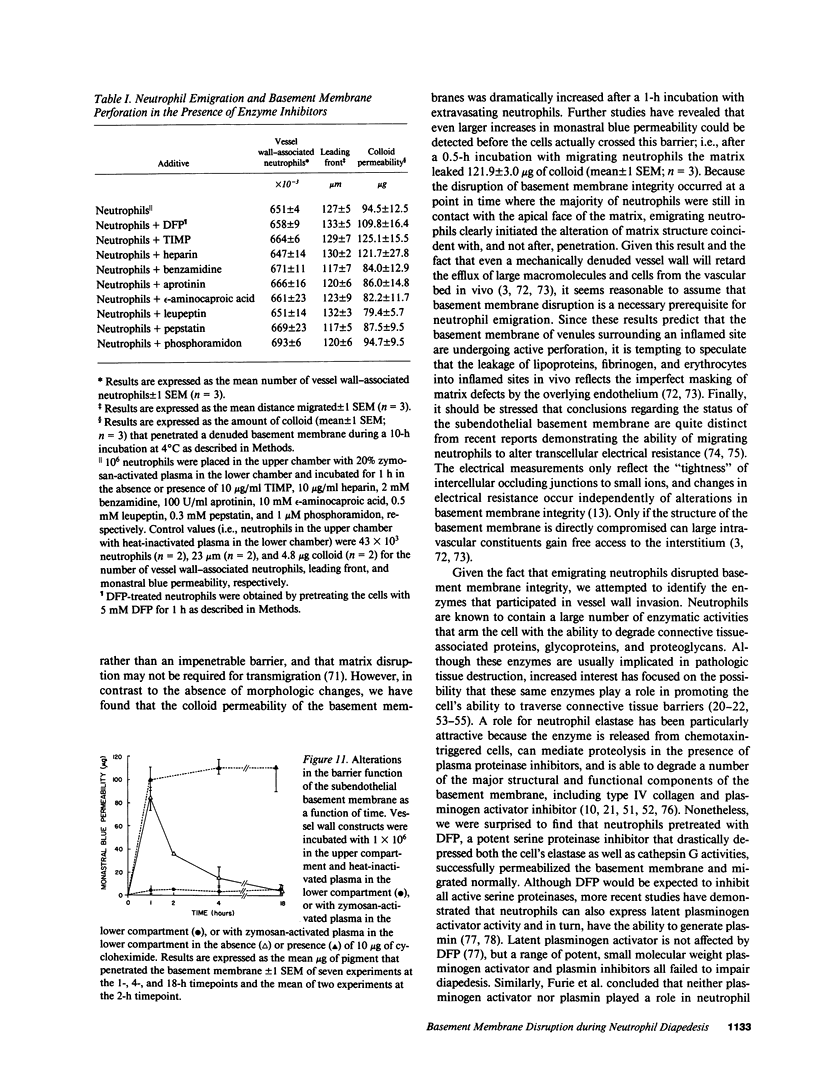
Abstract
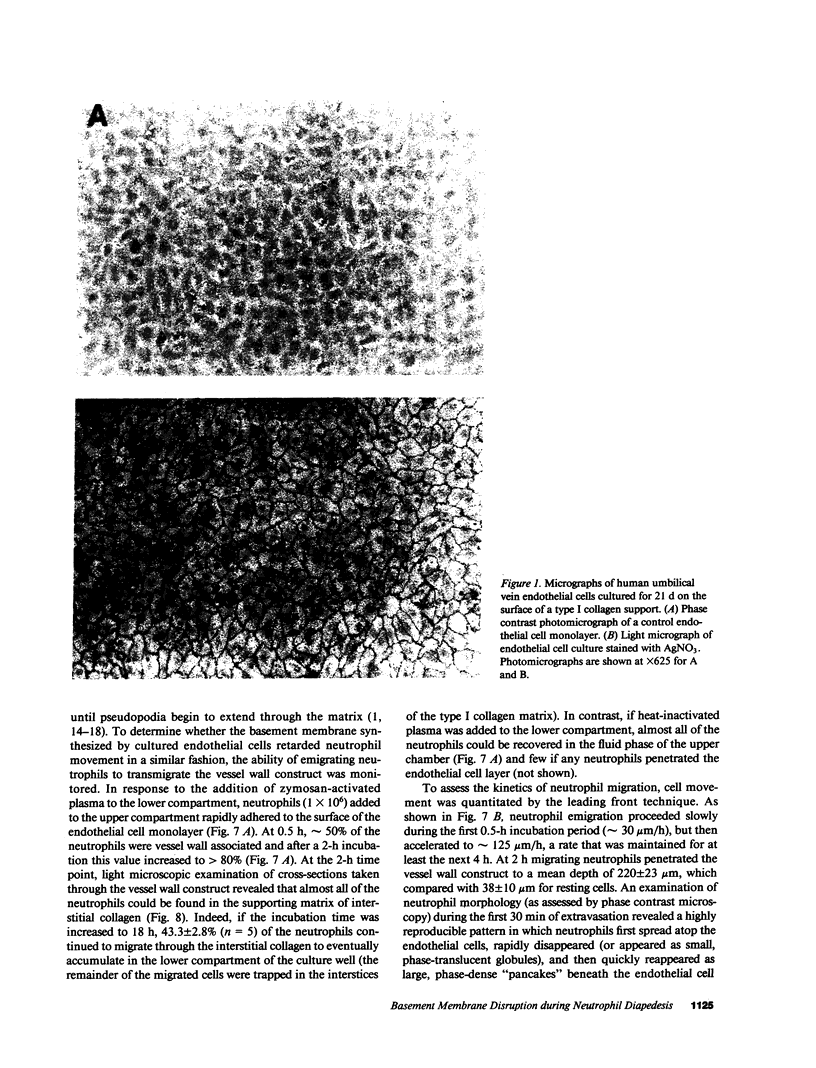
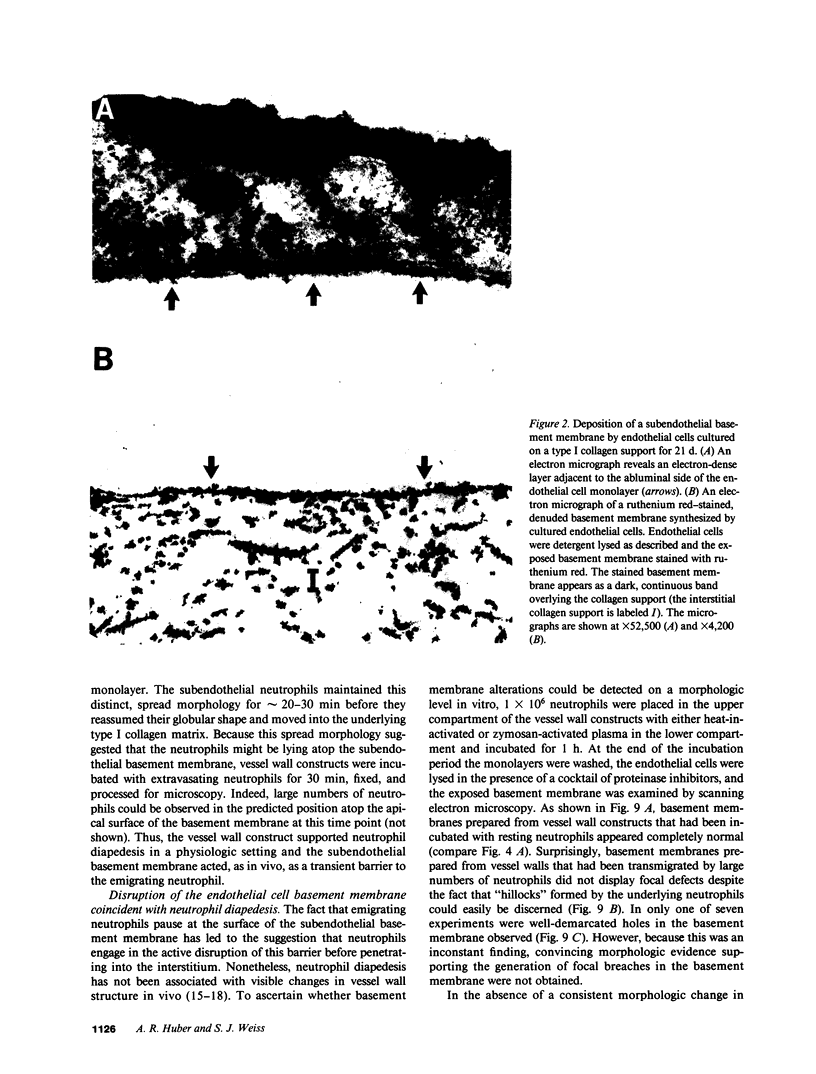
To examine the course of physiologic interactions between extravasating neutrophils and the subendothelial basement membrane, a model of the venular vessel wall was constructed by culturing human umbilical vein endothelial cells on a collagen matrix. After 21 d in culture, the endothelial cell monolayer displayed in vivo-like intercellular borders and junctions, deposited a single-layered, continuous basement membrane that was impenetrable to colloidal particles, and supported neutrophil extravasation in a physiologic manner. Using this model, we demonstrate that neutrophil transmigration in a plasma milieu was associated with a significant disruption of the retentive properties of the basement membrane in the absence of discernable morphologic changes. The loss of basement membrane integrity associated with neutrophil diapedesis was not dependent on neutrophil elastase or cathepsin G and was resistant to inhibitors directed against neutrophil collagenase, gelatinase, and heparanase. Despite the fact that this loss in matrix integrity could not be prevented, basement membrane defects were only transiently expressed before they were repaired by the overlying endothelium via a mechanism that required active protein and RNA synthesis. These data indicate that neutrophil extravasation and reversible basement membrane disruption are coordinated events that occur as a consequence of vessel wall transmigration.
Full text
PDF



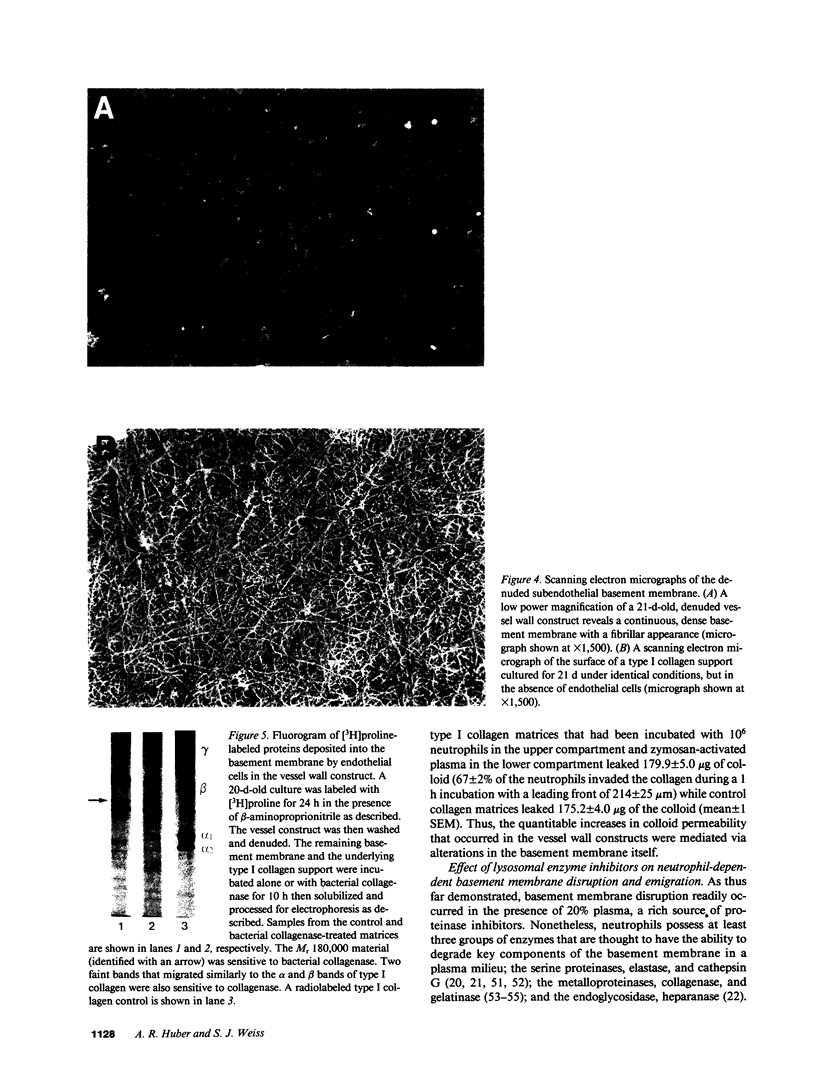
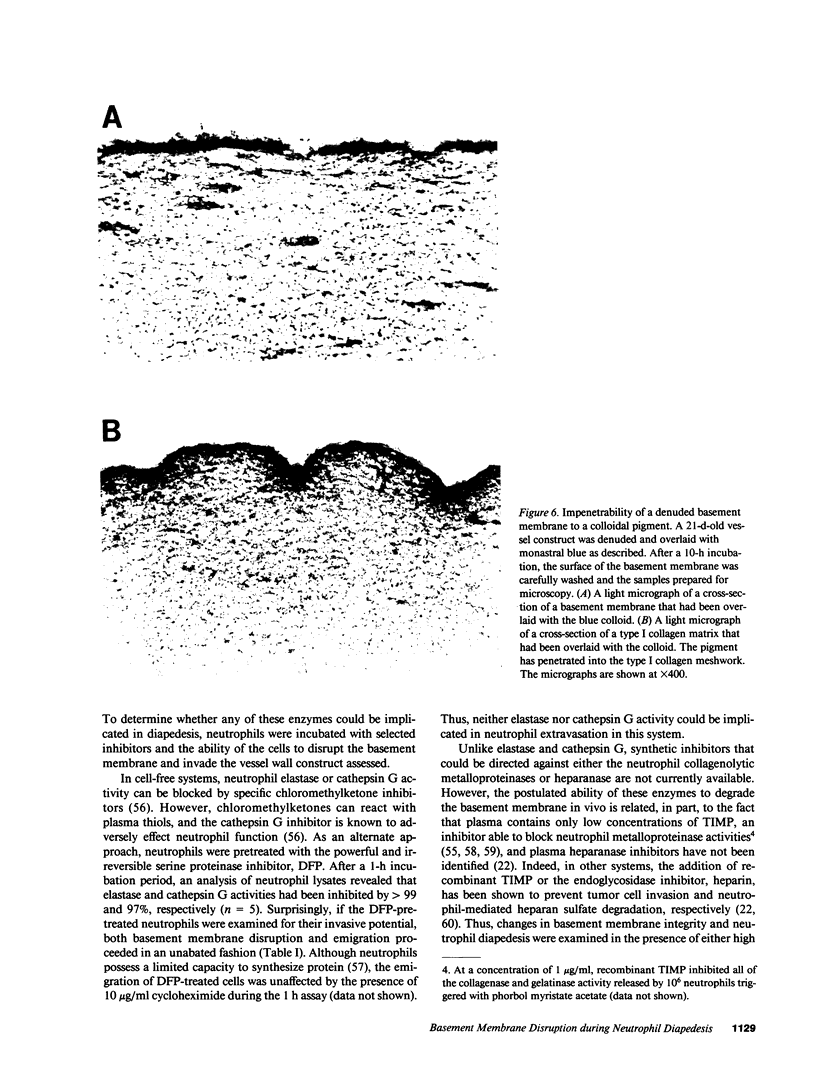
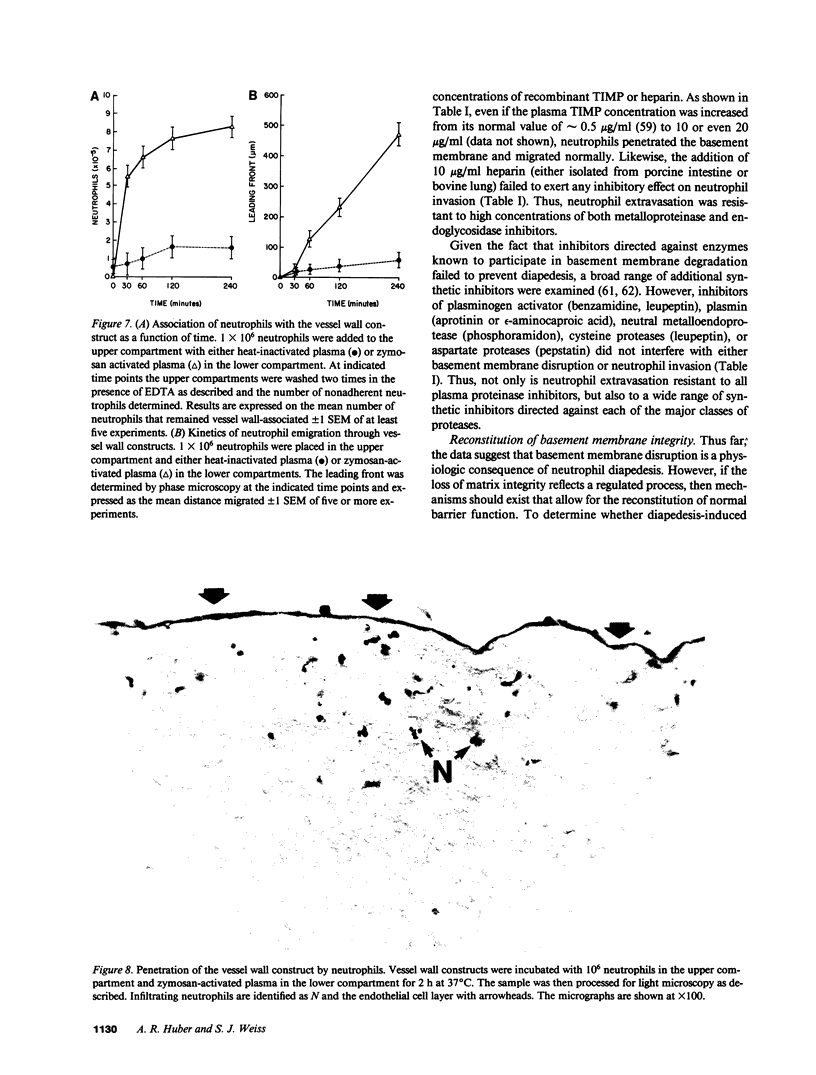

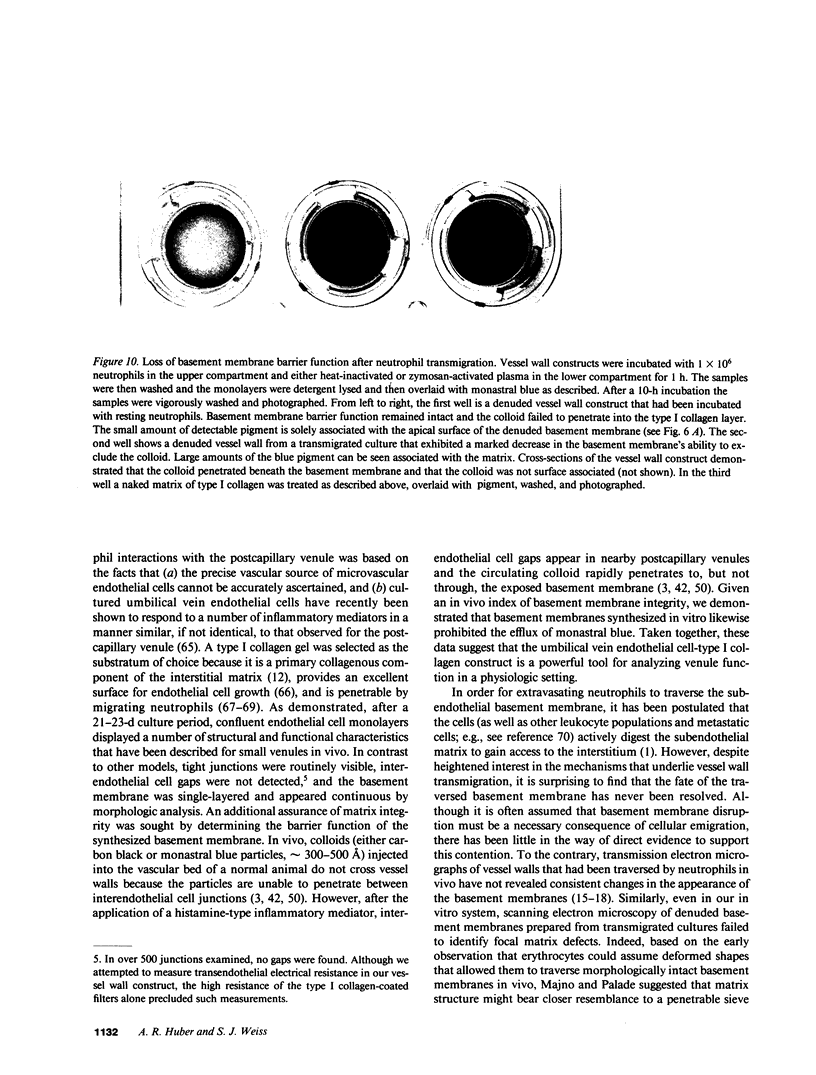



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Sampson P. M., Haselton F. R., McNiff J. M., Mueller S. N., Williams S. K., Fishman A. P., Levine E. M. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol (1985) 1988 Jan;64(1):308–322. doi: 10.1152/jappl.1988.64.1.308. [DOI] [PubMed] [Google Scholar]
- Azzarelli B., Lafuze J. Amniotic basement membrane: a barrier to neutrophil invasion. Am J Obstet Gynecol. 1987 May;156(5):1130–1136. doi: 10.1016/0002-9378(87)90125-6. [DOI] [PubMed] [Google Scholar]
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Bar-Ner M., Mayer M., Schirrmacher V., Vlodavsky I. Involvement of both heparanase and plasminogen activator in lymphoma cell-mediated degradation of heparan sulfate in the subendothelial extracellular matrix. J Cell Physiol. 1986 Aug;128(2):299–306. doi: 10.1002/jcp.1041280223. [DOI] [PubMed] [Google Scholar]
- Beesley J. E., Pearson J. D., Hutchings A., Carleton J. S., Gordon J. L. Granulocyte migration through endothelium in culture. J Cell Sci. 1979 Aug;38:237–248. doi: 10.1242/jcs.38.1.237. [DOI] [PubMed] [Google Scholar]
- Brown A. F. Neutrophil granulocytes: adhesion and locomotion on collagen substrata and in collagen matrices. J Cell Sci. 1982 Dec;58:455–467. doi: 10.1242/jcs.58.1.455. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Campbell M. A. Pericellular proteolysis by neutrophils in the presence of proteinase inhibitors: effects of substrate opsonization. J Cell Biol. 1988 Mar;106(3):667–676. doi: 10.1083/jcb.106.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., Schor S. L., Grant M. E. The biosynthesis of extracellular-matrix components by bovine retinal endothelial cells displaying distinctive morphological phenotypes. Biochem J. 1986 Apr 15;235(2):375–383. doi: 10.1042/bj2350375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. M., Chen W. T. Fibronectin-degrading proteases from the membranes of transformed cells. Cell. 1987 Jan 30;48(2):193–203. doi: 10.1016/0092-8674(87)90423-5. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- Cooper T. W., Eisen A. Z., Stricklin G. P., Welgus H. G. Platelet-derived collagenase inhibitor: characterization and subcellular localization. Proc Natl Acad Sci U S A. 1985 May;82(9):2779–2783. doi: 10.1073/pnas.82.9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Suter E. R., Majno G. The use of colloidal carbon as a tracer for vascular injury. A review. Vasc Dis. 1967 Apr;4(2):107–127. [PubMed] [Google Scholar]
- Crawford J. P., Movat H. Z., Minta J. O., Opas M. Acute inflammation induced by immune complexes in the microcirculation. Exp Mol Pathol. 1985 Apr;42(2):175–193. doi: 10.1016/0014-4800(85)90026-7. [DOI] [PubMed] [Google Scholar]
- Desrochers P. E., Weiss S. J. Proteolytic inactivation of alpha-1-proteinase inhibitor by a neutrophil metalloproteinase. J Clin Invest. 1988 May;81(5):1646–1650. doi: 10.1172/JCI113500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas J., Hechtman H. B., Shepro D. Endothelial-secreted arachidonic acid metabolites modulate polymorphonuclear leukocyte chemotaxis and diapedesis in vitro. Blood. 1988 Mar;71(3):771–779. [PubMed] [Google Scholar]
- Doukas J., Shepro D., Hechtman H. B. Vasoactive amines directly modify endothelial cells to affect polymorphonuclear leukocyte diapedesis in vitro. Blood. 1987 Jun;69(6):1563–1569. [PubMed] [Google Scholar]
- Fehr J., Moser R., Leppert D., Groscurth P. Antiadhesive properties of biological surfaces are protective against stimulated granulocytes. J Clin Invest. 1985 Aug;76(2):535–542. doi: 10.1172/JCI112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie M. B., Naprstek B. L., Silverstein S. C. Migration of neutrophils across monolayers of cultured microvascular endothelial cells. An in vitro model of leucocyte extravasation. J Cell Sci. 1987 Sep;88(Pt 2):161–175. doi: 10.1242/jcs.88.2.161. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Foidart J. M., Savion N. The production and localization of laminin in cultured vascular and corneal endothelial cells. J Cell Physiol. 1981 May;107(2):171–183. doi: 10.1002/jcp.1041070203. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. Extracellular matrix and control of proliferation of vascular endothelial cells. J Clin Invest. 1980 Jun;65(6):1351–1364. doi: 10.1172/JCI109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Migration of human neutrophils in hydrated collagen lattices. J Cell Sci. 1982 Dec;58:95–108. doi: 10.1242/jcs.58.1.95. [DOI] [PubMed] [Google Scholar]
- HURLEY J. V. ACUTE INFLAMMATION: THE EFFECT OF CONCURRENT LEUCOCYTIC EMIGRATION AND INCREASED PERMEABILITY ON PARTICLE RETENTION BY THE VASCULAR WALL. Br J Exp Pathol. 1964 Dec;45:627–633. [PMC free article] [PubMed] [Google Scholar]
- HURLEY J. V. An electron microscopic study of leucocytic emigration and vascular permeability in rat skin. Aust J Exp Biol Med Sci. 1963 Apr;41:171–186. doi: 10.1038/icb.1963.17. [DOI] [PubMed] [Google Scholar]
- HURLEY J. V., XEROS N. Electron microscopic observations on the emigration of leucocytes. Aust J Exp Biol Med Sci. 1961 Dec;39:609–623. doi: 10.1038/icb.1961.60. [DOI] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Harlan J. M., Schwartz B. R., Reidy M. A., Schwartz S. M., Ochs H. D., Harker L. A. Activated neutrophils disrupt endothelial monolayer integrity by an oxygen radical-independent mechanism. Lab Invest. 1985 Feb;52(2):141–150. [PubMed] [Google Scholar]
- Heiple J. M., Ossowski L. Human neutrophil plasminogen activator is localized in specific granules and is translocated to the cell surface by exocytosis. J Exp Med. 1986 Sep 1;164(3):826–840. doi: 10.1084/jem.164.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron G. S., Werb Z., Dwyer K., Banda M. J. Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J Biol Chem. 1986 Feb 25;261(6):2810–2813. [PubMed] [Google Scholar]
- Islam L. N., McKay I. C., Wilkinson P. C. The use of collagen or fibrin gels for the assay of human neutrophil chemotaxis. J Immunol Methods. 1985 Dec 17;85(1):137–151. doi: 10.1016/0022-1759(85)90282-0. [DOI] [PubMed] [Google Scholar]
- Joris I., DeGirolami U., Wortham K., Majno G. Vascular labelling with monastral blue B. Stain Technol. 1982 May;57(3):177–183. doi: 10.3109/10520298209066611. [DOI] [PubMed] [Google Scholar]
- Kalebic T., Garbisa S., Glaser B., Liotta L. A. Basement membrane collagen: degradation by migrating endothelial cells. Science. 1983 Jul 15;221(4607):281–283. doi: 10.1126/science.6190230. [DOI] [PubMed] [Google Scholar]
- Knudsen B. S., Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Binding of plasminogen to extracellular matrix. J Biol Chem. 1986 Aug 15;261(23):10765–10771. [PubMed] [Google Scholar]
- Kramer R. H., Bensch K. G., Davison P. M., Karasek M. A. Basal lamina formation by cultured microvascular endothelial cells. J Cell Biol. 1984 Aug;99(2):692–698. doi: 10.1083/jcb.99.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Fuh G. M., Bensch K. G., Karasek M. A. Synthesis of extracellular matrix glycoproteins by cultured microvascular endothelial cells isolated from the dermis of neonatal and adult skin. J Cell Physiol. 1985 Apr;123(1):1–9. doi: 10.1002/jcp.1041230102. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Fuh G. M., Karasek M. A. Type IV collagen synthesis by cultured human microvascular endothelial cells and its deposition into the subendothelial basement membrane. Biochemistry. 1985 Dec 3;24(25):7423–7430. doi: 10.1021/bi00346a059. [DOI] [PubMed] [Google Scholar]
- La Fleur M., Beaulieu A. D., Kreis C., Poubelle P. Fibronectin gene expression in polymorphonuclear leukocytes. Accumulation of mRNA in inflammatory cells. J Biol Chem. 1987 Feb 15;262(5):2111–2115. [PubMed] [Google Scholar]
- Laug W. E., Weinblatt M. E., Jones P. A. Endothelial cells degrade extracellular matrix proteins produced in vitro. Thromb Haemost. 1985 Aug 30;54(2):498–502. [PubMed] [Google Scholar]
- Levin E. G., Santell L. Association of a plasminogen activator inhibitor (PAI-1) with the growth substratum and membrane of human endothelial cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2543–2549. doi: 10.1083/jcb.105.6.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. H., MacCallum D. K., Jepsen A. The behavior of subcultivated stratified squamous epithelial cells on reconstituted extracellular matrices: initial interactions. Eur J Cell Biol. 1982 Nov;29(1):50–60. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Lohr K. M., Snyderman R. Amphotericin B alters the affinity and functional activity of the oligopeptide chemotactic factor receptor on human polymorphonuclear leukocytes. J Immunol. 1982 Oct;129(4):1594–1599. [PubMed] [Google Scholar]
- Luft J. H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966 Nov-Dec;25(6):1773–1783. [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHESI V. T. SOME ELECTRON MICROSCOPIC OBSERVATIONS ON INTERACTIONS BETWEEN LEUKOCYTES, PLATELETS, AND ENDOTHELIAL CELLS IN ACUTE INFLAMMATION. Ann N Y Acad Sci. 1964 Aug 27;116:774–788. doi: 10.1111/j.1749-6632.1964.tb52545.x. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M. Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochem Cytochem. 1986 Jan;34(1):85–91. doi: 10.1177/34.1.2416801. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A., Meza I., Beaty G., Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):736–745. doi: 10.1083/jcb.87.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner Y., Bar-Ner M., Yahalom J., Ishai-Michaeli R., Fuks Z., Vlodavsky I. Degradation of heparan sulfate in the subendothelial extracellular matrix by a readily released heparanase from human neutrophils. Possible role in invasion through basement membranes. J Clin Invest. 1985 Oct;76(4):1306–1313. doi: 10.1172/JCI112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messadi D. V., Pober J. S., Fiers W., Gimbrone M. A., Jr, Murphy G. F. Induction of an activation antigen on postcapillary venular endothelium in human skin organ culture. J Immunol. 1987 Sep 1;139(5):1557–1562. [PubMed] [Google Scholar]
- Migliorisi G., Folkes E., Pawlowski N., Cramer E. B. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am J Pathol. 1987 Apr;127(1):157–167. [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Milks L. C., Conyers G. P., Cramer E. B. The effect of neutrophil migration on epithelial permeability. J Cell Biol. 1986 Dec;103(6 Pt 2):2729–2738. doi: 10.1083/jcb.103.6.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Bretz U., Baggiolini M. Partial purification of collagenase and gelatinase from human polymorphonuclear leucocytes. Analysis of their actions on soluble and insoluble collagens. Biochem J. 1982 Apr 1;203(1):209–221. doi: 10.1042/bj2030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987 Oct;80(4):1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation J. L. A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol. 1983 Nov;58(6):347–351. doi: 10.3109/10520298309066811. [DOI] [PubMed] [Google Scholar]
- Ossanna P. J., Test S. T., Matheson N. R., Regiani S., Weiss S. J. Oxidative regulation of neutrophil elastase-alpha-1-proteinase inhibitor interactions. J Clin Invest. 1986 Jun;77(6):1939–1951. doi: 10.1172/JCI112523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli B., Weinstein R. S., Soble L. W., Alroy J. Freeze-fracture of monolayer cultures. J Cell Biol. 1977 Mar;72(3):763–769. doi: 10.1083/jcb.72.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Vassalli J. D., Montesano R., Orci L. Urokinase-type plasminogen activator is induced in migrating capillary endothelial cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2535–2541. doi: 10.1083/jcb.105.6.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez H. D., Chenoweth D. E., Goldstein I. M. Attachment of human C5a des Arg to its cochemotaxin is required for maximum expression of chemotactic activity. J Clin Invest. 1986 Dec;78(6):1589–1595. doi: 10.1172/JCI112751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipoly D. J., Crouch E. C. Degradation of native type IV procollagen by human neutrophil elastase. Implications for leukocyte-mediated degradation of basement membranes. Biochemistry. 1987 Sep 8;26(18):5748–5754. doi: 10.1021/bi00392a025. [DOI] [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Ross M. H., Grant L. On the structural integrity of basement membrane. Exp Cell Res. 1968 May;50(2):277–285. doi: 10.1016/0014-4827(68)90446-1. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L., Allen T. D. Effects of culture conditions on the proliferation, morphology and migration of bovine aortic endothelial cells. J Cell Sci. 1983 Jul;62:267–285. doi: 10.1242/jcs.62.1.267. [DOI] [PubMed] [Google Scholar]
- Shah S. V., Baricos W. H., Basci A. Degradation of human glomerular basement membrane by stimulated neutrophils. Activation of a metalloproteinase(s) by reactive oxygen metabolites. J Clin Invest. 1987 Jan;79(1):25–31. doi: 10.1172/JCI112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. O. Leukocytes in chemotactic-fragment-induced lung inflammation. Vascular emigration and alveolar surface migration. Am J Pathol. 1980 Nov;101(2):283–302. [PMC free article] [PubMed] [Google Scholar]
- Sibille Y., Merrill W. W., Cooper J. A., Jr, Polomski L., Gee J. B. Effects of a series of chloromethyl ketone protease inhibitors on superoxide release and the glutathione system in human polymorphonuclear leukocytes and alveolar macrophages. Am Rev Respir Dis. 1984 Jul;130(1):110–114. doi: 10.1164/arrd.1984.130.1.110. [DOI] [PubMed] [Google Scholar]
- Simionescu N. Cellular aspects of transcapillary exchange. Physiol Rev. 1983 Oct;63(4):1536–1579. doi: 10.1152/physrev.1983.63.4.1536. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F., Price T. H., Schwartz S. M., Dale D. C. Neutrophil-endothelial cell interactions on endothelial monolayers grown on micropore filters. J Clin Invest. 1981 Feb;67(2):584–587. doi: 10.1172/JCI110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., GRISHAM J. W. Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am J Pathol. 1961 Aug;39:239–256. [PMC free article] [PubMed] [Google Scholar]
- Wachtfogel Y. T., Abrams W., Kucich U., Weinbaum G., Schapira M., Colman R. W. Fibronectin degradation products containing the cytoadhesive tetrapeptide stimulate human neutrophil degranulation. J Clin Invest. 1988 May;81(5):1310–1316. doi: 10.1172/JCI113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Urban-Pickering M., Marder V. J. Von Willebrand protein binds to extracellular matrices independently of collagen. Proc Natl Acad Sci U S A. 1984 Jan;81(2):471–475. doi: 10.1073/pnas.81.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Curnutte J. T., Regiani S. Neutrophil-mediated solubilization of the subendothelial matrix: oxidative and nonoxidative mechanisms of proteolysis used by normal and chronic granulomatous disease phagocytes. J Immunol. 1986 Jan;136(2):636–641. [PubMed] [Google Scholar]
- Weiss S. J., Peppin G. J. Collagenolytic metalloenzymes of the human neutrophil. Characteristics, regulation and potential function in vivo. Biochem Pharmacol. 1986 Oct 1;35(19):3189–3197. doi: 10.1016/0006-2952(86)90412-0. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz J. I., Huang A. J., Landman S. L., Nicholson S. C., Silverstein S. C. Elastase-mediated fibrinogenolysis by chemoattractant-stimulated neutrophils occurs in the presence of physiologic concentrations of antiproteinases. J Exp Med. 1987 Dec 1;166(6):1836–1850. doi: 10.1084/jem.166.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Ruben G. C. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol. 1987 Dec;105(6 Pt 1):2559–2568. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot P. G., Reinders J. H., Sixma J. J. Perturbation of human endothelial cells by thrombin or PMA changes the reactivity of their extracellular matrix towards platelets. J Cell Biol. 1987 Mar;104(3):697–704. doi: 10.1083/jcb.104.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]