Abstract
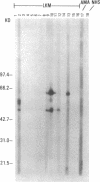
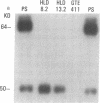
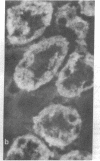
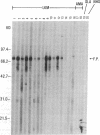
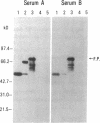
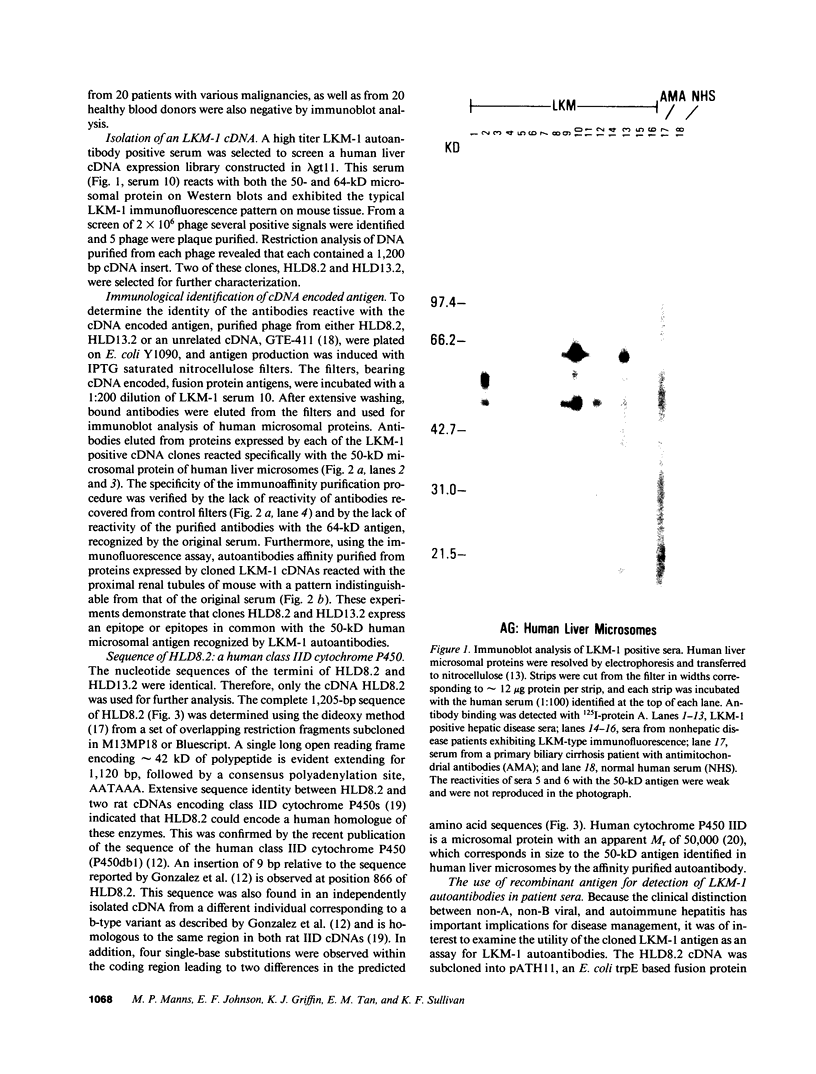
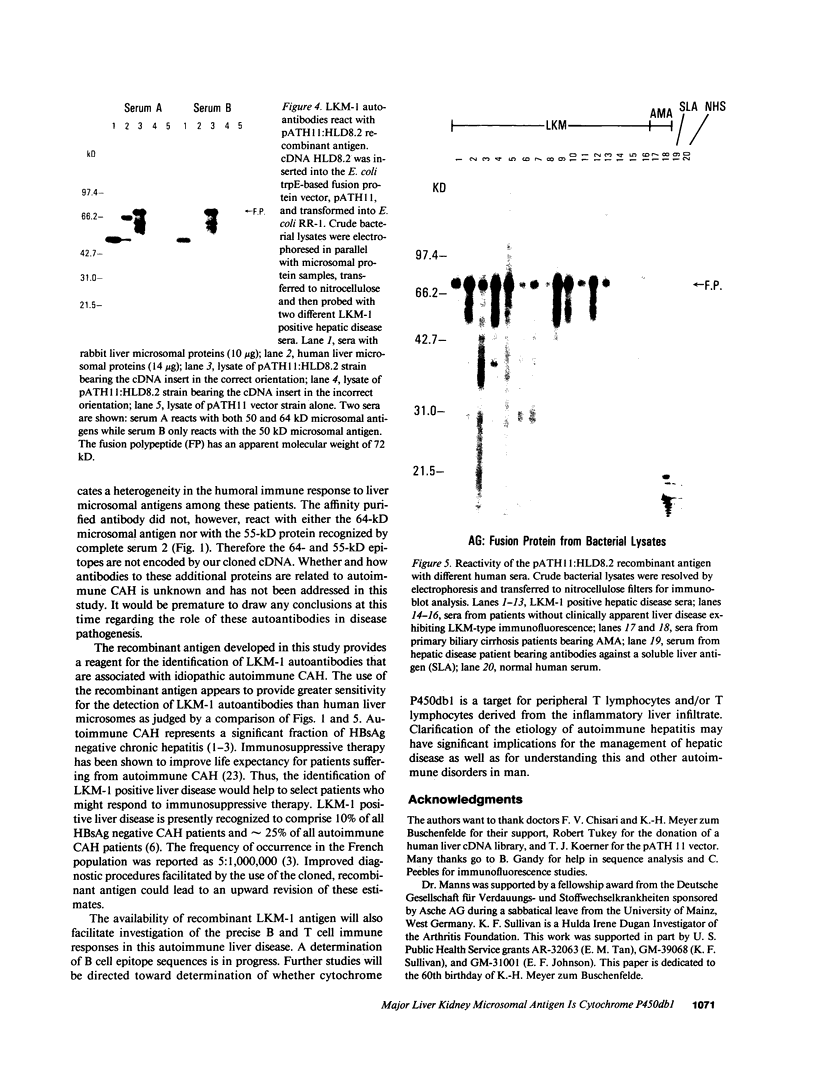
Type 1, liver kidney microsomal autoantibodies (LKM-1) are associated with a subgroup of idiopathic autoimmune type, chronic active hepatitis (CAH). The antigenic specificity of LKM-1 autoantibodies from 13 patients was investigated by immunoblot analysis of human liver microsomal proteins. Polypeptides of 50, 55, and 64 kD were detected with these antisera. A high titer LKM-1 serum was selected to screen a human liver lambda gt11 cDNA expression library, resulting in the isolation of several complementary (c)DNA clones. Autoantibodies affinity purified from proteins expressed by two of the immunopositive cDNA clones, HLD8.2 and HLD13.2, specifically react with a 50-kD protein of human liver microsomes and display immunofluorescence staining of the proximal renal tubular epithelia characteristic of LKM-1 sera. Determination of the sequence of HLD8.2 revealed that it encodes a recently described cytochrome P450db1. A bacterial fusion protein constructed from HLD8.2 proved to be a specific and sensitive diagnostic reagent. All sera from patients with LKM-1 positive liver disease react with this fusion protein. No reaction was seen, however, for sera from patients with other types of autoimmune liver diseases, viral hepatitis, systemic immunological disorders, or healthy controls.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez F., Bernard O., Homberg J. C., Kreibich G. Anti-liver-kidney microsome antibody recognizes a 50,000 molecular weight protein of the endoplasmic reticulum. J Exp Med. 1985 May 1;161(5):1231–1236. doi: 10.1084/jem.161.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaune P., Dansette P. M., Mansuy D., Kiffel L., Finck M., Amar C., Leroux J. P., Homberg J. C. Human anti-endoplasmic reticulum autoantibodies appearing in a drug-induced hepatitis are directed against a human liver cytochrome P-450 that hydroxylates the drug. Proc Natl Acad Sci U S A. 1987 Jan;84(2):551–555. doi: 10.1073/pnas.84.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWLING D. C., MACKAY I. R., TAFT L. I. Lupoid hepatitis. Lancet. 1956 Dec 29;271(6957):1323–1326. doi: 10.1016/s0140-6736(56)91483-0. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Tan E. M. Human autoantibody-reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987 Dec 1;166(6):1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Matsunaga T., Nagata K., Meyer U. A., Nebert D. W., Pastewka J., Kozak C. A., Gillette J., Gelboin H. V., Hardwick J. P. Debrisoquine 4-hydroxylase: characterization of a new P450 gene subfamily, regulation, chromosomal mapping, and molecular analysis of the DA rat polymorphism. DNA. 1987 Apr;6(2):149–161. doi: 10.1089/dna.1987.6.149. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Skoda R. C., Kimura S., Umeno M., Zanger U. M., Nebert D. W., Gelboin H. V., Hardwick J. P., Meyer U. A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988 Feb 4;331(6155):442–446. doi: 10.1038/331442a0. [DOI] [PubMed] [Google Scholar]
- Gueguen M., Meunier-Rotival M., Bernard O., Alvarez F. Anti-liver kidney microsome antibody recognizes a cytochrome P450 from the IID subfamily. J Exp Med. 1988 Aug 1;168(2):801–806. doi: 10.1084/jem.168.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut J., Catin T., Dayer P., Kronbach T., Zanger U., Meyer U. A. Debrisoquine/sparteine-type polymorphism of drug oxidation. Purification and characterization of two functionally different human liver cytochrome P-450 isozymes involved in impaired hydroxylation of the prototype substrate bufuralol. J Biol Chem. 1986 Sep 5;261(25):11734–11743. [PubMed] [Google Scholar]
- Homberg J. C., Abuaf N., Bernard O., Islam S., Alvarez F., Khalil S. H., Poupon R., Darnis F., Lévy V. G., Grippon P. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of "autoimmune" hepatitis. Hepatology. 1987 Nov-Dec;7(6):1333–1339. doi: 10.1002/hep.1840070626. [DOI] [PubMed] [Google Scholar]
- Idle J. R., Smith R. L. Polymorphisms of oxidation at carbon centers of drugs and their clinical significance. Drug Metab Rev. 1979;9(2):301–317. doi: 10.3109/03602537908993896. [DOI] [PubMed] [Google Scholar]
- Maddrey W. C. Subdivisions of idiopathic autoimmune chronic active hepatitis. Hepatology. 1987 Nov-Dec;7(6):1372–1375. doi: 10.1002/hep.1840070631. [DOI] [PubMed] [Google Scholar]
- Manns M., Gerken G., Kyriatsoulis A., Staritz M., Meyer zum Büschenfelde K. H. Characterisation of a new subgroup of autoimmune chronic active hepatitis by autoantibodies against a soluble liver antigen. Lancet. 1987 Feb 7;1(8528):292–294. doi: 10.1016/s0140-6736(87)92024-1. [DOI] [PubMed] [Google Scholar]
- Manns M., Meyer zum Büschenfelde K. H., Slusarczyk J., Dienes H. P. Detection of liver-kidney microsomal autoantibodies by radioimmunoassay and their relation to anti-mitochondrial antibodies in inflammatory liver diseases. Clin Exp Immunol. 1984 Sep;57(3):600–608. [PMC free article] [PubMed] [Google Scholar]
- Meier U. T., Meyer U. A. Genetic polymorphism of human cytochrome P-450 (S)-mephenytoin 4-hydroxylase. Studies with human autoantibodies suggest a functionally altered cytochrome P-450 isozyme as cause of the genetic deficiency. Biochemistry. 1987 Dec 15;26(25):8466–8474. doi: 10.1021/bi00399a065. [DOI] [PubMed] [Google Scholar]
- Odièvre M., Maggiore G., Homberg J. C., Saadoun F., Couroucé A. M., Yvart J., Hadchouel M., Alagille D. Seroimmunologic classification of chronic hepatitis in 57 children. Hepatology. 1983 May-Jun;3(3):407–409. doi: 10.1002/hep.1840030320. [DOI] [PubMed] [Google Scholar]
- Rizzetto M., Swana G., Doniach D. Microsomal antibodies in active chronic hepatitis and other disorders. Clin Exp Immunol. 1973 Nov;15(3):331–344. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schalm S. W. Treatment of chronic active hepatitis. Liver. 1982 Jun;2(2):69–76. doi: 10.1111/j.1600-0676.1982.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Schwab G. E., Raucy J. L., Johnson E. F. Modulation of rabbit and human hepatic cytochrome P-450-catalyzed steroid hydroxylations by alpha-naphthoflavone. Mol Pharmacol. 1988 May;33(5):493–499. [PubMed] [Google Scholar]
- Smith M. G., Williams R., Walker G., Rizzetto M., Doniach D. Hepatic disorders associated with liver-kidney microsomal antibodies. Br Med J. 1974 Apr 13;2(5910):80–84. doi: 10.1136/bmj.2.5910.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch W., Cossel L., Dargel R. The immunoelectron-microscopical demonstration of antibodies against endoplasmic reticulum (microsomes) in chronic aggressive hepatitis and liver cirrhosis. Immunology. 1977 Jun;32(6):941–945. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger U. M., Hauri H. P., Loeper J., Homberg J. C., Meyer U. A. Antibodies against human cytochrome P-450db1 in autoimmune hepatitis type II. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8256–8260. doi: 10.1073/pnas.85.21.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]