Abstract
During pressure-load hypertrophy of the adult heart in vivo, there is up-regulation of the mRNA encoding skeletal alpha-actin, the sarcomeric actin iso-mRNA characteristic of mature skeletal muscle and the fetal/neonatal heart. We have shown previously that during alpha 1-adrenergic receptor-stimulated hypertrophy of cultured rat heart myocytes, the induction of skeletal alpha-actin mRNA is greater than that of the mRNA encoding cardiac alpha-actin, the sarcomeric actin iso-mRNA characteristic of the adult heart. To determine if this actin iso-mRNA switch during cardiac hypertrophy reflects changes in the transcriptional status of the myocyte nucleus, we quantified the rate of transcription of actin mRNAs and total RNA, using an in vitro run-on transcription assay with nuclei isolated from the cultured myocytes after stimulation with norepinephrine (NE). Transcription of skeletal alpha-actin was increased at 3 h after NE, reached a maximum 6.1-fold increase at 12 h, and returned to the control level at 24 h. The EC50 for NE was 200 nM, and pharmacologic studies indicated alpha 1-receptor specificity. Transcription of cardiac alpha-actin was also increased rapidly by NE (maximum 4.6-fold vs. control at 3 h). However, cardiac alpha-actin transcription had returned to the control level at 6 h, when NE-stimulated skeletal alpha-actin transcription was still increasing. Transcription of the cytoskeletal (beta) actin gene was not changed significantly by NE treatment. Total RNA transcription was not increased until 6 h after NE (1.5-fold vs. control) and remained elevated through 24 h. Inhibition of protein synthesis did not attenuate NE-stimulated actin gene transcription. Thus the alpha 1-adrenoceptor mediates a rapid, transient, and selective increase in transcription of the sarcomeric actin isogenes during cardiac myocyte hypertrophy. Skeletal alpha-actin, the fetal/neonatal isogene, is induced preferentially to cardiac alpha-actin, the adult isogene. The different kinetics of actin isogene and total RNA transcription and the independence of transcription from protein synthesis suggest that transcriptional induction via the alpha 1 receptor is complex and may involve preexisting regulatory factors. These results are the first to demonstrate that the alpha 1-adrenergic receptor is a molecular mediator of transcriptional changes underlying an isogene switch that is known to be associated with cardiac myocyte hypertrophy.
Full text
PDF

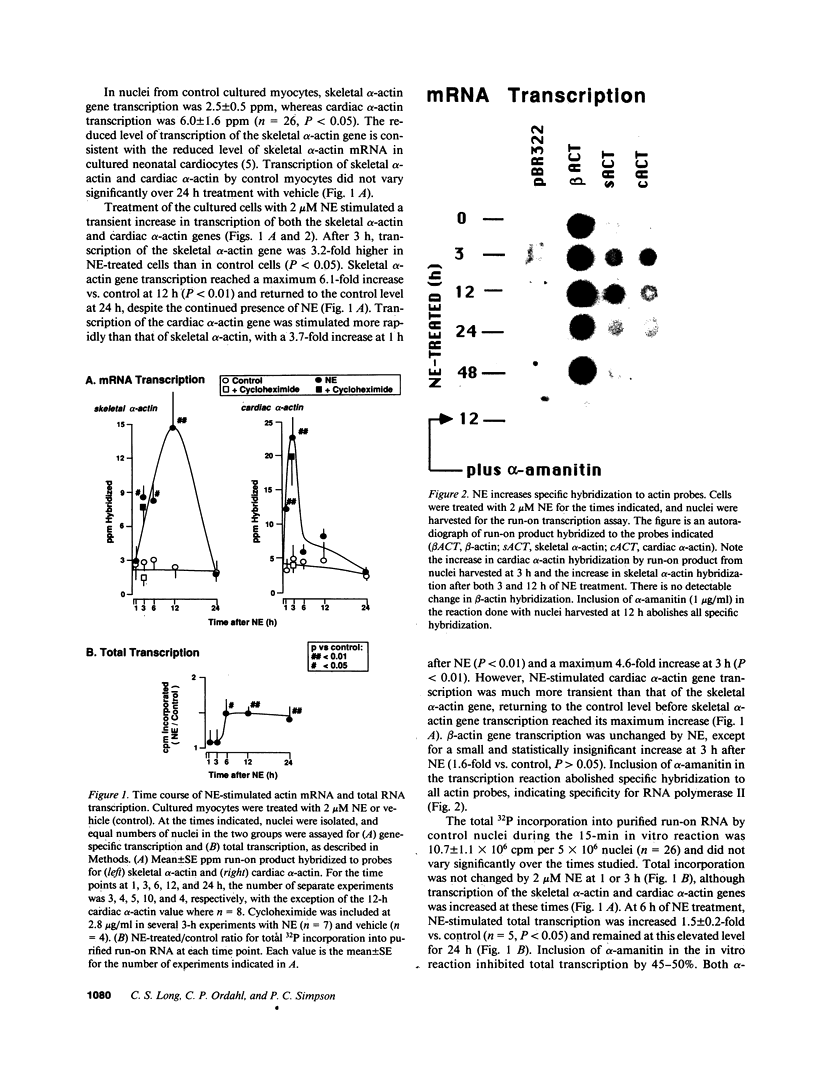
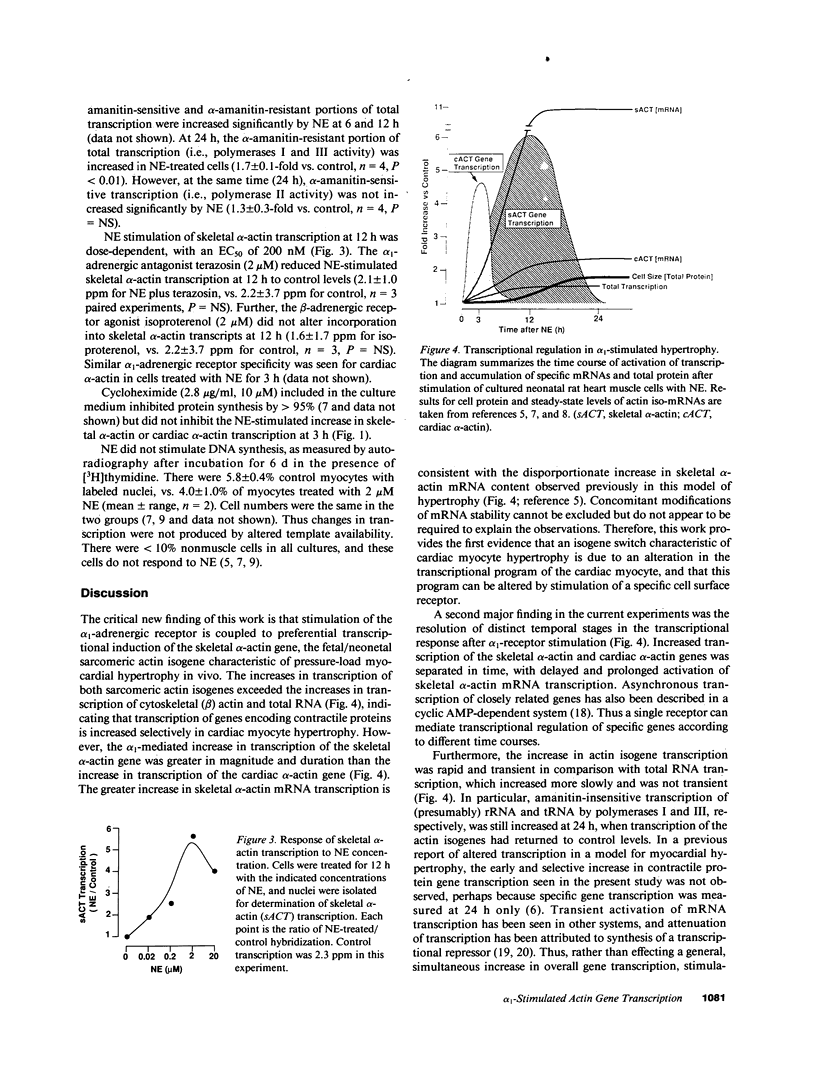

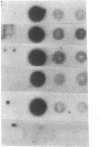
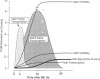
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishopric N. H., Simpson P. C., Ordahl C. P. Induction of the skeletal alpha-actin gene in alpha 1-adrenoceptor-mediated hypertrophy of rat cardiac myocytes. J Clin Invest. 1987 Oct;80(4):1194–1199. doi: 10.1172/JCI113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Blau H., Kedes L. alpha-skeletal and alpha-cardiac actin genes are coexpressed in adult human skeletal muscle and heart. Mol Cell Biol. 1983 Nov;3(11):1985–1995. doi: 10.1128/mcb.3.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Larner A. C., Chaudhuri A., Darnell J. E., Jr Transcriptional induction by interferon. New protein(s) determine the extent and length of the induction. J Biol Chem. 1986 Jan 5;261(1):453–459. [PubMed] [Google Scholar]
- Lee H. R., Henderson S. A., Reynolds R., Dunnmon P., Yuan D., Chien K. R. Alpha 1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells. Effects on myosin light chain-2 gene expression. J Biol Chem. 1988 May 25;263(15):7352–7358. [PubMed] [Google Scholar]
- Long C. S., Ordahl C. P. Transcriptional repression of an embryo-specific muscle gene. Dev Biol. 1988 May;127(1):228–234. doi: 10.1016/0012-1606(88)90205-9. [DOI] [PubMed] [Google Scholar]
- Mayer Y., Czosnek H., Zeelon P. E., Yaffe D., Nudel U. Expression of the genes coding for the skeletal muscle and cardiac actions in the heart. Nucleic Acids Res. 1984 Jan 25;12(2):1087–1100. doi: 10.1093/nar/12.2.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Milsted A., Cox R. P., Nilson J. H. Cyclic AMP regulates transcription of the genes encoding human chorionic gonadotropin with different kinetics. DNA. 1987 Jun;6(3):213–219. doi: 10.1089/dna.1987.6.213. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Mulvihill E. R., Palmiter R. D. Relationship of nuclear estrogen receptor levels to induction of ovalbumin and conalbumin mRNA in chick oviduct. J Biol Chem. 1977 Mar 25;252(6):2060–2068. [PubMed] [Google Scholar]
- Murdoch G. H., Potter E., Nicolaisen A. K., Evans R. M., Rosenfeld M. G. Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature. 1982 Nov 11;300(5888):192–194. doi: 10.1038/300192a0. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P. The skeletal and cardiac alpha-actin genes are coexpressed in early embryonic striated muscle. Dev Biol. 1986 Oct;117(2):488–492. doi: 10.1016/0012-1606(86)90315-5. [DOI] [PubMed] [Google Scholar]
- Schwartz K., de la Bastie D., Bouveret P., Oliviéro P., Alonso S., Buckingham M. Alpha-skeletal muscle actin mRNA's accumulate in hypertrophied adult rat hearts. Circ Res. 1986 Nov;59(5):551–555. doi: 10.1161/01.res.59.5.551. [DOI] [PubMed] [Google Scholar]
- Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983 Aug;72(2):732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P., Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982 Jan;50(1):101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985 Jun;56(6):884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- Spindler S. R., Mellon S. H., Baxter J. D. Growth hormone gene transcription is regulated by thyroid and glucocorticoid hormones in cultured rat pituitary tumor cells. J Biol Chem. 1982 Oct 10;257(19):11627–11632. [PubMed] [Google Scholar]