Anim. 1988;22:195–201. doi: 10.1258/002367788780746467. [DOI] [PubMed] [Google Scholar]
- 31.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Efron B, Tibshirani R. On testing the significance of sets of genes. 2006. Stanford tech report rep, http://www-stat.stanford.edu/~tibs/ftp/GSA.pdf.
- 34.Neumuller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 2009;23:2675–99. doi: 10.1101/gad.1850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 36.Ikuta K, Takemura K, Sasaki K, et al. Expression of multidrug resistance proteins and accumulation of cisplatin in human non-small cell lung cancer cells. Biol Pharm Bull. 2005;28:707–12. doi: 10.1248/bpb.28.707. [DOI] [PubMed] [Google Scholar]
- 37.Nomura M, Matsunami T, Kobayashi K, et al. Involvement of ABC transporters in chemosensitivity of human renal cell carcinoma, and regulation of MRP2 expression by conjugated bilirubin. Anticancer Res. 2005;25:2729–35. [PubMed] [Google Scholar]
- 38.Weaver DA, Crawford EL, Warner KA, Elkhairi F, Khuder SA, Willey JC. ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer. 2005;4:18–22. doi: 10.1186/1476-4598-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christgen M, Geffers R, Ballmaier M, et al. Down-regulation of the fetal stem cell factor SOX17 by H33342: a mechanism responsible for differential gene expression in breast cancer side population cells. J Biol Chem. 2010;285:6412–18. doi: 10.1074/jbc.M109.082941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margueron R, Justin N, Ohno K, et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461:762–67. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen X, Liu Y, Hsu YJ, et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo Y, Shen L, Cheng AS, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–50. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 43.Abbosh PH, Montgomery JS, Starkey JA, et al. Dominant-negative histone H3 lysine 27 mutant derepresses silenced tumor suppressor genes and reverses the drug-resistant phenotype in cancer cells. Cancer Res. 2006;66:5582–91. doi: 10.1158/0008-5472.CAN-05-3575. [DOI] [PubMed] [Google Scholar]
- 44.Wei Y, Xia W, Zhang Z, et al. Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Mol Carcinog. 2008;47:701–6. doi: 10.1002/mc.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang Y, Zhang X, Horton JR, et al. Structural basis for G9a-like protein lysine methyltransferase inhibition by BIX-01294. Nat Struct Mol Biol. 2009;16:312–17. doi: 10.1038/nsmb.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan J, Yang X, Zhuang L, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–63. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.