Abstract

The syntheses of D,L-geissoschizol, D,L-corynantheidol, D,L-dihydrocorynantheol, D,L-protoemetinol, and D,L-3-epi-protoemetinol have been accomplished from a single synthetic intermediate.
Keywords: Natural product synthesis, secologanin, geissoschizol, corynantheidol, dihydrocorynantheol, protoemetinol, 3-epi-protoemetinol
Introduction
The densely functionalized monoterpene secologanin glucoside (1) has been shown to be the common biosynthetic precursor of several structurally diverse classes of natural products.1 The simple secoiridoids arise from minor modifications to the secologanin core and have been reported to display analgesic,2 anti-inflammatory,3 anti-arthritic,4 anti-allergenic,5 antibacterial,6 and antiviral7 activities. Strictosidine synthase catalyzes the coupling of secologanin glucoside with tryptamine (2) to produce strictosidine (3),8 which is the common precursor of more than two hundred and fifty structurally diverse naturally occurring alkaloids including important therapeutic agents such as quinine, vinblastine, and reserpine. Additional classes of natural products arise from the coupling of secologanin glucoside with dopamine (4) producing the tetrahydroisoquinoline deacetylipecoside (5).9 Modification of 5 gives rise to alkaloids such as the antitumor agent tubulosine10 and emetine, which displays antiprotozoic11 activity and may be useful in the treatment of lymphatic leukemia.12
Synthetic access to the secoiridoids and the secologanin alkaloid derivatives has traditionally been pursued on a case-by-case basis by developing novel approaches for a small number of structurally similar synthetic targets. The Cook13 and Martin14 laboratories have pursued more general synthetic strategies allowing access to a broad selection of secologanin tryptamine alkaloids via a single synthetic strategy. Although highly efficient and productive, these strategies confine their synthetic scope to the tryptamine-derived alkaloids. We have envisioned a more general strategy involving the large scale synthesis of a functionalized intermediate, which can be rapidly modified to allow access to the simple secoiridoids as well as the tryptamine- and dopamine-derived alkaloids. We recently demonstrated that lactone 6 was a viable intermediate for the synthesis of the simple secoiridoid oleocanthal (7) Scheme 2.15 Herein we wish to report the use of lactone 6 as a key intermediate in the synthesis of the secologanin tryptamine alkaloids D,L-geissoschizol (8), D,L-corynantheidol (9), and D,L-dihydrocorynantheol (10) as well as the secologanin dopamine alkaloids D,L-3-epi-protoemetinol (11) and D,L-protoemetinol (12) (Figure 1).
Scheme 2.
Previous conversion of 6 to oleocanthal (7).
Figure 1.
Target natural products.
Figure 2 illustrates our retrosynthetic strategy. We planned to form the arylpiperidine rings of the target alkaloids (13) via late-stage Bischler-Napieralski cyclization of a hydroxyl-protected lactam (14). We predicted that this cyclization would proceed with complete cis-diastereoselectivity based on observations made by Martin and coworkers while working with a similar system.16 We envisioned the requisite lactam ring of 14 arising from the cyclization of the methyl ester moiety and the secondary amine of a compound of type 15. This secondary amine would be produced through the reductive amination of an appropriately functionalized aldehyde (16) and tryptamine or a dopamine-derived primary amine. The desired oxidation state and relative stereochemistry of the ethyl side chain could be established via selective reduction of the olefin of lactone 6 followed by the reduction of the resulting lactone.
Figure 2.
Retrosynthetic analysis.
Results and Discussion
Scheme 3 illustrates our synthesis of D,L-geissoschizol (8). Reduction of the lactone carbonyl of 6 followed by reductive amination of the crude aldehyde/lactol mixture both successfully merged the tryptamine and secoiridoid portions of the molecule as well as forming the lactam ring of intermediate 18, presumably via amine 17, which was not isolated. Protection of the hydroxyl functionality of 18 as the acetate ester followed by Bischler-Napieralski cyclization gave acetate 19 as a single isomer, which was deprotected to yield D,L-geissoschizol (8) in good yield.
Scheme 3.
Synthesis of geissoschizol (8).
Next, we turned our attention to the alkaloids bearing reduced ethyl side chains, corynantheidol (9) and dihydrocorynantheol (10). As part of their 1992 synthesis of these and other related alkaloids, Lounasmaa and coworkers reported the successful hydrogenation of several geissoschizol isomers.17 Unfortunately, poor diastereoselectivity was observed for these reductions regardless of the geissoschizol isomer or reduction method employed. These observations lead us to pursue a strategy, which introduces the C3 stereogenic centers earlier in the synthesis. We speculated that either of the desired ethyl side chain stereochemistries could be accessed via selective reduction of lactone 6. As illustrated in Scheme 4, catalytic hydrogenation of the olefin of lactone 6 produces exclusively the cis-isomer of the reduced lactone (21). This selectivity is presumably due to the delivery of hydrogen to the least sterically encumbered face of the olefin. Conjugate reduction, conversely, produces exclusively the thermodynamically favored trans-isomer of the reduced lactone (20). With the ability to selectively produce lactones 20 and 21 we turned our attention to the completion of some representative natural alkaloid syntheses.
Scheme 4.
Selective reduction of lactone 6
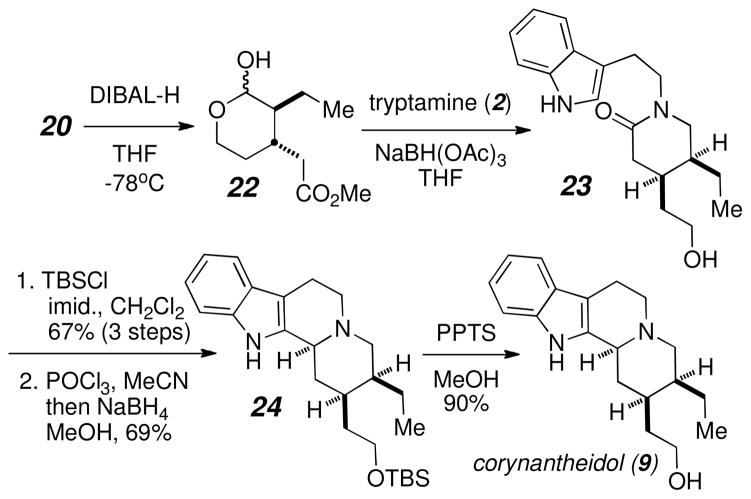
Following a similar protocol to that deployed for our synthesis of geissoschizol, our synthesis of corynantheidol (9) (Scheme 5) began with the DIBAL-H reduction of lactone 20 followed by a one-step reductive amination/lactam cyclization to give alcohol 23. Alcohol protection and Bischler-Napieralski cyclization gave tetracycle 24 in good yield and as a single isomer. Removal of the alcohol protecting group with PPTS/MeOH gave D,L-corynantheidol (9) in excellent yield.
Scheme 5.
Synthesis of corynantheidol (9).
Our attempts to access dihydrocorynantheol (10) began via employment of an identical method to that used for its epimer corynantheidol (Scheme 6). Reduction of lactone 6 gave a mixture of lactol isomers (25), which was immediately subjected to reductive amination conditions producing the desired lactam (26) and its C3 epimer (23) as a minor product. Protection of the alcohol and Bischler-Napieralski cyclization of this mixture gave a mixture of two tetracyclic epimers (24 and 27) that were readily separated by silica gel flash chromatography. Separate deprotection of 27 and 24 gave D,L-dihydrocorynantheol (10) and D,L-corynantheidol (9) respectfully.
Scheme 6.
Synthesis of dihydrocorynantheol (9).
With control of the secoiridoid portion of the secologanin-derived alkaloids established, we began our investigation of the secoiridoids derived from dopamine. Fortunately very little modification to the procedures developed was necessary for this purpose. Thus, substituting 2-(3,4-dimethoxyphenyl)ethanamine (29) for tryptamine and adjusting Bischler-Napieralski reaction temperature to compensate for a slightly less reactive aromatic nucleophile, the synthesis of D,L-3-epi-protoemetinol (11) was accomplished by the procedure developed for the synthesis of D,L-corynantheidol (9) (Scheme 7) and D,L-protoemetinol (12) was synthesized by the procedure developed for the synthesis of D,L-dihydrocorynantheol (10) (Scheme 8).
Scheme 7.
Synthesis of 3-epi-protoemetinol (11).
Scheme 8.
Synthesis of protoemetinol (12).
The syntheses described herein of the tryptamine-derived secologanin alkaloids geissoschizol, corynantheidol, and dihydrocorynantheol and the dopamine-derived secologanin alkaloids protoemetinol and 3-epi-protoemetinol taken in context with our previously reported synthesis of oleocanthal, demonstrate the potentially broad utility of lactone 6. The careful manipulation of lactone 6 delivered rapid access to members of, to date, three very distinct classes of secologanin-derived natural products. Current efforts to produce lactone 6 in an enantioselective manner are under investigation.
Experimental Section
Synthesis of (E)-1-(2-(1H-indol-3-yl)ethyl)-5-ethylidene-4-(2-hydroxyethyl)piperidin-2-one (18)
To a flame dried 50 mL round-bottomed flask containing 248 mg (1.25 mmol, 1 equiv.) lactone 6 was added 20 mL of dry THF and this solution was cooled to −78°C. To this solution was dropwise added 1.50 mL (1.2 equiv.) of a 1.0 M solution of DIBAl-H in THF and the reaction was allowed to stir for 60 min at this temperature before the dropwise addition of 2 mL dry MeOH and warming to ambient temperature. To this reaction mixture was added 25 mL of a saturated aqueous solution of Rochelle’s salt and the mixture was stirred for 30 min at ambient temperature. This mixture was extracted thrice with EtOAc, dried over Na2SO4, and concentrated to yield a colorless oil, which was immediately used without purification.
To the above produced residue was added 360 mg (2.25 mmol, 1.5 equiv) tryptamine, 15 mL dry THF, and then 954 mg (4.50 mmol, 3 equiv.) NaBH(OAc)3. After stirring at ambient temperature for 48 hr the reaction was added to NaHCO3(sat.) and extracted thrice with CH2Cl2. Combined organic layers were dried over Na2SO4 and concentrated to afford a brown oil which was purified by silica gel chromatography eluting with 5% to 20% MeOH in EtOAc to yield 220 mg (56%, 2 steps) of compound 18 as a white foam.
1HNMR (300 MHz, CDCl3): δ 9.00 (brs, 1H), 7.62 (d, J = 7.8, 1H), 7.33 (d, J = 8.1, 1H) 7.13 (m, 2H), 6.97 (s, 1H), 5.28 (q, J = 5.7, 1H), 3.44–3.82 (m, 6H), 3.04 (q, J = 7.5, 2H), 2.97 (m, 1H) 2.26–2.56 (m, 2H), 1.57 (dd, J = 6.6, 1.5, 3H), 1.40–1.56 (m, 2H); 13CNMR (75 MHz, CDCl3): δ169.7, 136.4, 132.6, 127.4, 122.5, 121.7, 120.8, 119.1, 118.6, 112.3, 111.4, 60.3, 52.5, 47.5, 37.9, 35.2, 29.9, 22.9, 12.8; IR (NaCl, film): 3289, 1620 cm−1; HRMS (+TOF): [M+H]+ 313.1911 calcd for C19H25N2O2, found: 313.1916; Rf = 0.18 (5% MeOH in EtOAc).
Synthesis of (E)-2-(1-(2-(1H-indol-3-yl)ethyl)-5-ethylidene-2-oxopiperidin-4-yl)ethyl acetate
To a 10 mL round bottomed flask containing 65.0 mg (0.208 mmol, 1 equiv.) alcohol 18 dissolved in 1 mL dry CH2Cl2 and 1 mL dry pyridine was added 30.0 μL (0.416 mmol, 2 equiv.) AcCl followed by a single crystal of DMAP. The reaction was stirred at ambient temperature for 1 hr before being added to NaHCO3(sat.), and extracted thrice with CH2Cl2, dried over Na2SO4, and concentrated. The crude residue was purified by silica gel chromatography eluting with 1% to 10% MeOH in CH2Cl2 to yield 70 mg (95%) of the desired product as a pale yellow oil.
1HNMR (300 MHz, CDCl3) δ 9.01 (bs, 1H), 7.63 (d, J = 7.8, 1H), 7.32 (d, J = 8.1, 1H), 7.15 (t, J = 6.6, 1H), 7.08 (t, J = 7.2, 1H), 6.97 (s, 1H), 5.33 (q, J = 6.9, 1H), 4.01-3.46 (m, 6H), 3.04 (t, J = 7.5, 2H), 2.97 (m, 1H), 2.50 (dd, J = 17.1, 5.7, 1H), 2.39 (dd, J = 16.8, 2.1, 1H), 2.01 (s, 3H), 1.63 (m, 2H), 1.56 (dd, J = 6.9, 1.5, 3H); 13CNMR (75 MHz, CDCl3) δ 171.3, 169.2, 136.6, 132.0, 127.6, 122.6, 122.0, 121.8, 119.3, 118.8, 112.6, 111.6, 62.5, 52.6, 47.9, 38.3, 31.3, 30.3, 23.2, 21.2, 12.9; IR (NaCl, film): 1736, 1628 cm−1; HRMS (+TOF): [M+H]+ 355.2016 calcd for C21H27N2O3, found: 355.2014; Rf = 0.27 (5% MeOH in CH2Cl2).
Synthesis of 2-((2R,12bS,E)-3-ethylidene-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl)ethyl acetate (19)
To 192 mg (0.542 mmol, 1 equiv.) of the above produced acetate dissolved in 5 mL dry benzene was added 100 μL (1.08 mmol, 2 equiv.) freshly distilled POCl3. The reaction was heated to reflux for 1.5 hr before being concentrated under reduced pressure. The resulting residue was taken up in 5 mL dry MeOH and cooled to 0°C before 50 mg NaBH4 was added and the reaction was removed from the ice bath and stirred for 15 min. The reaction was then added to 0.5 M NaOH, extracted thrice with CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by silica gel chromatography eluting with 5% MeOH in EtOAc to provide 119 mg (65%) of the desired product as a pale yellow foam.
1HNMR (300 MHz, CDCl3) δ 8.40 (bs, 1H), 7.45 (d, J = 7.2, 1H), 7.34 (d, J = 7.2, 1H), 7.09 (m, 2H), 5.51 (q, J = 6.9, 1H), 4.24-3.86 (m, 2H), 3.73 (d, J = 10.5, 1H), 3.04 (m, 3H), 2.64 (m, 3H), 2.08 (s, 3H), 2.05-1.70 (m, 5H), 1.58 (d, J = 6.9, 3H); 13CNMR (75 MHz, CDCl3) δ 171.5, 136.3, 135.5, 135.0, 127.5, 122.1, 121.4, 119.4, 118.2, 111.1, 108.5, 62.9, 60.1, 55.4, 53.0, 35.3, 31.0, 30.7, 21.9, 21.3, 12.9; IR (NaCl, film): 1735 cm−1; HRMS (+TOF): [M+H]+ 339.2073 calcd for C21H27N2O2, found: 339.2072; Rf = 0.45 (5% MeOH in EtOAc).
Synthesis of (+/−)-geissoshizol (8)
To a 10 mL round bottomed flask containing 50.0 mg (0.148 mmol, 1 equiv.) acetate 19 dissolved in 1 mL MeOH and 0.5 mL H2O was added 245 mg (1.77 mmol, 12 equiv.) K2CO3. The reaction was stirred at ambient temperature for 2 hr, added to brine, extracted thrice with CH2Cl2, dried over Na2SO4, and concentrated. The resulting oil was purified by silica gel chromatography eluting with 10% MeOH in CH2Cl2 to yield 39.0 mg (89%) of the desired product as a white solid.
1HNMR (400 MHz, CDCl3) δ 7.91 (bs, 1H), 7.44 (d, J = 7.6, 1H), 7.32 (d, J = 7.6, 1H), 7.15-7.06 (m, 2H), 5.51 (q, J = 6.8, 1H), 3.67 (m, 3H), 3.25-2.97 (m, 5H), 2.72 (m, 1H), 2.62 (m, 1H), 2.08-1.46 (m, 5H), 1.62 (d, J = 6.8, 3H); 13CNMR (75 MHz, CDCl3) δ 136.4, 136.2, 134.8, 127.5, 121.7, 121.4, 119.5, 118.2, 110.9, 108.6, 61.3, 60.1, 55.3, 52.8, 35.4, 35.2, 31.0, 21.8, 12.9; IR (NaCl, film): 3233, 2851, 2790, 2745 cm−1; HRMS (+TOF): [M+H]+ 297.1961 calcd for C19H25N2O, found: 297.1961; Rf = 0.14 (5% MeOH in EtOAc). Spectroscopic properties agree in all respects with those previously reported.13e,18
Synthesis of lactone 20
To a 250 mL round-bottomed flask contining 1.10 g (5.55 mmol, 1 equiv.) of lactone 6 dissolved in 56 mL dry THF at −78°C was added 6.10 mL (1.1 equiv.) of a 1.0 M solution of L-Selectride in THF. The reaction was allowed to stir at −78°C for 60 min and was then added to NH4Cl(sat.), extracted thrice with EtOAc, dried over Na2SO4, concentrated, and purified by silica gel chromatography eluting with 2: 1 hex./EtOAc to yield 918 mg (83%) of the title compound as a colorless oil.
1HNMR (300 MHz, CDCl3): δ 4.26 (m, 2H), 3.66 (s, 3H), 2.50 (m, 2H), 2.26 (m, 2H), 2.04 (m, 1H), 1.87 (m, 1H), 1.65 (m, 2H), 0.94 (t, J = 7.2, 3H); 13CNMR (75 MHz, CDCl3) major conformer: δ 173.1, 172.2, 67.1, 51.9, 46.6, 38.7, 31.8, 28.4, 22.7, 10.9; IR (NaCl, film): 1732 cm−1; HRMS (+TOF): [M+H]+ 201.1121 calcd for C10H17O4, found: 201.1123; Rf = 0.52 (1: 1 hex./EtOAc)
Synthesis of 1-(2-(1H-indol-3-yl)ethyl)-4-(2-(tert-butyldimethylsilyloxy)ethyl)-5-ethylpiperidin-2-one
A 100 mL round-bottomed flask containing 505 mg (2.52 mmol, 1 equiv.) lactone 20 dissolved in 25 mL dry THF was cooled to −78°C. To this cooled mixture was slowly added 2.77 mL (2.77 mmol, 1.1 equiv.) of a 1.0 M solution of DIBAL-H in THF. The reaction was stirred for 30 min at −78°C followed by the dropwise addition of 2 mL MeOH. The reaction was allowed to warm to ambient temperature and was then added to a saturated aqueous solution of NaK tartrate and extracted thrice with EtOAc. Combined organic layers were dried over Na2SO4, concentrated, and purified by silica gel flash chromatography eluting with 1: 1 hex./EtOAc to yield 459 mg of an inseparable mixture of the desired lactol mixture (22) as a colorless oil.
To a 50 mL round-bottomed flask containing the above prepared lactol mixture dissolved in 23 mL dry THF was added 546 mg (3.41 mmol, 1.5 equiv.) tryptamine and then 1.44 g (6.81 mmol, 3 equiv.) NaBH(OAc)3. The reaction was allowed to stir at ambient temperature for 48 hr before being added to NaHCO3(sat.), extracted thrice with EtOAc, dried over Na2SO4, and concentrated. The resulting residue containing crude 23 was carried forward without purification.
To the crude alcohol (23) prepared above, in a 50 mL round-bottomed flask was added 22.7 mL dry CH2Cl2 followed by 411 mg (2.72 mmol, 1.2 equiv.) TBSCl and then 309 mg (4.54 mmol, 2 equiv.) imidazole. The reaction was allowed to stir at ambient temperature for 12 hr before being added to brine, extracted into CH2Cl2, dried over Na2SO4 and concentrated. The resultant residue was purified by silica gel flash chromatography eluting with 1: 1 hex./EtOAc to yield 720 mg (67%, 3 steps) of the desired O-TBS ether derivative of 23 as a tan foam.
1HNMR (300 MHz, CDCl3): δ 8.94 (brs, 1H), 7.66 (d, J = 7.5, 1H), 7.34 (d, J = 7.5, 1H), 7.17 (t, J = 7.2, 1H), 7.10 (t, J = 7.2, 1H), 6.98 (s, 1H), 3.65 (m, 4H), 3.13 (dd, J = 12.3, 5.1, 1H), 3.03 (m, 3H), 2.37 (qd, J = 17.7, 6.0, 2H), 2.07 (m, 1H), 1.68 (m, 1H), 1.54 (m, 1H), 1.14–1.34 (m, 3H), 0.90 (s, 9H), 0.83 (t, J = 7.2, 3H), 0.05 (s, 6H); 13CNMR (75MHz, CDCl3): δ 169.5, 136.4, 127.4, 122.3, 121.7, 119.0, 118.6, 112.5, 111.4, 60.6, 50.6, 48.3, 38.3, 36.4, 31.5, 31.1, 26.0, 23.0, 20.5, 18.3, 11.8, −5.3; IR (NaCl, film): 3260, 1622 cm−1; HRMS (+TOF): [M+H]+ 429.2932 calcd for C25H41N2O2Si, found: 429.2937; Rf = 0.36 (1:1 hex./EtOAc)
Synthesis of tetracycle 24
To 160 mg (0.373 mmol, 1 equiv.) of the above-produced O-TBS ether derivative of 23 dissolved in 4 mL dry MeCN was added 1.21 mL (14.9 mmol, 40 equiv.) dry pyridine followed by 278 μL (2.98 mmol, 8 equiv.) freshly distilled POCl3. The reaction was stirred for 16 hr at 40°C and concentrated under reduced pressure. To the resultant residue was added 4 mL dry MeOH. This solution was cooled to 0°C, 142 mg (3.73 mmol, 10 equiv.) NaBH4 was added, and the reaction was stirred at 0°C 15min before being added to NaHCO3(sat.), extracted into CH2Cl2, dried over Na2SO4, and concentrated. Purification by silica gel flash chromatography eluting with 2: 1 hex./EtOAc yielded 107 mg (69%) of the desired product (24) as a pale yellow oil.
1HNMR (300 MHz, CDCl3): δ 7.82 (bs, 1H), 7.47 (d, J = 7.2, 1H), 7.30 (d, J = 7.2, 1H), 7.11 (m, 2H), 3.72 (t, J = 6.6, 2H), 3.24 (m, 1H), 3.01 (m, 3H), 2.68 (m, 1H), 2.38 (d, J = 11.4, 1H) 1.88 (m, 2H), 1.53 (m, 4H), 1.27 (m, 3H), 0.95 (s, 9H), 0.91 (m, 3H), 0.11 (s, 6H); 13CNMR (75 MHz, CDCl3): δ 136.0, 135.4, 127.6, 121.3, 119.4, 118.2, 110.9, 108.1, 66.7, 61.5, 60.6, 53.5, 39.7, 36.5, 36.4, 32.2, 29.8, 26.2, 18.5, 17.9, 12.8, −5.1; IR (NaCl, film): 2796, 2747cm−1; HRMS (+TOF): [M+H]+ 413.2951 calcd. for C25H41N2OSi, found: 413.2948; Rf = 0.46 (4: 1 hex./EtOAc).
Synthesis of (+/−)-corynantheidol (9)
To a 25 mL round-bottomed flask containing 40 mg (0.097 mmol, 1 equiv.) tetracycle 24 was added 1 mL MeOH followed by 49 mg (0.19 mmol, 2 equiv.) PPTS. The reaction was stirred 12 hr at ambient temperature, added to 1N NaOH, extracted twice with CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by silica gel flash chromatography eluting with 5% to 20% MeOH in CH2Cl2 to yield 26 mg (90%) corynantheidol (9) as a white foam.
1HNMR (300 MHz, CDCl3): δ 8.14 (bs, 1H), 7.45 (d, J = 6.6, 1H), 7.31 (d, J = 7.2, 1H), 7.10 (m, 2H), 3.73 (m, 2H), 3.13 (dd, J = 10.8, 0.6, 1H), 2.99 (m, 3H), 2.66 (m, 1H), 2.54 (m, 1H), 2.32 (d, J = 9.9, 1H), 1.85 (m, 2H), 1.58-1.48 (m, 5H), 1.26 (m, 2H), 0.91 (t, J = 7.2, 3H); 13CNMR (75 MHz, CDCl3): δ 136.1, 135.4, 127.5, 121.2, 119.3, 118.1, 110.9, 107.9, 60.8, 60.5, 57.9, 53.6, 39.7, 36.4, 36.0, 31.9, 21.8, 17.8, 12.8; IR (NaCl, film): 3412, 3277, 2800, 2749 cm−1; HRMS (+TOF): [M+H]+ 299.2118 calcd. for C19H27N2O, found: 299.2120; Rf = 0.25 (5% MeOH in CH2Cl2). Spectroscopic properties agreed in all respects with those previously reported.13e,17,18
Synthesis of lactone 21
To a 100 mL round-bottomed flask containing 1.07 g (5.40 mmol, 1 equiv.) lactone 6 was added 25 mL dry MeOH then 574 mg 10% Pd/C. Hydrogen gas was bubbled through this mixture for 5 min and then the reaction was vigorously stirred for 4 hr at ambient temperature under balloon pressure of H2. The reaction was then filtered through a thin pad of Celite, concentrated, and purified by silica gel flash chromatography eluting with 1: 1 hex./EtOAc to yield 1.07 g (>99%) of the title compound as a colorless oil. (Note: The trans isomer (20) was not detected by 1HNMR, 13CNMR or TLC.)
1HNMR (300 MHz, CDCl3): (mixture of rotamers) δ 4.26 (m, 2H), 3.63 (s, 1.5H), 3.62 (s, 1.5H), 2.74-2.36 (m, 2H), 2.29-1.99 (m, 3H), 1.89-1.52 (m, 3H), 1.35 (m, 1H), 1.20 (m, 1H), 0.94 (t, J = 7.5, 1.5H), 0.92 (t, J = 7.2, 1.5H); 13CNMR (75 MHz, CDCl3): (mixture of rotamers) δ 174.2, 173.4, 172.7, 172.4, 68.6, 67.3, 66.1, 65.6, 52.7, 52.1, 51.9, 46.7, 44.5, 38.9, 38.7, 36.1, 34.3, 33.7, 32.0, 30.3, 29.2, 28.5, 28.0, 27.0, 22.9, 22.8, 20.8, 20.2, 14.0, 12.3, 12.0, 11.1; IR (NaCl, film): 1732 cm−1; HRMS (+TOF): [M+H]+ 201.1121 calcd for C10H17O4, found: 201.1126; Rf = 0.39 (1: 1 hex./EtOAc).
Synthesis of lactams 26 and 23
To a 100 mL flame dried round bottomed flask was added 1.08 g (5.40 mmol, 1 equiv.) lactone 21 dissolved in 25 mL dry THF. This mixture was cooled to −78°C before the slow addition of 5.94 mL (5.94 mmol, 1.1 equiv) of a 1.0 M solution of DIBAl-H in THF. The reaction was stirred at −78°C for 30 min befor the addition of 2 mL dry MeOH. The reaction was allowed to warm to ambient temperature before being added to a saturated solution of Rochelle’s salt, extracted twice into CH2Cl2, dried over Na2SO4, and concentrated to yield the desired mixture of lactols 21 as colorless oil, which was immediately used without further purification. (Note: The undesired trans isomer (20) was not detected in this crude mixture, however, subjecting this crude mixture to silica gel chromatography did result in the isolation of 20.)
To a 100 mL round bottomed flask containing the above produced lactol mixture 21 dissolved in 27 mL dry THF was added 649 mg (4.05 mmol, 1.5 equiv.) tryptamine followed by 1.72 g (8.10 mmol, 3 equiv.) NaBH(OAc)3. The reaction was allowed to stir at ambient temperature for 24 hr before being added to NaHCO3(sat.), extracted twice into CH2Cl2, dried over Na2SO4, and concentrated to yield a mixture of alcohols as a tan oil, which was used without further purification.
To a 100 mL round bottomed flask containing the above produced crude mixture of alcohols dissolved in 27 mL dry CH2Cl2 was added 488 mg (3.24 mmol, 1.2 equiv.) TBSCl followed by 368 mg (5.40 mmol, 2 equiv.) imidazole. The reaction was stirred at ambient temperature for 16 hr before being added to brine, extracted twice into CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by silica gel flash chromatography eluting with 1: 1 to 0: 1 hex./EtOAc to yield 542 mg (47%, 3 steps) of the desired mixture of isomers as a tan foam.
1HNMR (300 MHz, CDCl3): δ 8.78 (bs, 1H), 7.66 (d, J = 7.5, 1H), 7.35 (d, J = 7.8, 1H), 7.13 (m, 2H), 6.98 (s, 1H), 3.64 (m, 4H), 3.22-2.86 (m, 4H), 2.28–2.59 (m, 2H), 2.07 (m, 1H), 1.70-1.10 (m, 6H), 0.90 (s, 9H), 0.80 (m, 4H), 0.05 (s, 6H); 13CNMR (75MHz, CDCl3): δ 170.0, 169.7, 136.6, 127.7, 122.5, 122.0, 119.3, 118.9, 113.0, 112.9, 111.6, 60.9, 60.6, 51.6, 50.8, 48.5, 48.4, 39.4, 38.5, 36.6, 36.4, 33.1, 31.8, 31.3, 26.2, 23.9, 23.4, 23.2, 20.7, 18.5, 12.1, 11.1; IR (NaCl, film): 3254, 1626 cm−1; HRMS (+TOF): [M+H]+ 429.2932 calcd for C25H41N2O2Si, found: 429.2933; Rf = 0.30 (1: 1 hex./EtOAc)
Synthesis of tetracycle 27
To a 100 mL round bottomed flask containing the above produced crude mixture of alcohols dissolved in 27 mL dry CH2Cl2 was added 488 mg (3.24 mmol, 1.2 equiv.) TBSCl followed by 368 mg (5.40 mmol, 2 equiv.) imidazole. The reaction was stirred at ambient temperature for 16 hr before being added to brine, extracted twice into CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by silica gel flash chromatography eluting with 1: 1 to 0: 1 hex./EtOAc to yield 542 mg (47%, 3 steps) of the desired mixture of isomers as a tan foam.
1HNMR (300 MHz, CDCl3): δ 8.78 (bs, 1H), 7.66 (d, J = 7.5, 1H), 7.35 (d, J = 7.8, 1H), 7.13 (m, 2H), 6.98 (s, 1H), 3.64 (m, 4H), 3.22-2.86 (m, 4H), 2.28–2.59 (m, 2H), 2.07 (m, 1H), 1.70-1.10 (m, 6H), 0.90 (s, 9H), 0.80 (m, 4H), 0.05 (s, 6H); 13CNMR (75MHz, CDCl3): δ 170.0, 169.7, 136.6, 127.7, 122.5, 122.0, 119.3, 118.9, 113.0, 112.9, 111.6, 60.9, 60.6, 51.6, 50.8, 48.5, 48.4, 39.4, 38.5, 36.6, 36.4, 33.1, 31.8, 31.3, 26.2, 23.9, 23.4, 23.2, 20.7, 18.5, 12.1, 11.1; IR (NaCl, film): 3254, 1626 cm−1; HRMS (+TOF): [M+H]+ 429.2932 calcd for C25H41N2O2Si, found: 429.2933; Rf = 0.30 (1: 1 hex./EtOAc)
To a 25 mL round bottomed flask containing 373 mg (0.870 mmol, 1 equiv.) of the above produced mixture of lactams dissolved in 9 mL dry MeCN was added 1.40 mL (17.4 mmol, 20 equiv.) dry pyridine followed by 406 μL (4.35 mmol, 5 equiv.) POCl3. The reaction was heated to 40°C for 3 hr before being concentrated, taken up in 9 mL dry MeOH and cooled to 0°C. To this solution was added 33 mg NaBH4 and the reaction was allowed to warm to ambient temperature over 15 min before being added to NaHCO3(sat.), extracted into EtOAc, dried over Na2SO4, and concentrated. The crude residue was purified by silica gel flash chromatography eluting with 4: 1 to 0: 1 hex./EtOAc to yield 142 mg (40%) of the desired trans product 27 as a tan foam along with 113 mg (31%) of the cis product 24 as a tan foam.
1HNMR (300 MHz, CDCl3): δ 7.76 (bs, 1H), 7.47 (d, J = 7.2, 1H), 7.3 (d, J = 7.2, 1H), 7.11 (m, 2H), 3.73 (m, 2H), 3.13 (m, 3H), 3.00 (m, 1H), 2.72 (m, 1H), 2.59 (td, J = 11.1, 5.4, 1H), 2.19 (d, J = 11.7, 1H), 2.10 (t, J = 10.8, 1H), 1.94 (m, 1H), 1.68 (m, 1H), 1.26–1.51 (m, 4H), 1.16 (m, 1H), 0.932 (s, 12H), 0.10 (s, 6H); 13CNMR (75MHz, CDCl3): δ136.1, 135.1, 127.6, 121.4, 119.5, 118.3, 110.8, 108.3, 61.1, 60.7, 60.0, 53.4, 41.9, 37.3, 35.9, 35.8, 26.1, 23.6, 21.8, 18.5, 11.2, −5.0.; HRMS (+TOF): [M+H]+ 413.2983 calcd for C25H41N2O2Si, found: 413.2985; Rf = 0.17 (4: 1 hex./EtOAc)
Synthesis of (+/−)-dihydrocorynantheol (10)
To a 25 mL round bottomed flask containing 71 mg (0.17 mmol, 1 equiv.) compound 27 was added 1 mL dry MeOH followed by a spatula tip of PPTS. The reaction was heated at reflux for 3 hr, added to 1 N NaOH, extracted twice with CH2Cl2, dried over Na2SO4, and concentrated. The resultant residue was purified by silica gel flash chromatography eluting with 10% to 20% MeOH in CH2Cl2 to yield 46 mg (90%) of the title compound as a white foam.
1HNMR (300 MHz, CDCl3): δ 9.04 (bs, 1H), 7.41 (d, J = 7.5, 1H), 7.29 (d, J = 7.8, 1H), 7.08 (m, 2H), 3.61 (t, J = 6.0, 2H), 3.58 (bs, 1H), 3.00 (m, 4H), 2.69 (m, 1H), 2.47 (m, 1H), 2.16 (m, 1H), 1.88 (t, J = 11.4, 1H), 1.79 (m, 1H), 1.53 (m, 1H), 1.39 (m, 1H), 0.97–1.29 (m, 5H), 0.84 (t, J = 7.2, 3H); 13CNMR (75MHz, CDCl3): δ 136.4, 134.5, 127.1, 121.3, 119.3, 118.1, 111.3, 107.3, 60.1, 59.9, 53.1, 50.5, 41.3, 37.0, 35.1, 34.9, 23.4, 21.4, 11.0; IR (NaCl, film): 3256, 2813, 2757 cm−1; HRMS (+TOF): [M+H]+ 299.2118 calcd for C19H27N2O, found: 299.2116; Rf = 0.16 (10% MeOH in CH2Cl2). Spectroscopic properties agree in all respects with those previously reported.17, 18
Synthesis of 1-(3,4-dimethoxyphenethyl)-5-ethyl-4-(2-hydroxyethyl)piperidin-2-one (30)
To a 25 mL round-bottomed flask containing 85 mg (0.42 mmol, 1 equiv.) 20 was added 4.2 mL dry THF, 114 mg (0.631 mmol, 1.5 equiv.) 2-(3,4-dimethoxyphenyl)ethanamine (29), and then 178 mg (0.840 mmol, 2 equiv.) NaBH(OAc)3. The reaction was allowed to stir at ambient temperature for 48 hr before being added to NaHCO3(sat.), extracted thrice with EtOAc, dried over Na2SO4, and concentrated. Purification by silica gel flash chromatography eluting with 5% to 20% MeOH in CH2Cl2 to yield 95 mg (67%, 92% BRSM) of the desired product as a colorless oil.
1HNMR (300 MHz, CDCl3): δ 6.69–6.76 (m, 3H), 3.82 (s, 3H), 3.80 (s, 3H) 3.58 (m, 3H), 3.44 (m, 1H), 3.06 (dd, J = 12.0, 4.8, 1H), 2.93 (dd, J = 12.3, 7.5, 1H), 2.81 (m, 1H), 2.75 (t, J = 7.5, 2H), 2.27 (m, 2H), 2.02 (m, 1H), 1.66 (m, 1H), 1.53 (m, 1H), 1.11–1.32 (m, 3H), 0.82 (t, J = 7.2, 3H). 13CNMR (75MHz, CDCl3): δ 169.4, 148.8, 147.5, 131.5, 120.7, 112.0, 111.2, 60.2, 55.9, 50.6, 49.1, 38.4, 36.3, 33.0, 31.5, 31.4, 20.5, 12.0; IR (NaCl, film): 3406, 1621 cm−1; HRMS (+TOF): [M+H]+ 336.2169 calcd for C19H30NO4, found: 336.2174; Rf = 0.23 (5% MeOH in CH2Cl2).
Synthesis of 2-(-1-(3,4-dimethoxyphenethyl)-5-ethyl-2-oxopiperidin-4-yl)ethyl acetate
To a 10 mL round bottomed flask containing 45 mg (0.13 mmol, 1 equiv.) alcohol 30 dissolved in 1 mL dry CH2Cl2 and 1 mL pyridine was added 19 μL (0.027 mmol, 2 equiv) AcCl followed by a single crystal of DMAP. The reaction was stirred for 30 min at ambient temperature before being added to NaHCO3(sat.), extracted into CH2Cl2, dried over Na2SO4, and concentrated. Purification by silica gel flash chromatography eluting with 10% MeOH in CH2Cl2 yielded 50 mg (99%) of the desired product as a pale yellow oil.
1HNMR (300 MHz, CDCl3) δ 6.74 (m, 3H), 4.06 (m, 2H), 3.84 (s, 3H), 3.82 (s, 3H), 3.52 (m, 2H), 3.08 (dd, J = 12.3, 4.8, 1H), 2.96 (dd, J = 12.3, 7.2, 1H), 2.79 (t, J = 7.8, 2H), 2.31 (qd, J = 17.4, 6.3, 2H), 2.02 (s, 3H), 2.00 (m, 1H), 1.67 (m, 2H), 1.26 (m, 3H), 0.84 (t, J = 7.5, 3H); 13CNMR (75 MHz, CDCl3) δ 171.2, 168.9, 149.0, 147.7, 131.7, 120.9, 112.2, 111.4, 62.6, 56.1, 50.7, 49.3, 38.4, 36.4, 33.2, 32.3, 28.1, 21.2, 20.4, 12.1; IR (NaCl, film): 1737, 1640 cm−1; HRMS (+TOF): [M+H]+ 378.2275 calcd for C21H32NO5, found: 378.2280.
Synthesis of 2-((2R,3S,11bS)-3-ethyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl)ethyl acetate (31)
To a 25 mL round bottomed flask containing 190 mg (0.503 mmol, 1 equiv.) 2-(-1-(3,4-dimethoxyphenethyl)-5-ethyl-2-oxopiperidin-4-yl)ethyl acetate dissolved in 5 mL dry benzene was added 94.0 μL (1.06 mmol, 2 equiv.) freshly distilled POCl3. The reaction was heated to reflux for 2 hr before being concentrated, taken up in 5 mL dry MeOH, and cooled to 0°C. To this stirred solution was carefully added 19 mg (0.50 mmol, 1 equiv.) NaBH4 and the reaction was allowed to warm to ambient temperature for 15 min. The reaction was added to NaHCO3(sat.), extracted thrice with CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by flash chromatography (10%w/w NEt3 on silica gel) eluting with 4: 1 to 0: 1 hex./EtOAc to yield 142 mg (78%) of the desired product as a colorless oil.
1HNMR (300 MHz, CDCl3): δ 6.66 (s, 1H), 6.55 (s, 1H), 4.16 (t, J = 6.3, 2H), 3.83 (s, 3H), 3.82 (s, 3H), 3.11-2.94 (m, 3H), 2.82 (dd, J = 10.8, 6, 1H), 2.55 (dd, J = 15.6, 3.0, 1H), 2.41 (td, J = 11.7, 3.9, 1H), 2.25 (dd, J = 11.4, 2.4, 1H), 2.06 (s, 3H), 1.99 (m, 1H), 1.82 (m, 1H), 1.67 (m, 3H), 1.45 (m, 1H), 1.27 (m, 2H), 0.90 (t, J = 7.2, 3H); 13CNMR (75MHz, CDCl3): δ 171.5, 147.4, 147.2, 130.7, 127.2, 111.6, 108.0, 63.5, 63.1, 59.1, 56.3, 56.0, 53.2, 39.0, 37.5, 34.0, 32.3, 29.6, 21.3, 17.6, 12.8; IR (NaCl, film): 2802, 2748 (Bohlmann bands), 1737 cm−1; HRMS (+TOF): [M+H]+ 362.2326 calcd for C21H32NO4, found: 362.2331.
Synthesis of (+/−)-3-epi-protoemetinol (11)
To a 10 mL round bottomed flask containing 95.0 mg (0.263 mmol, 1 equiv.) tetracycle 31 was added 2 mL MeOH, 1 mL H2O and finally 436 mg (3.15 mmol, 12 equiv.) anhydrous K2CO3. The reaction was stirred for 2 hr at ambient temperature before being added to brine, extracted thrice into CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by silica gel flash chromatography eluting with 2% to 10% MeOH in CH2Cl2 to yield 84 mg (>99%) of the desired product as a white foam.
1HNMR (300 MHz, CDCl3): δ 6.67 (s, 1H), 6.56 (s, 1H), 3.83 (s, 3H), 3.82 (s, 3H), 3.71 (t, J = 6.8, 2H), 3.12-2.94 (m, 3H), 2.82 (dd, J = 10.8, 6.0, 1H), 2.56 (dd, J = 15.9, 3.0, 1H), 2.41 (td, J = 11.7, 3.9, 1H), 2.25 (dd, J = 11.4, 2.4, 1H), 1.99 (m, 1H), 1.87 (m, 2H), 1.59 (m, 3H), 1.45 (m, 1H), 1.26 (m, 2H), 0.90 (t, J = 7.5, 3H); 13CNMR (75MHz, CDCl3): δ 147.5, 147.2, 130.9, 127.3, 111.7, 108.2, 63.6, 61.0, 59.3, 56.3, 56.0, 53.3, 39.2, 36.9, 36.6, 34.0, 29.6, 17.7, 12.9; IR (NaCl, film): 3387, 2803, 2749 cm−1(Bohlmann bands); HRMS (+TOF): [M+H]+ 320.2220 calcd for C19H30NO3, found: 320.2221; Rf = 0.54 (10% MeOH in CH2Cl2). All spectral data was in agreement with previous reports.19
Synthesis of a mixture of alcohols 30 and 33
To a 25 mL flame dried round bottomed flask containing 410 mg (2.05 mmol, 1 equiv.) lactone 21 dissolved in 10 mL dry THF at −78°C was dropwise added 2.15 mL (2.15 mmol, 1.05 equiv.) of a 1.0 M solution of DIBAL-H in THF. The reaction was stirred at −78°C for 30 min before being added to a saturated solution of Rochelle’s salt, extracted thrice with CH2Cl2, dried over Na2SO4, and concentrated to yield 351 mg (84%) of a mixture of lactol isomers, which was used without further purification. To a 50 mL round bottomed flash containing 290 mg (1.43 mmol, 1 equiv) of the above produced mixture of lactol isomers dissolved in 7 mL dry THF was added 390 mg (2.15 mmol, 1.5 equiv.) 2-(3,4-dimethoxyphenyl)ethanamine (29) dissolved in 7 mL dry THF. To this mixture was added 909 mg (4.29 mmol, 3 equiv.) NaBH(OAc)3 and the reaction was stirred at ambient temperature for 24 hr before being added to NaHCO3(sat.), extracted into CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by silica gel chromatography eluting with 5% to 20% MeOH in CH2Cl2 to yield 195 mg (41%) of the desired mixture of products.
1HNMR (300MHz, CDCl3): (mixture of epimers) δ 7 (m, 3H), 3.79 (m, 8H), 3.58 (m, 3H), 3.48 (m, 1H), 3.00 (m, 2H), 2.73 (t, J = 7.5, 2H, 2.52-2.18 (m, 2H), 1.97 (m, 1H), 1.72-1.51 (m, 2H), 1.19 (m, 3H), 0.77 (m, 3H); 13CNMR (75MHz, CDCl3): (mixture of epimers) δ: 169.9, 169.6 149.0, 147.7, 131.7, 120.9, 120.8, 112.2, 112.0, 111.4, 60.3, 59.8, 56.1, 51.7, 50.8, 49.4, 41.0, 39.6, 38.6, 36.4, 36.0, 35.3, 33.3, 33.2, 31.7, 31.6, 23.8, 20.7, 12.2, 11.2; IR (NaCl, film): 3385, 1621 cm−1; HRMS (+TOF): [M+H]+ 336.2169 calcd for C19H30NO4, found: 336.2174; Rf = 0.33 (5% MeOH in EtOAc).
Synthesis of 2-((2R,3R,11bS)-3-ethyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl)ethyl acetate (34)
To a 25 mL round bottomed flask containing 195 mg (0.549 mmol, 1 equiv.) of a mixture of alcohols 30 and 33 dissolved in 5 mL dry CH2Cl2 and 1 mL dry pyridine was added 76 mL (1.10 mmol, 2 equiv.) AcCl followed by a single crystal of DMAP. The reaction was stirred at ambient temperature for 30 min before being added to NaHCO3(sat.), extracted into CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by silica gel flash chromatography eluting with 1% to 10% MeOH in CH2Cl2 to yield 204 mg (>99%) of the desired mixture of products.
To a 25 mL round bottomed flask containing 164 mg (0.434 mmol, 1 equiv.) of the above produced acetate mixture dissolved in 5 mL dry benzene was added 81.0 μL (0.869 mmol, 2 equiv.) freshly distilled POCl3. The reaction was heated to reflux for 2 hr before being concentrated, taken up in 5 mL dry MeOH, and cooled to 0°C. To this stirred solution was carefully added 17 mg (0.43 mmol, 1 equiv.) NaBH4 and the reaction was allowed to warm to ambient temperature for 15 min. The reaction was added to NaHCO3(sat.), extracted thrice with CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by flash chromatography (10%w/w NEt3 on silica gel) eluting with 4: 1 to 0: 1 hex./EtOAc to yield 64 mg (41%) of the desired product (34) as a colorless oil as well as 55 mg (35%) of the C3 epimer (31).
1HNMR (300 MHz, CDCl3): δ 6.66 (s, 1H), 6.56 (s, 1H), 4.17 (t, J = 6.3, 2H), 3.85 (s, 3H), 3.83 (s, 3H), 3.03 (m, 4H), 2.61 (dd, J = 15.6, 3.0, 1H), 2.45 (td, J = 11.4, 3.9, 1H), 2.31 (m, 1H), 2.05 (s, 3H), 2.00 (m, 2H), 1.66 (m, 1H), 1.42 (m, 3H), 1.27-1.07 (m, 2H), 0.90 (t, J = 7.5, 3H); 13CNMR (75 MHz, CDCl3): δ 171.4, 147.6, 147.3, 130.1, 126.9, 111.6, 108.2, 62.8, 61.6, 56.3, 56.0, 52.7, 41.4, 38.2, 37.4, 31.9, 29.4, 23.7, 21.3, 11.3; IR (NaCl, film): 2802, 2748 (Bohlmann bands), 1737 cm−1; HRMS (+TOF): [M+Na]+ 384.2145 calcd. for C21H31NNaO4, found: 384.2147.
Synthesis of (+/−)-protoemetinol (12)
To a 10 mL round bottomed flask containing 61.0 mg (0.169 mmol, 1 equiv.) acetate 34 was added 1 mL MeOH, 0.5 mL H2O, and finally 280 mg (2.03 mmol, 12 equiv.) anhydrous K2CO3. The reaction was stirred for 2 hr at ambient temperature before being added to brine, extracted thrice into CH2Cl2, dried over Na2SO4, and concentrated. The resulting residue was purified by silica gel flash chromatography eluting with 2% to 10% MeOH in CH2Cl2 to yield 54 mg (>99%) of the desired product as a colorless oil.
1HNMR (400 MHz, CDCl3): δ 6.66 (s, 1H), 6.55 (s, 1H), 3.82 (s, 3H), 3.81 (s, 3H), 3.72 (m, 2H), 3.14-2.94 (m, 4H), 2.60 (d, J = 16.4, 1H), 2.46 (td, J = 11.6, 4.0, 1H), 2.32 (d, J = 13.2, 1H), 2.01 (m, 1H), 1.90 (m, 1H), 1.63 (m, 1H), 1.41 (m, 3H), 1.23 (m, 2H), 1.09 (m, 1H), 0.89 (t, J = 7.6, 3H). 13CNMR (75MHz, CDCl3): δ 147.5, 147.2, 129.9, 126.7, 111.5, 108.4, 62.8, 61.5, 60.4, 56.2, 55.9, 52.5, 41.2, 37.7, 37.2, 35.9, 29.1, 23.5, 11.2; IR (NaCl, film): 3373, 2801, 2751 cm−1 (Bohlmann bands); HRMS (+TOF): [M+H]+ 320.2220 calcd for C19H30NO3, found: 320.2224; Rf = 0.46 (10% MeOH in CH2Cl2). All spectral data was in agreement with previous reports.19,20
Supplementary Material
Scheme 1.
Biosynthesis of secologanin derived natural products.
Acknowledgments
We gratefully acknowledge financial support from the National Institutes of Health (Grant GM068011). Mass spectra were obtained on instruments supported by the National Institutes of Health Shared Instrumentation Grant No. GM49631. We also gratefully acknowledge an Eli Lilly Graduate Fellowship to BJE from Eli Lilly. This paper is dedicated to Professor Raymond L. Funk of Pennsylvania State University on the occasion of his 60th birthday.
Footnotes
Supporting Information Available. Additional experimental details and spectra of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- 1.(a) Cordell GA. Lloydia. 1974;37(2):219. [PubMed] [Google Scholar]; (b) Stöckigt J, Zenk MH. J Chem Soc Chem Commun. 1977:646–648. [Google Scholar]; (c) Kutchan TM. Phytochemistry. 1993;32:493–506. doi: 10.1016/s0031-9422(00)95128-8. [DOI] [PubMed] [Google Scholar]
- 2.Beauchamp G, Keast R, Morel D, Liu J, Pika J, Han Q, Lee C, Smith AB, III, Breslin PAS. Nature. 2005;437:45–46. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- 3.(a) Ju HK, Moon TC, Lee E, Baek SH, An RB, Bae K, Son KH, Kim HP, Kang SS, Lee SH, Son JK, Chang HW. Planta Medica. 2003;69:950–953. doi: 10.1055/s-2003-45107. [DOI] [PubMed] [Google Scholar]; (b) Park KS, Chang IM. Planta Medica. 2004;70:778–779. doi: 10.1055/s-2004-827211. [DOI] [PubMed] [Google Scholar]; (c) Benito PB, Lnaza AMD, Sen AMS, Galindez JSD, Matellano LF, Gomez AS, Martinez MJA. Planta Medica. 2000;66:324–328. [Google Scholar]; (d) Cimanga K, Hermans N, Apers S, Miert SV, Heuvel HVD, Claeys M, Pieters L, Vlietinck A. J Nat Prod. 2003;66:97–102. doi: 10.1021/np020215h. [DOI] [PubMed] [Google Scholar]
- 4.(a) Wegener T. Z Phytother. 1998;19:284–294. [Google Scholar]; (b) Boje K, Lechtenberg M, Nahrstedt A. Planta Medica. 2003;69:820–825. doi: 10.1055/s-2003-43225. [DOI] [PubMed] [Google Scholar]; (c) Chrubasik S, Junck H, Breitschwerdt H, Conradt C, Zappe H. Eur J Anasthesiol. 1999;16:118–129. doi: 10.1046/j.1365-2346.1999.00435.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa M, Ueda T, Matsuda H, Yamahara J, Murakami N. Chem Pharm Bull. 1994;42:1691–1693. doi: 10.1248/cpb.42.1691. [DOI] [PubMed] [Google Scholar]
- 6.Kumarasamy Y, Nahar L, Cox PJ, Jaspars M, Sarker SD. Phytomedicine. 2003;10(4):344–347. doi: 10.1078/094471103322004857. [DOI] [PubMed] [Google Scholar]
- 7.(a) Bermejo P, Abad MJ, Diaz AM, Fernandez L, Santos JD, Sanches S, Villaescusa L, Carrasco L, Irurzun A. Planta Medica. 2002;68:106–110. doi: 10.1055/s-2002-20238. [DOI] [PubMed] [Google Scholar]; (b) Chen JL, Blanc P, Stoddart CA, Bogan M, Rozhon EJ, Parkinson N, Ye Z, Cooper R, Balick M, Nanakorn W, Kernan MR. J Nat Prod. 1998;61:1295–1297. doi: 10.1021/np980086y. [DOI] [PubMed] [Google Scholar]; (c) Suksmrarn S, Wongkrajang K, Kirtikara K, Suksmrarn A. Planta Medica. 2003;69:877–879. doi: 10.1055/s-2003-43223. [DOI] [PubMed] [Google Scholar]
- 8.Treimer JF, Zenk MH. Eur J Biochem. 1979;101:225–233. doi: 10.1111/j.1432-1033.1979.tb04235.x. [DOI] [PubMed] [Google Scholar]
- 9.(a) Fujii T, Ohba M. In: The Alkaloids. Brossi A, editor. Vol. 22. Academic Press; New York: 1983. pp. 1–50. [Google Scholar]; (b) De-Eknamkul W, Ounaroon A, Tanahashi T, Kutchan TM, Zenk MH. Phytochemistry. 1997;45(3):477–484. [Google Scholar]
- 10.Ma WW, Anderson JE, McKenzie AT, Byrn SR, McLaughlin JL, Hudson MS. J Nat Prod. 1990;53:1009–1014. doi: 10.1021/np50070a041. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales-Garza MT, Martlin SA, Mata-Cardena BD, Said-Fernandez S. J Pharm Pharmacol. 1993;45:144. doi: 10.1111/j.2042-7158.1993.tb03701.x. [DOI] [PubMed] [Google Scholar]
- 12.Liou YF, Hall IH, Lee KH. J Pharm Sci. 1982;71:745–749. doi: 10.1002/jps.2600710707. [DOI] [PubMed] [Google Scholar]
- 13.(a) Yu P, Wang T, Yu F, Cook JM. Tett Lett. 1997;38:6819–6822. [Google Scholar]; (b) Wang T, Yu P, Li J, Cook JM. Tetrahedron Lett. 1998;39:8009–8012. [Google Scholar]; (c) Yu P, Cook JM. J Org Chem. 1998;63(25):9160–9161. [Google Scholar]; (d) Li J, Yu P, Peterson A, Weber R, Soerens D, Grubisha D, Bennet D, Cook JM. J Am Chem Soc. 1999;121:6998. [Google Scholar]; (e) Yu S, Berner M, Cook JM. J Am Chem Soc. 2000;122:7827–7828. [Google Scholar]; (f) Wang T, Cook JM. Org Lett. 2000;2(14):2057–2059. doi: 10.1021/ol000095+. [DOI] [PubMed] [Google Scholar]; (g) Liu X, Deschamp JR, Cook JM. Org Lett. 2002;4:3339–3342. doi: 10.1021/ol020101x. [DOI] [PubMed] [Google Scholar]; (h) Liao X, Zhou H, Wearing XZ, Ma J, Cook JM. Org Lett. 2005;7:3501–3504. doi: 10.1021/ol051208y. [DOI] [PubMed] [Google Scholar]; (i) Liao X, Zhou H, Yu J, Cook JM. J Org Chem. 2006;71:8884–8890. doi: 10.1021/jo061652u. [DOI] [PubMed] [Google Scholar]
- 14.(a) Martin SF, Benage B, Hunter JE. J Am Chem Soc. 1988;110:5925–5927. [Google Scholar]; (b) Martin SF, Benage B, Geraci LS, Hunter JE, Mortimore M. J Am Chem Soc. 1991;113:6161–6171. [Google Scholar]; (c) Martin SF, Chen KX, Eary CT. Org Lett. 1999;1:79–81. doi: 10.1021/ol990554a. [DOI] [PubMed] [Google Scholar]; (d) Deiters A, Chen K, Eary T, Martin SF. J Am Chem Soc. 2003;125:4541–4550. doi: 10.1021/ja0296024. [DOI] [PubMed] [Google Scholar]
- 15.English BJ, Williams RM. Tetrahedron Lett. 2009;50(23):2713–2715. doi: 10.1016/j.tetlet.2009.03.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deiters A, Pettersson M, Martin SF. J Org Chem. 2006;71:6547–6561. doi: 10.1021/jo061032t. [DOI] [PubMed] [Google Scholar]
- 17.Louansmaa M, Jokela R, Tirkkonen B, Miettinen J, Halonen M. Heterocycles. 1992;34(2):321–339. [Google Scholar]
- 18.Lounasmaa M, Jokela R. Heterocycles. 1990;31(7):1351–1358. [Google Scholar]; (b) Kametani T, Kanaya N, Honda T. Heterocycles. 1981;16(11):1937–1946. [Google Scholar]; (c) Beard RL, Meyers AI. J Org Chem. 1991;56:2091–2096. [Google Scholar]; (d) Itoh T, Yokoya M, Miyauchi K, Nagata K, Ohsawa A. Org Lett. 2006;8:1533. doi: 10.1021/ol0530575. [DOI] [PubMed] [Google Scholar]; (e) Wenkert E, Guo M, Pestchanker MJ, Shi YJ, Vankar YD. J Org Chem. 1989;54:1166–1174. [Google Scholar]
- 19.Nuhant P, Raikar SB, Wypych JC, Delpech B, Marazano C. J Org Chem. 2009;74:9413–9421. doi: 10.1021/jo9019545. [DOI] [PubMed] [Google Scholar]
- 20.(a) Battersby AB, Kapil BS, Bhakuni DS, Popli SP, Merchant JR, Salgar SS. Tetrahedron Lett. 1966:4965–4971. [Google Scholar]; (b) Chang JK, Chang BR, Chuang YH, Chang NC. Tetrahedron. 2008;64:9685–9688. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.