Abstract
Conventional leather processing involving depilation of animal hide by lime and sulphide treatment generates considerable amounts of chemical waste causing severe environmental pollution. Enzymatic depilation is an environmentally friendly process and has been considered to be a viable alternative to the chemical depilation process. We isolated an extracellular protease from Pseudomonas aeruginosa strain MCM B-327 with high depilation activity using buffalo hide as a substrate. This 33 kDa protease generated a peptide mass fingerprint and de novo sequence that matched perfectly with LasB (elastase), of Pseudomonas aeruginosa. In support of this data a lasB mutant of MCM B-327 strain lacked depilatory activity and failed to produce LasB. LasB heterologously over-produced and purified from Escherichia coli also exhibited high depilating activity. Moreover, reintroduction of the lasB gene to the P. aeruginosa lasB mutant via a knock-in strategy also successfully restored depilation activity thus confirming the role of LasB as the depilating enzyme.
Introduction
Leather-making, is a by-product of the meat industry and reduces potential waste as well as contributing to economic growth [1]. Current leather-processing procedures generate a considerable amount of chemical waste during all stages of processing and cause serious environmental pollution [2]. In the conventional pre-tanning process, depilation of animal hide is done by employing lime and sulphide. These two chemicals alone account for 70% of the total pollution in terms of biological oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solids (TDS) and total suspended solids (TSS) [3]. The alkaline nature of tannery effluents and the high sulphide content pollute ground water sources and cause serious health problems to the tannery workers and people living in the vicinity of leather-processing industries [4], [5].
A number of attempts have been made to find alternative methods for depilation of animal hide. The use of microbial enzymes, especially extracellular proteases have proved to be highly effective in depilating of animal hides [6]. Though a number of bacterial and fungal strains are known to grow on hides, only a few of them have been shown to produce extracellular proteases with depilatory activity [7], [8], [9], [10], [11]. In principle, the proteases having high depilatory properties with mild or no collagenolytic activity are considered to be the best proteases for depilating animal hide [12], [13], [14], [15], [16].
Pseudomonas aeruginosa MCM B-327 isolated from vermicompost pit soil has been shown to produce a depilating protease with no significant collagenolytic activity and its potential use in depilating buffalo hide has been successfully demonstrated [17]. The use of strain MCMB-327 is restricted to large-scale production of the depilating protease since P. aeruginosa is an opportunistic pathogen. If the candidate gene coding for the depilating protease could be identified and expressed in any one of the GRAS (Generally Regarded As Safe) organisms, the recombinant enzyme could be safely used to substitute for chemical treatment in the leather industry. In this study, we report the cloning of a candidate gene coding for the depilatory protease and present evidence using gene knock-out and knock-in strategies, showing that the depilatory activity is due to the product of the lasB gene. Heterologously expressed and purified LasB and a variant secreted as an extracellular protein successfully depilated buffalo hide, showing its utility in the leather industry.
Materials and Methods
Media and bacterial growth conditions
The bacterial strains and plasmids used in this study are given in Table 1. Unless otherwise stated Pseudomonas aeruginosa MCM B-327 was grown on nutrient agar medium at 30°C. However, for optimal depilating enzyme activity P. aeruginosa MCM B-327 was grown on tryptone-soy medium (pH 7.0), which typically contains 1% (w/v) tryptone and 1% (w/v) soyabean meal. E. coli was grown in LB medium (pH 7.0) at 37°C. When required, the antibiotics ampicillin (100 µg/ml), kanamycin (50 µg/ml), gentamycin (10 µg/ml) and chloramphenicol (30 µg/ml) were added to the growth medium at the indicated concentration.
Table 1. Bacterial strains and plasmids.
| Strain/Plasmid | Genotype or phenotype | Reference |
| P. aeruginosa MCM B-327 | Amr, Cmr wild type strain | [17] |
| P. aeruginosa MCM B-327-B1 | Ampr, CmrGmr Derivative of wild type P. aeruginosa MCM B-327 strain generated by replacing lasB with lasB::gm | This work |
| P. aeruginosa MCM B-327-B2 | Ampr, Cmr,Gmr , Kmr, LasB negative mutant of P. aeruginosa MCM B-327-B1 with pMMBD | This work |
| E.coli DH5α | supE44 ΔlacU169 (ϕ80 lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi1 relA1 | [18] |
| pGEMT-Easy | Ampr, Cloning vector with 5′ T overhangs | Promega |
| pMMB206 | Cmr, Broad host range mobilzable vector | [19] |
| pMMB-Km | Kmr, broad host range expression vector created by replacing cmr gene with Kmr gene in pMMB206. | This work |
| pUC18 | Ampr, high copy number cloning vector | [20] |
| pSUP202 | Ampr, Cmr, Tcr, Mob+ | 41 |
| pGEMD | Ampr, 2 kb lasB fragment cloned in pGEMT-Easy vector. | This work |
| pGEMD153 | Ampr, 2 kb lasB mutant coding for LasB T-153I in pGEMT-Easy vector | This work |
| pMMBD | Kmr, 2 kb lasB cloned in pMMB-Km vector. | This work |
| pUCD | Ampr, 2 kb lasB cloned in pUC18 vector as an EcoRI fragment. | This work |
| pUCDG | Ampr, Gmr , lasB::gm cloned in pUC18 | This work |
| pSUPDG | Ampr, Gmr,Tetr, lasB::gm cloned in pSUP202 as an EcoRI fragment. | This work |
DNA manipulations
All plasmids and genomic DNA used in this study were isolated using Qiagen miniprep / DNAeasy kits following the manufacturer's protocols. Restriction enzymes and T4 DNA ligase were purchased from MBI Fermentas. Routine DNA manipulations were performed following standard procedures described elsewhere [22].
Production of protease
Pseudomonas aeruginosa MCM B-327 was grown in tryptone-soyabean medium (pH 7.0) for 72 h at 30°C with rotary shaking at 200 rpm. After incubation, the culture was centrifuged at 15000g for 20 min to remove cells. The spent medium was saturated with 60% ammonium sulphate. Ammonium sulphate precipitate was dissolved in 50 mM Tris buffer (pH 8.0) and dialysed against five liters of the same buffer.
Protease assay
The protease activity was determined by following standard protocols with slight modifications [23]. To measure protease activity in the spent medium, the medium was mixed in 1∶ 4 ratio with freshly prepared casein (0.625% w/v) solution and incubated at 37°C for 30 min. The reaction was stopped by addition of 5 ml of 5% (w/v) trichloroacetic acid and the protease activity was measured by determining the rate of enzymatic hydrolysis of casein by the Folin Ciocalteu method [24], [25]. One unit of protease activity was defined as the amount of enzyme required to liberate 1 µg of tyrosine per min at 37°C.
Casein agar plate assay
Secretion of extracellular protease was monitored by performing a casein-agar plate assay. Colonies to be tested for their ability to secrete extracellular protease were streaked on casein containing agar plates prepared by using autoclaved casein agar solution (1.5% w/v), or skimmed milk powder and agar (2%) in Tris-HCl buffer (50 mM, pH 7.0). After streaking, the plates were incubated at 30°C and were frequently monitored for the formation of a clear zone due to hydrolysis of the casein. If a clear zone was formed within 6 h of incubation it was taken as an indication of the secretion of extracellular protease.
Assay of depilating activity
The depilating activity of the extracellular protease was tested on a fresh animal hide obtained from a local slaughterhouse. Initially the thoroughly washed fresh animal hide was cut into 2 cm2 pieces and soaked with 50 mM Tris-Cl (pH 8.0) after placing them separately in a sterile petri-dish. The plates were then incubated for 16 h after applying 200 U of enzyme uniformly to the animal hide. After incubation, the hair from the hide was scraped with a sterile spatula to monitor depilating activity. The hide piece treated with 50 mM Tris buffer (pH 8.0) instead of protease served as negative control.
Development of a zymogram assay on native PAGE
About 200 µg of total ammonium sulfate-precipitated protein was resuspended in sample buffer (50 mM Tris, pH 8.0, 5% w/v sucrose, 0.1% w/v bromophenol blue) before loading a portion on two identical native 7.5% polyacrylamide gels [26]. After electrophoresis, one of the gels was stained with Coomassie Brilliant Blue R-250 and the other was used for developing a zymogram. The unstained gel was soaked in 1% (w/v) casein solution for 1 h and thoroughly washed with distilled water before staining with Coomassie Brilliant Blue R-250. The gel was then destained and clear zones generated due to proteolytic activity were documented by scanning using an Imagescanner (GE Healthcare).
Purification of depilating enzyme
The dialyzed ammonium sulphate precipitate was loaded on a manually packed DEAE sepharose column equilibrated with 50 mM Tris-Cl (pH 8.0) containing 0.18 M NaCl. The protein was eluted at a flow-rate of 0.5 ml/min with a linear gradient of 1 M NaCl in 50 mM Tris buffer (pH 8.0). All fractions obtained from DEAE chromatography and the flow-through were tested for depilating activity. The active fractions were analyzed by SDS-PAGE [27].
Gel Permeation Chromatography
The protein found in the active fractions was concentrated by re-precipitating with ammonium sulphate (60% saturation) and was dissolved in 5ml of 50 mM Tris buffer (pH 8.0) and loaded onto a manually packed Sephacryl 200 HR column (XK 16/100) fitted to an AKTA basic FPLC system (GE Healthcare). The column was equilibrated with 7 column volumes of 50 mM Tris buffer (pH 8.0) and chromatography was performed at a flow-rate of 0.5 ml/min. The fractions collected were tested for depilatory activity and the active fractions were analyzed on SDS-PAGE [27].
Two Dimensional gel electrophoresis
All chemicals and IPG strips used for two-dimensional (2D) electrophoresis were obtained from GE Healthcare, USA. 100 µg of pure depilating enzyme obtained from gel permeation chromatography was precipitated with methanol-chloroform (4∶1) and dissolved in 350 µl of sample buffer (7 M urea, 4% (w/v) CHAPS, 2% (v/v) pharmalyte, 40 mM DTT) before loading on IPG strips (18 cm, pH 3–10). The strips were then actively rehydrated for 12 h at 20 V and IEF was performed on an Ettan IPGphor3 system using a four-step programme [500 V for 30 min (gradient), 500 V for 30 min (step), 8000 V for 3 h (gradient); and electrophoresis was continued for 40,000 Vh]. After focusing, strips were equilibrated with equilibration buffer I (75 mM Tris, 6 M urea, 2% (w/v) SDS, 20% (v/v) glycerol, 2% (w/v) DTT) and then with equilibration buffer II (75 mM Tris, 6 M urea, 2%(w/v) SDS, 20%(v/v) glycerol, 2% (w/v) idoacetamide) each for 15 min. The second dimension electrophoresis was performed on 12.5% (w/v) denaturing polyacrylamide gels in an Ettan DALTsix system (GE Healthcare) at a constant voltage of 200 V. The gels were stained with Coomassie Brilliant Blue R-250 and results were recorded by scanning the stained gel before picking the spots.
MALDI-MS
The protein spots were processed and digested with trypsin following procedures described elsewhere [28]. The tryptic peptides were dissolved in 2 µl of 50% (v/v) acetonitrile (ACN) containing 1% (w/v) trifluoroacetic acid (TFA) and mixed with 2 µl of 1% (w/v) cyano-4-hydroxycinnamic acid (HCCA) dissolved in 50% (w/v) ACN and 1% (w/v) TFA. Finally, 1 µl tryptic peptides were mixed with 1% (w/v) HCCA was applied to the MALDI target plate. Peptides were analyzed using MALDI-TOF-TOF Autoflex (Bruker Daltonics) in reflectron mode. MS/MS of selected peptides was performed by LIFT. The spectra were calibrated by Pepmix (Bruker Daltonics).
Protein Identification
The spectral data were analyzed using Biotools software and searches were performed for protein identification using the MASCOT search engine (www.matrixscience.com) against the Swiss-Prot (http://www.expasy.ch/sprot) and the NCBInr (http://www.ncbi.nlm.nih.gov/) databases. The following search parameters were used: trypsin is the enzyme used and one missed cleavage was allowed, the peptide tolerance was set at ±0.5 Da, carbamidomethylation and oxidized methionine were set as fixed and variable modifications, respectively. MS/MS data was analyzed using Biotools software and a mass tolerance of ±0.2 Da was used.
Amplification and cloning of the gene encoding the depilating protease
The sequence of the lasB gene along with its upstream region was taken from the Pseudomonas genome database (www.pseudomonas.com) and primers DHEF (5′-CTAGCTGCCACCTGCTTTTC-3′) and DHER (5′-TGAACTTTAGACCGGGTTCG -3′) were designed using the Primer3 software (http://frodo.wi.mit.edu/). PCR was performed using the genomic DNA of P. aeruginosa MCM B-327 as the template. The conditions used were 95°C for 5 min, followed by 30 cycles of 95°C for 1 min, 56°C for 1 min and 72°C for 2 min and an extension at 72°C for 20 min. The 1.5 kb amplicon was cloned into the pGEMT-Easy vector (Promega) and recombinant clones were confirmed by restriction digestion followed by DNA sequencing.
Generation of lasB mutants of P. aeruginosa MCM B-327
The complete lasB gene was isolated as an EcoRI fragment and ligated into pUC18 digested with EcoRI to obtain pUCD. In recombinant plasmid pUCD, there is an unique NotI site in the coding sequence of lasB. In order to generate insertionally inactivated lasB (lasb::gen) the gentamycin gene was obtained as NotI fragment from plasmid pGEN150 (our unpublished work) and ligated into a NotI digested pUCD plasmid. The lasB::gm gene was isolated as an EcoRI fragment and ligated into the mobilizable suicide vector pSUP202 previously digested with EcoRI to obtain pSUPDG. E.coli S17-1 harbouring pSUPDG was used as donor to mobilize lasB::gm into P. aeruginosa. Conjugation was performed using standard procedures [29]. Exconjugants were selected on plates containing Km (50 µg/ml) and Gm (10 µg/ml). The exconjugants were then screened for proteolytic activity by plating them on casein-agar plates. Replacement of lasB with lasB::gm through homologous recombination was confirmed by performing PCR using lasB-specific primers.
Expression of LasB variant
A mutant of lasB encoding a LasB variant in which the threonine at amino acid position 153 was substituted with isoleucine was generated by PCR mutagenesis [30]. Plasmid pGEMD was used as template, the forward primer (5′-CCGGTCATCTTGCAAGCCGCGGTC-3′) contained the desired mutation and the reverse primer (5′-GACCGCGGCTTGCAAGATGACC GG-3′) was complimentary to the forward primer. The PCR reaction was performed for 18 cycles following procedures described elsewhere [30]. The PCR product was then digested with DpnI before transforming plasmids into E. coli DH5α. The colonies that appeared on an ampicillin plate were replica-plated onto casein-agar plates to identify colonies that gave a clear zone due to production of extracellular LasB. Plasmids isolated from these colonies were sequenced to confirm the presence of the desired mutation in lasB gene.
Results
In P. aeruginosa MCM B-327, secretion of the depilating enzyme initiated only in early stationary phase (after 48 h of incubation) and its accumulation in spent medium showed a steady increase till the culture reached the late stationary phase (72 h of incubation). Further incubation had no influence on production of the depilating activity [17]. Interestingly, along with the depilating enzyme activity a bluish-green pigment accumulated in the medium, which turned brown on further incubation giving a greenish-brown appearance to the spent medium.
The depilating enzyme is an extracellular protease
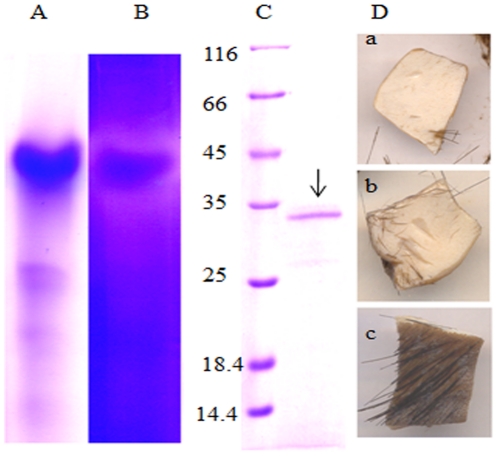
The spent medium collected from a culture grown for 72 h was initially brought to various levels of saturation with ammonium sulphate. The proteins precipitated during each stage of saturation were independently tested for both protease and depilating activity. Only the protein precipitate obtained from the spent medium saturated to 60% ammonium sulphate showed the presence of a protease with depilating activity. A zymogram developed for the proteins found in this fraction revealed the existence of a single clear zone around a major protein band (Figure 1 A & B). No other clear zones were seen in the entire zymogram, indicating the existence of a single protease complex in the 60% ammonium sulphate precipitate. To gain further insights into the depilating activity the protein band tested to be protease-positive in the zymogram was electro-eluted from the native gel and was applied to a fresh buffalo hide. The electro-eluted protein gave a single band of 33 kDa on SDS-PAGE and successfully depilated animal hide providing direct evidence for the depilatory properties of the extracellular protease (Figure 1C & D).
Figure 1. Identification of depilating protease of Pseudomonas aeruginosa MCM B-327.
Panel A and B represent native PAGE and the corresponding zymogram of extracellular proteins of Pseudomonas aeruginosa MCM B-327. Panel C. SDS-PAGE showing the molecular mass of the depilating protease electro-eluted from the zymogram. Lane 1 represents protein molecular mass markers. The 33 kDa depilating protease band found in lane 2 is shown with an arrow. Depilating activity shown by crude extracellular protease (a), protease electro-eluted from the zymogram (b) and a control hide kept by adding buffer instead of protease are shown in panel D.
Purification of the depilating protease
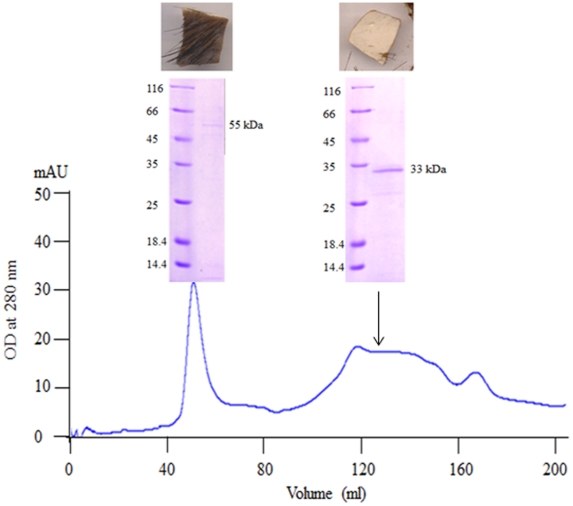
After identifying the depilating enzyme as an extracellular protease of 33 kDa, further experiments were done to establish its identity. Initially the extracellular protease was purified using conventional ion-exchange chromatography. When loaded onto a DEAE anion-exchange column most of the depilatory protease activity was found in the flow through. However, the impurities that contributed to the brown colour in the culture filtrate and other proteins with no protease activity bound to the DEAE column. Therefore, the depilatory protease activity found in the flow-through, along with other impurities, was concentrated by precipitating with ammonium sulphate (60%). The proteins found in the precipitate were then separated on a Sephacryl S-200 gel-filtration column to achieve further purification. After chromatography, two major peaks were obtained and they were designated as peak-I and peak-II (Figure 2). When proteins found in these peaks were analyzed on SDS-PAGE, a 54 and 33 kDa proteins were found in peak I and peak II, respectively. Further, proteins found in both peak-I and peak-II were independently tested to determine which one of these two peaks possessed depilatory activity. The 54 kDa protein obtained from peak-I showed no depilatory activity, whereas the 33 kDa protein found in peak-II showed a strong depilatory activity. The size of the protein matched the size of the depilating protease electro-eluted from the zymogram (Figure 1C) providing primary evidence to show that the purified protein was the depilating protease that gave a clear zone in the zymogram.
Figure 2. Purification of extracellular depilating protease from Pseudomonas aeruginosa MCM B-327: Peak I and II show elution of 55 kDa and 33 kDa proteins from the gel filtration column.
Depilating activity is found only with 33 kDa protein.
Identification of the depilating protease
The purified, depilating enzyme was subjected to two-dimensional PAGE to determine the purity of the depilating protease. Three closely associated protein spots were found on 2D gels in the 33 kDa range (Figure S1, panel A). All three closely associated spots were observed in the pI range of 6.5–7.0. In order to identify these polypeptides each spot was manually excised and subjected to trypsin digestion. MALDI-TOF and MALDI-TOF-TOF were performed to obtain peptide mass fingerprint (PMF) (Figure S1, panel B) and MS/MS data (Figure S1, panels C–F). All three spots showed identical PMF profiles suggesting that the three closely associated spots on 2D gels were products of the same gene. The observed differences in pI values of the protein spots might be due to posttranslational modifications. Further, when the peptide mass profiles were used to search the protein database significant scores were obtained for elastase, the product of the lasB gene of P. aeruginosa. The MS/MS data obtained for four prominent m/z peaks showed significant scores for LasB (Figure S1, panels C–F), further confirms that the depilating protease as the product of the lasB gene of P. aeruginosa MCM B-327.
The depilating protease is the product of the lasB gene
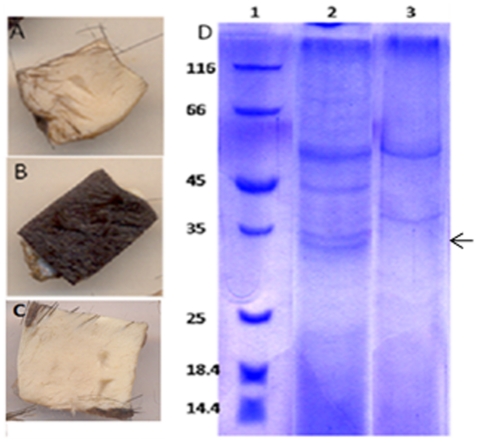
After establishing the identity of the polypeptides, a parallel approach was taken to prove the role of the lasB gene product in depilation of animal hide. The first approach was to test if the depilating protease is produced by the lasB knockout strain of P. aeruginosa MCM B-327, while the second one was to demonstrate depilating activity with the heterologously produced LasB. The lasB mutant was generated by introducing the insertionally inactivated lasB into P. aeruginosa MCM B-327 as described in the methods section [21]. In most of the gentamycin-resistant exconjugants, the wild-type lasB gene was replaced with the lasB::gm insertion through homologous recombination. PCR amplification using lasB-specific primers gave an amplicon whose size was similar to that of lasB::gm (data not shown). The lasB mutant was then used to check if it produced depilating activity. As expected, in the lasB mutant, neither depilating activity nor protease activity could be detected in the spent medium (Figure 3B). Further, no 33 kDa polypeptide corresponding to the extracellular protease was seen on SDS-PAGE confirming that the lasB mutant of P. aeruginosa failed to produce the depilating protease (Figure 3D).
Figure 3. Depilating of animal hide by extracellular proteins obtained from P.aeruginosa MCM B-327 (panel A) P.aeruginosa MCM B-327-B1 (panel B) and P.aeruginosa MCM B-327-B2 (panel C).
Extracellular protein profile of P. aeruginosa MCM B-327 (lane 2) and knockout strain (lane 3) is shown in panel D. Absence of the 33 kD LasB is shown with an arrow.
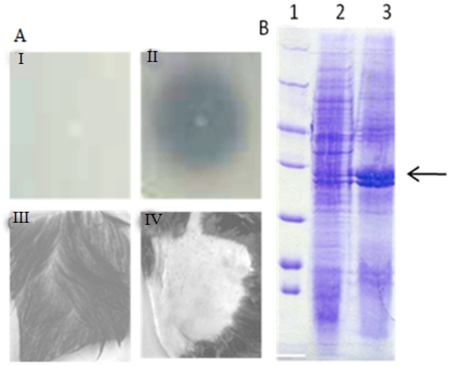
To test for gain-of-function, E. coli DH5α was transformed with plasmid pGEMD in which the lasB gene of P. aeruginosa MCM B-327 was expressed from its own promoter. Growth of these cultures was very slow and our attempts to achieve maximum growth by prolonged incubation of the culture resulted in cell lysis (data not shown). The cell lysate prepared from these cultures had a significant amount of caseinolytic and depilating activities, suggesting successful expression of active lasB in E. coli (pGEMD) (data not shown). However, when these cultures were plated on casein-agar no clear zone was observed indicating that LasB was not excreted as an extracellular protein (Figure 4A (I)). Previous studies revealed that introduction of a Thr153Ile substitution resulted in secretion of LasB in E. coli as an extracellular protein [31]. Therefore, we generated such a variant by substituting threonine at amino acid position 153 with isoleucine (see methods section). The E.coli cells expressing the LasB variant, as determined by extracellular caseinolytic activity, produced a significant amount of extracellular LasB (Figure 4A (II) & 4B). When tested, the LasB variant successfully depilated animal hide at a level comparable with the wild-type LasB produced by Pseudomonas aeruginosa MCM B-327 (Figure 4A (IV)).
Figure 4. Panel A shows caseinolytic activity of (I) E.coli DH5α (pGEMD) and (II) E.coli DH5α (pGEMD153) and depilating activity of extracellular proteins produced by E. coli DH5α (pGEMD) (III) and E. coli DH5α (pGEMD153) (IV) Panel B shows SDS-PAGE profile of extracellular proteins of E.coli DH5α (lane 1) and E.coli DH5α (pGEMD153) (lane 2).
The loss of depilating activity in the lasB mutant of P. aeruginosa MCM B-327 and the gain of depilating function by an E. coli strain, which otherwise produce no depilating proteases, provide substantial evidence for the involvement of LasB in depilating animal hide. In order to provide final confirmation, a lasB knock-in strain, P. aeruginosa MCM B-327-B2 was generated by mobilizing the lasB gene cloned in a broad-host range vector into the lasB mutant B-327-B1 [29]. The knock-in strain of P. aeruginosa MCM B-327-B2 restored both production of extracellular LasB and depilating activity providing strong evidence to show that LasB is responsible for the depilation of animal hide in Pseudomonas aeruginosa MCM B-327 (Figure 3C).
Discussion
Protein secretion in prokaryotes, especially in pathogenic P. aeruginosa has attracted attention of a number of investigators working to understand host-pathogen interactions [32], [33], [34]. According to an in silico prediction, nearly 19.4% of the total proteome is exported into the extracellular milieu by using various protein secretion pathways. Among the extracellular proteins, proteases play a predominant role in colonizing the host after infection [35]. In P. aeruginosa, LasB represents nearly 60% of the extracellular protein and generates multiple forms during the course of its maturation [36], [37]. Moreover, lasB is one of the virulence factors whose expression is regulated by a quorum-sensing signal molecule N-acyl homoserine lactone [38], [39].
Extracellular proteases of P. aeruginosa have been used in a number of industrial applications [40], [41]. They are given different names based on the assay substrates used for monitoring of activity [42], [43], [44]. Recent studies have successfully demonstrated use of extracellular protease in degradation of feather waste generated from the poultry industry [45]. Our earlier studies have clearly shown involvement of an extracellular protease of P. aeruginosa MCM B-327 in depilating animal hide without causing any damage to the leather [17]. The present study has identified the extracellular protease as the product of lasB. The lasB knock-out and knock-in strains of P. aeruginosa have shown convincingly that the depilating protease is LasB. In the light of these studies we have revisited the work done by Lin and his coworkers [18]. The authors have successfully shown involvement of an extracellular protease of P. aeruginosa, designated as keratinase, in solubilization of feather waste from poultry industry. The sequence of the corresponding gene presented by the authors was aligned with the sequence of LasB reported in this study. Interestingly, 100% similarity was seen between these two proteins suggesting that keratinase reported by them is LasB [42]. The N-terminal sequence of the mature protein (AEAGGPGG), the signal peptide and the pro-peptide coincide perfectly with the LasB sequence reported in the present study.
The quorum-sensing signaling mechanism is known to influence expression of genes that affect cellular physiology in a number of ways. LasB expression has been shown to be under the control of 3OC12-HSL [46]. Binding of the 3OC12-HSL-LasR complex to the sites designated as OP1 and OP2 boxes found upstream of the lasB promoter were shown to influence positively its expression during early and late stationary phase [47]. This lasB expression pattern coincides with our observation, where accumulation of LasB was shown in the spent medium collected from early and late stationary phases. This evidence further supports our observation that the depilating enzyme is LasB.
The results of heterologous expression studies presented here differ slightly from the published reports, where LasB is shown expressed as an active extracellular protein [43], [48]. In our experience, the extracellular LasB produced in Pichia pastoris was found to be inactive. Further, certain recent studies have shown expression of LasB in E.coli as an extracellular protein [42], [44]. In our experience, the lasB gene cloned in pGEMT-Easy has produced considerable amount of LasB. However, most of it accumulated inside the cells causing growth inhibition and subsequent cell lysis. In our study, only the LasB variant, LasB T153I was secreted effectively into the spent medium (Figure 4B). There is no significant difference in the depilating activity of LasB produced either from the wild-type strain of P. aeruginosa MCM B-327 or from E. coli. Both LasB and its variant have shown identical depilating properties. As the LasB variant was secreted as an extracellular protein in E. coli it can be successfully used for large-scale production and subsequent application in the leather-processing industry.
Supporting Information
Analysis of depilating protease of Pseudomonas aeruginosa MCM B-327 by TwoD electrophoresis (Panel A). Peptide mass fingerprint (PMF) of depilating protease is shown in panel B. Panels C to F represent MS/MS profiles of peaks with m/z values of 1167.604, 1610.825, 2082.871 and 2560.216, respectively.
(TIF)
Acknowledgments
We thank Dr. Gary Sawers for reading the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by CSIR- NMITLI, New Delhi through the grant 5/258/13A/2005-NMITLI to DS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Germann HP. 1999. The ecology of leather production – Present state and development trends; Proceedings of the XXV IULTCS Congress: Chennai.
- 2.Ludvik J. The scope for decreasing pollution load in leather processing. Vienna: United Nations Industrial Development Organization. US/RAS/92/120/11-51; 2000. [Google Scholar]
- 3.Marsal A, Cot J, Boza EG, Celma PJ, Manich AM. Oxidizing unhairing process with hair recovery. Part I. experiments on the prior hair immunization. J Soc Leather Technol Chem. 1999;83:310–315. [Google Scholar]
- 4.Ramasami T, Rao JR, Chandrababu NK, Parthasarathi K, Rao PG, et al. Beamhouse and tanning operations: process chemistry revisited. J Soc Leather Technol Chem. 1999;83:39–45. [Google Scholar]
- 5.Ramasami T. Emerging leather processing strategies for waste minimization. . In: Buljan J, editor. Background information and cleaner technologies in raw material preservation and in the Beamhouse processes. UNIDO; 1998. pp. 183–197. [Google Scholar]
- 6.Puvanakrishnan R, Dhar SC. Enzymes in Dehairing. Chennai, India: NICLAI Publication; 1988. Enzyme technology in beamhouse practice, pp. 92–120. [Google Scholar]
- 7.Yates JR. Studies in depilation. II. Structural changes in the wool follicle during bacterial wool loosening (‘sweating’). Australian J Biol Sci. 1968;21:361–374. doi: 10.1071/bi9681249. [DOI] [PubMed] [Google Scholar]
- 8.Yates JR. Studies in depilation. part X. the mechanism of the enzyme depilation process. J Soc Leather Technol Chem. 1972;56:158–175. [Google Scholar]
- 9.Mukhopadhyay RP, Chandra AL. Protease of a keratinolytic Streptomycete to unhair goat skin. Ind J of Expt Biol. 1993;31:557–558. [PubMed] [Google Scholar]
- 10.Nilegaonkar SS, Zambare VP, Kanekar PP, Dhakephalkar PK, Sarnaik SS. Production and partial characterization of dehairing protease from Bacillus cereus MCM B-326. Biores Technol. 2007;98:1238–1245. doi: 10.1016/j.biortech.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Zambare VP, Nilegaonkar SS, Kanekar PP. Production of an alkaline protease by Bacillus cereus MCM B-326 and its application as a dehairing agent. World J Microbiol Biotechnol. 2007;23:1569–1574. [Google Scholar]
- 12.Anbu P, Gopinath SCB, Hilda A, Lakshmi Priya T, Annadurai G. Purification of keratinase from poultry farm isolate-Scopulariopsis brevicaulis and statistical optimization of enzyme activity. Enzyme Microb Technol. 2005;36:639–647. [Google Scholar]
- 13.Friedrich J, Gradišar H, Vrecl M, Pogaènik A. In vitro degradation of porcine skin epidermis by a fungal keratinase of Doratomyces microsporus. Enzyme Microb Technol. 2005;36:455–460. [Google Scholar]
- 14.Giongo JL, Lucas FS, Casarin F, Heeb P, Brandelli A. Keratinolytic proteases of Bacillus species isolated from the Amazon basin showing remarkable de-hairing activity. World J Microbiol Biotechnol. 2007;23:375–382. [Google Scholar]
- 15.Macedo AJ, da Silva WOB, Gava R, Driemeier D, Henriques JAP, et al. Novel keratinase from Bacillus subtilis S14 exhibiting remarkable dehairing capabilities. Appl Environ Microbiol. 2005;71:594–596. doi: 10.1128/AEM.71.1.594-596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HY, Liu DM, Liu Y, Cheng CF, Ma QY, et al. Screening and mutagenesis of a novel Bacillus pumilus strain producing alkaline protease for dehairing. Lett Appl Microbiol. 2007;44:1–6. doi: 10.1111/j.1472-765X.2006.02039.x. [DOI] [PubMed] [Google Scholar]
- 17.Zambare VP, Nilegaonkar SS, Kanekar PP. A novel extracellular protease from Pseudomonas aeruginosa MCM B-327: Enzyme production and its partial characterization. New Biotechnology (in press) 2010 doi: 10.1016/j.nbt.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 19.Morales VM, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 20.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: Nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 21.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 23.Kunitz M. Crystalline soyabean trypsin inhibitor II. General properties. J Gen Physiol. 1947;30:291–310. doi: 10.1085/jgp.30.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall ARJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Kanekar PP, Nilegaonkar SS, Sarnaik SS, Kelkar AS. Optimization of protease activity of alkaliphilic bacteria isolated from an alkaline lake in India. Biores Technol. 2002;85:87–93. doi: 10.1016/s0960-8524(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 26.Schägger H. Native electrophoresis for isolation of mitochondrial oxidative phosphorylation protein complexes. Methods Enzymol. 1995;260:190–202. doi: 10.1016/0076-6879(95)60137-6. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Pandeeti EVP, Chinnaboina MR, Siddavattam D. Benzoate mediated changes on expression profile of soluble proteins in Serratia sp. DS001. Lett Appl Micobiol. 2009;48:566–571. doi: 10.1111/j.1472-765X.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 29.Figureurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandey JP, Gorla P, Manavathi B, Siddavattam D. mRNA secondary structure modulates the translation of organophosphate hydrolase (OPH) in E. coli. Mol Biol Rep. 2009;34:13–31. doi: 10.1007/s11033-007-9200-5. [DOI] [PubMed] [Google Scholar]
- 31.Braun P, Bitter W, Tommassen J. Activation of Pseudomonas aeruginosa elastase in Pseudomonas putida by triggering dissociation of the propeptide-enzyme complex. Microbiology. 2000;146:2565–2572. doi: 10.1099/00221287-146-10-2565. [DOI] [PubMed] [Google Scholar]
- 32.Yahr TL, Goranson J, Frank DW. Exoenzymes of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- 33.Hauser AR, Fleiszig S, Kang PJ, Mostov K, Engel JN. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect Immun. 1998;66:1413–1420. doi: 10.1128/iai.66.4.1413-1420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandkvist M. Type II secretion and pathogenesis. Infect Immun. 2001;69:3523–3535. doi: 10.1128/IAI.69.6.3523-3535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewenza S, Gardy JL, Brinkman FSL, Hancock REW. Genome-wide identification of Pseudomonas aeruginosa exported proteins using a consensus computational strategy combined with a laboratory-based PhoA fusion screen. Genome Res. 2005;15:321–329. doi: 10.1101/gr.3257305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nouwens AS, Willcox MDP, Walsh BJ, Cordwell SJ. Proteomic comparison of membrane and extracellular proteins from invasive (PAO1) and cytotoxic (6206) strains of Pseudomonas aeruginosa. Proteomics. 2002;2:1325–1346. doi: 10.1002/1615-9861(200209)2:9<1325::AID-PROT1325>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Braun P, Ockhuijsen C, Eppens E, Koster M, Bitter W, et al. Maturation of Pseudomonas aeruginosa elastase. Formation of the disulfide bonds. J Biol Chem. 2001;276:26030–26035. doi: 10.1074/jbc.M007122200. [DOI] [PubMed] [Google Scholar]
- 38.Holder IA, Neely NA. Pseudomonas elastase acts as a virulence factor in burned hosts by Hageman factor-dependent activation of the host kinin cascade. Infect Immun. 1989;57:3345–3348. doi: 10.1128/iai.57.11.3345-3348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nouwens AS, Beatson SA, Whitchurch CB, Walsh BJ, Schweizer HP, et al. Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiol. 2003;149:1311–1322. doi: 10.1099/mic.0.25967-0. [DOI] [PubMed] [Google Scholar]
- 40.Najafi MF, Deobagkar DN, Mehrvarz M, Deobagkar DD. Enzymatic properties of a novel highly active and chealator resistant protease from a Pseudomonas aeruginosa PD100. Enzyme Microb Technol. 2006;39:1433–1440. [Google Scholar]
- 41.Bayoudh AN, Gharsallah M, Chamkha A, Dhouib S, Ammar, et al. Purification and characterization of an alkaline protease from Pseudomonas aeruginosa MN1. J Ind Microbiol Biotechnol. 2000;24:291–295. [Google Scholar]
- 42.Lin H, Yin L, Jiang S. Cloning, expression, and purification of Pseudomonas aeruginosa keratinase in Escherichia coli AD494 (DE3) pLysS expression system. J Agric and Food Chem. 2009;57:3506–3511. doi: 10.1021/jf803752j. [DOI] [PubMed] [Google Scholar]
- 43.Lin H, Yin L, Jiang S. Functional expression and characterization of keratinase from Pseudomonas aeruginosa in Pichia pastoris. J Agric and Food Chem. 2009;57:5321–5325. doi: 10.1021/jf900417t. [DOI] [PubMed] [Google Scholar]
- 44.Sharma R, Gupta R. Extracellular expression of keratinase kerP from Pseudomonas aeruginosa in E. coli. Biotechnol Lett. 2010 doi: 10.1007/s10529-010-0361-2. DOI 10.1007/s10529-010-0361-2. [DOI] [PubMed] [Google Scholar]
- 45.Brandelli A. Bacterial keratinases: Useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol. 2008;1:105–116. [Google Scholar]
- 46.Rust L, Pesci EC, Iglewski BH. Analysis of the Pseudomonas aeruginosa elastase (lasB) regulatory region. J Bacteriol. 1996;179:557–562. doi: 10.1128/jb.178.4.1134-1140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson RM, Zimprich CA, Rust L. A second operator is involved in Pseudomonas aeruginosa elastase (lasB) activation. J Bacteriol. 1999;181:6264–6270. doi: 10.1128/jb.181.20.6264-6270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin X, Xu W, Huang K, Mei X, Liang Z, et al. Cloning, expression and characterization of recombinant elastase from Pseudomonas aeruginosa in Pichia pastoris. Protein Expr Purif. 2009;63:69–74. doi: 10.1016/j.pep.2007.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of depilating protease of Pseudomonas aeruginosa MCM B-327 by TwoD electrophoresis (Panel A). Peptide mass fingerprint (PMF) of depilating protease is shown in panel B. Panels C to F represent MS/MS profiles of peaks with m/z values of 1167.604, 1610.825, 2082.871 and 2560.216, respectively.
(TIF)