Abstract
Background
The mechanisms by which isoflurane injured the developing brain are not clear. Recent work has demonstrated that it is mediated in part by activation of p75 neurotrophin receptor (p75NTR). p75NTR activates RhoA, a small GTPase that can depolymerize actin. It is therefore conceivable that inhibition of RhoA or prevention of cytoskeletal depolymerization might attenuate isoflurane neurotoxicity. This study was conducted to test these hypotheses using primary cultured neurons and hippocampal slice cultures from neonatal mouse pups.
Methods
Primary neuron cultures (days in vitro, DIV4-7) and hippocampal slice cultures from postnatal day 4-7 mice were exposed to 1.4% isoflurane (4 h). Neurons were pretreated either with TAT-Pep5, an intracellular inhibitor of p75NTR, the cytoskeletal stabilizer Jasplakinolide or their corresponding vehicles. Hippocampal slice cultures were pretreated with TATPep5 prior to isoflurane exposure. RhoA activation was evaluated by immunoblot. Cytoskeletal depolymerization and apoptosis were evaluated with immunofluorescence microscopy using drebrin and cleaved caspase-3 (cl-Csp3) staining respectively.
Results
RhoA activation was increased following 30 min and 120 min of isoflurane exposure in neurons; TAT-Pep5 (10 μM) decreased isoflurane - mediated RhoA activation at both time intervals. isoflurane decreased drebrin immunofluorescence and enhanced cl-Csp3 in neurons, effects that were attenuated by pretreatment with either Jasplakinolide (1 μM) or TAT-Pep5. TAT-ßPep5 attenuated the isoflurane-mediated decrease in phalloidin immunofluorescence. TAT-Pep5 significantly attenuated isoflurane-mediated loss of drebrin immunofluorescence in hippocampal slices.
Conclusion
Isoflurane results in RhoA activation, cytoskeletal depolymerization, and apoptosis. Inhibition of RhoA activation or prevention of downstream actin depolymerization significantly attenuated isoflurane-mediated neurotoxicity in developing neurons.
Introduction
Anesthetic exposure to neonatal animals during the critical period of synaptogenesis triggers widespread neuronal apoptosis and leads to subsequent neurocognitive dysfunction in adulthood 1-5. During normal synaptogenesis, neurons form synaptic connections with target neurons and receive neurotrophic support that is critical for consolidation and maturation of synapses and for cell survival. Neurons that fail to make appropriate synaptic connections lose neurotrophic support and undergo apoptosis. This is part of the normal “pruning” process and an essential component of network formation 6-9.
A key neurotrophin for synaptogenesis is brain derived neurotrophic factor (BDNF). Like other neurotrophic factors, BDNF is secreted as a proneurotrophin (proBDNF) from synaptic vesicles and is proteolytically cleaved by the protease plasmin within the synaptic cleft to form mature BDNF (mBDNF) 10-13. Depending on proteolytic cleavage, BDNF can serve either a prosurvival or a proapoptotic function 10,12,14. Signaling by mBDNF through tropomyosin receptor kinase B enhances neurite growth, stimulates maturation and stabilization of nascent synapses, and causes cell differentiation. In contrast, proBDNF activation of p75 neurotrophin receptor (p75NTR) induces apoptosis and actin cytoskeletal depolymerization 11,15.
In developing neurons, the actin cytoskeleton plays a key role in neurite formation 16. Actin is the most prominent cytoskeletal protein present at both pre- and postsynaptic terminals. Activation of RhoA, a small GTPase that regulates the state of actin cytoskeletal polymerization, can inhibit axonal elongation and cause growth cone collapse 17-19. Interestingly, p75NTR-mediated signaling results in activation of RhoA and subsequent actin depolymerization 20. Recently, we demonstrated that isoflurane neurotoxicity is partly mediated by shifting the balance of BDNF signaling toward proBDNF/p75NTR 15. In that study, we demonstrated that pretreatment with TAT-Pep5, an intracellular p75NTR inhibitor that prevents the activation of RhoA, attenuated isoflurane-mediated reduction in filopodial spines and nascent synapses, and decreased neuronal apoptosis. It is therefore conceivable that isoflurane-mediated proBDNF/p75NTR activation of RhoA results in actin cytoskeletal depolymerization and loss of nascent synapses. Synaptic loss would subsequently lead to neuronal apoptosis. The present study was performed to test that hypothesis.
Materials and Methods
All studies performed on animals were approved by Veteran Affairs San Diego Institutional Animal Care and Use Committee (San Diego, California) and conform to the guidelines of Public Health Service Policy on Human Care and Use of Laboratory Animals.
Preparation of Neuronal Cell Cultures
Neonatal mouse neurons (The Jackson Laboratory, Bar Harbor, ME) were isolated using a papain dissociation kit (Worthington Biochemical, Lakewood, NJ) as previously described 15. At each session, neurons were isolated from 12-20 neonatal pups, 1-3 days old (PND1-3) and grown in vitro for 4 to 7 days (DIV4-7). Experiments performed on a single neuronal isolation (12-20 pups) constitute a sample size of one (n = 1). Hippocampal slices were prepared from PND4-7 mouse pups. Briefly, both left and right hippocampi were dissected at 4°C and were sectioned at 400 μm using a vibratome 1000 plus (Vibratome, Bannockburn, IL). Slices were then placed in culture plate inserts (Millipore, Billerica, MA) above 1 ml of neuronal media. Neurons and slices were cultured in Neuobasal A media supplemented with B27 (2%), 250 mM GLUTMax1, and penicillin/streptomycin (1%) as previously described 15,21. Neurons were cultured on poly-D-lysine/laminin (2 μg/cm2) coated plates or coverslips at 37°C in 5% CO2 for 4-7 days prior to experiments. Cleaved-caspase 3 (Cl-Csp3) (Cell Signaling, Danvers, MA) and drebrin (Abcam, Cambridge, MA) were used to detect apoptosis and to delineate the F-actin cytoskeleton respectively via immunofluorescence deconvolution microscopy. Cl-Csp3 and drebrin staining intensities were normalized to the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes/Invitrogen, Carlsbad, CA). Antibodies to activated RhoA were obtained from Santa Cruz Biotech. RhoA activation was quantified by densitometry and normalized to glyceraldehyde 3-phosphate dehydrogenase (Imgenex; San Diego, CA). The p75NTR inhibitor, TAT-Pep5 [H-YGRKKRRQRRR-CFFRGGFFNHNPRYC-OH] and jasplakinolide, a marine sponge cyclodepsipeptide that stabilizes the actin cytoskeleton, were obtained from CalBiochem (Gibbstown, NJ).
Anesthetic Neurotoxicity Model
Primary neuronal cultures and acute hippocampal slice cultures were placed in a plexi-glass chamber within an incubator and exposed to 1.4% isoflurane, delivered from a calibrated vaporizer from 15 min to 4 h, in a gas mixture of 5% CO2 balanced with air, at a flow rate of 2 L/min. The concentration of isoflurane was monitored continuously by a Datex Capnomac (DRE Medical, Inc., Louisville, KY). The temperature in the incubator was maintained at 37°C.
Protein Extraction and Western Blot Analysis
Proteins in cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% acrylamide gels (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore) by electroelution. Membranes were blocked in 20 mM phosphate-buffered saline (PBS) Tween (1%) containing 4% bovine serum albumin and incubated with primary antibody overnight at 4°C as previously described 15. Primary antibodies were visualized using secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotech) and ECL reagent (Amersham Pharmacia Biotech, Piscataway, NJ). All displayed bands were expected to migrate to the appropriate size and were determined by comparison to molecular weight standards (Santa Cruz Biotech). Image J* was used for densitometric analysis of immunoblots with normalization of RhoA to glyceraldehyde 3-phosphate dehydrogenase.
Immunofluorescence and Deconvolution Microscopy
Neurons were prepared for immunofluorescence microscopy as previously described 15,21. Antibodies used for immunofluorescence were cleaved caspase-3 and drebrin; caspase-3 and drebrin were normalized to the nuclear stain DAPI; phalloidin-594 was normalized to DAPI. Hippocampal slices or cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, incubated with 100 mM glycine (pH. 7.4) for 10 min to quench aldehyde groups, permeabilized in buffered Triton X-100 (0.1%) for 10 min, blocked with 1% bovine serum albumin/PBS/Tween (0.05%) for 20 min and then incubated with primary antibodies (1:100) in 1% bovine serum albumin/PBS/Tween (0.05%) for 24 h at 4°C. Excess antibody was removed by incubation with PBS/Tween (0.1%) for 15 min, and the samples were incubated with fluorescein isothiocyanate or Alexaconjugated secondary antibody (1:250) for 1 h. To remove excess secondary antibody, cells were washed six times at 5 min intervals with PBS/Tween (0.1%) and incubated for 20 min with the nuclear stain DAPI (1:5000) diluted in PBS. Cells were then washed for 10 min with PBS and mounted in gelvatol for microscopic imaging. Deconvolution images were obtained as described elsewhere 21 and captured with a DeltaVision deconvolution microscope system (Applied Precision, Inc., Issaquah, WA). The system includes a Photometrics CCD (Photometrics, Tucson, AZ) mounted on a Nikon TE-200 (Nikon, Melville, NY) inverted epi-fluorescence microscope. Between 30 and 80 optical sections spaced by ~0.1-0.3 μm were taken. Exposure times were set such that the camera response was in the linear range for each fluorophore. Lenses included 100× (NA 1.4), 60× (NA 1.4) and 40× (NA 1.3). The data sets were deconvolved and analyzed using SoftWorx software (Applied Precision, Inc.) on a Silicon Graphics Octane workstation (SGI, Freemont, CA). Image analysis was performed with Data Inspector program in SoftWorx. Maximal projection volume views or single optical sections were visualized. Pixels were assessed quantitatively by CoLocalizer Pro 1.0 software (Colocalization Research Software, Japan and Switzerland). Statistical analysis was performed using Prism 4 (GraphPad Software, La Jolla, CA).
Cytoskeletal Depolymerization Quantification
The drebrin pixels (green) were normalized to nuclear stained pixels (blue) 21. Drebrin is a filamentous F-actin binding protein that stabilizes the actin cytoskeleton within neuritic processes. A reduction in neuritic processes is indicated by decreased drebrin protein expression. Twenty neurons were counted per preparation.
Statistical Analysis
All parametric data were analyzed by one-way analysis of variance (ANOVA) or two-tailed unpaired t-tests with Bonferroni's correction as indicated. Significance was set at p < 0.05. Statistical analysis was performed using Prism 4 (GraphPad Software). Sample size (n=) represents the amount of times the experiments were repeated on separate neuronal cell cultures preparations derived from 12-20 PND1-3 pups.
Results
TAT-Pep5 attenuates isoflurane-mediated RhoA activation in DIV4-7 primary mouse neurons
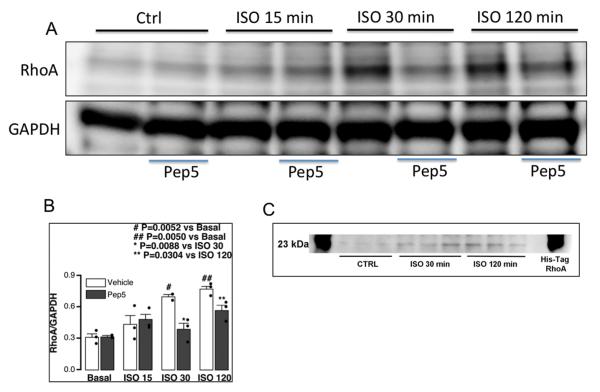
Mixed cortical and hippocampal neurons were isolated from PND1-2 mouse pups and were grown in culture 4-7 days (DIV4-7) and RhoA activation was assessed with or without TAT-Pep5 (fig. 1). Isoflurane (1.4%; 15 min, 30 min, 120 min) exposure resulted in significantly increased (n = 3; # p = 0.0052 vs. basal, ## p = 0.0050 vs. basal) levels of activated RhoA at both 30 min and 120 min compared to basal (figs. 1A and B). Pre-treatment with TAT-Pep5 (15 min; 10 μM) prior to isoflurane exposure significantly attenuated RhoA activation (n = 3; * p = 0.0088 vs. isoflurane 30, ** p = 0.0304 vs. isoflurane 120) at both 30 min and 120 min (figs. 1A and B). An immunoblot showing that the RhoA band (~23-25 kDa) corresponds to the His-Tag RhoA loaded control (fig. 1C).
Fig. 1.
Isoflurane exposure increases RhoA activation in DIV5 neurons in vitro. Primary neurons (4-7 days in vitro – DIV4-7) were exposed to 1.4% isoflurane for 15, 30, and 120 min with and without pretreatment with TAT-Pep5. Immunoblot analysis (A) demonstrated that RhoA was enhanced at both 30 min and 120 min following isoflurane exposure (n = 3; # p = 0.0052 vs. basal, ## p = 0.005 vs. basal) compared to control (Ctrl). Pretreatment with TAT-Pep5 (15 min; 10 μM) significantly decreased isoflurane-mediated RhoA activation at both 30 min and 120 min (n = 3; * p = 0.0088 vs. isoflurane 30, ** p = 0.0304 vs. isoflurane 120). Quantitation of the data is represented in the figure panel (B). An immunoblot showing that the RhoA band (~23-25 kDa) corresponds to the His-Tag RhoA loaded control (C). RhoA values were normalized to glyceraldehyde 3-phosphate dehydrogenase. Error bars, standard error of the mean (s.e.m.).
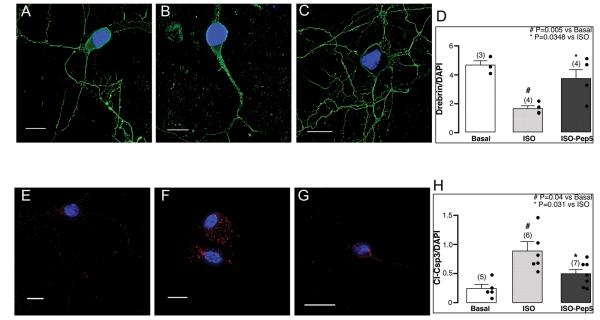
TAT-Pep5 attenuates isoflurane-mediated decrease in neuritic processes and enhanced neuronal apoptosis
Previous work has shown that RhoA regulates actin dynamics in neurons and causes growth cone collapse 17-19, we hypothesized that isoflurane would disrupt neuritic processes through p75NTR activation of RhoA. DIV4-7 primary mouse neurons were treated with or without TAT-Pep5 (10 μM, 15 min) prior to isoflurane exposure (1.4%, 2 h) and stained with drebrin, an F-actin binding protein, the apoptotic marker cleaved caspase 3 (cl-Csp3), and the nuclear marker DAPI (fig. 2). Basally neurons displayed prominent drebrin immunofluorescence (fig. 2A). Exposure to isoflurane resulted in a significant (n = 4; # p = 0.005 vs. basal) reduction in neuritic processes as indicated by reduced drebrin immunofluorescence (fig. 2B), an effect attenuated by TAT-Pep5 (fig. 2C, * p = 0.0348 vs. isoflurane). Quantitation is shown in fig. 2D. Basally neurons expressed minimal cl-Csp3 (fig. 2E). Isoflurane induced a significant (n = 5-7; # p = 0.04 vs. basal) increase in cl-Csp3 expression (fig. 2F), an effect significantly attenuated by TAT-Pep5 (fig. 2G, * p = 0.031 vs. isoflurane), a pharmacologic agent that prevents p75NTR activation of RhoA. Quantitation is shown in fig. 2H. These findings extend the notion that p75NTR activation plays a central role in isoflurane-mediated neurotoxicity.
Fig. 2.
Isoflurane exposure decreases neuritic processes and enhances neuronal apoptosis in DIV4-7 primary neurons. Primary neurons (4-7 days in vitro – DIV4-7) were exposed to 1.4% isoflurane for 2 h with and without pretreatment with TAT-Pep5 (15 min, 10 μM) and incubated with antibodies for drebrin (neuronal F-actin binding protein) (A-C, quantitation shown in panel D), the apoptotic marker, cleaved caspase 3 (cl-Csp3) (E-G, quantitation shown in panel H), and the nuclear marker DAPI. DIV4-7 neurons exposed to isoflurane exhibited a significant reduction (n = 4; # p = 0.005 vs. basal) in dendritic filopodial spines as indicated by decreased drebrin immunofluorescence along dendritic shafts (B) compared to control (Ctrl, A); isoflurane significantly enhanced cl-Csp-3 within the cell body (n = 5-7; # p = 0.04 vs. basal) (F) compared to Ctrl (E). Pretreatment with TAT-Pep5 significantly (n = 4) blocked the isoflurane-mediated decrease in drebrin (C, * p = 0.0348 vs. isoflurane) and the increase in cl-Csp3 (n = 5-7; * p = 0.031 vs. isoflurane) (G). Drebrin (green pixels) along dendrites is normalized to DAPI (blue pixels) or cl-Csp3 (red pixels) is normalized to DAPI. Scale bar, 10 μm. Error bars, standard error of the mean (s.e.m.).
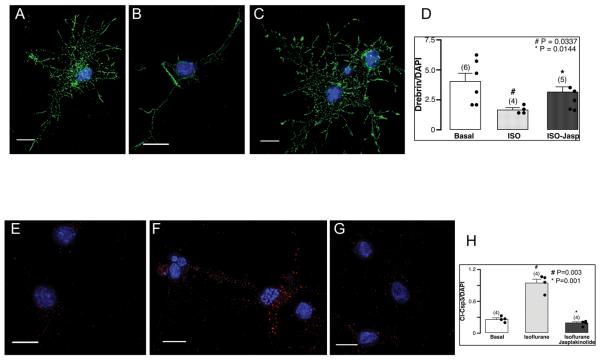
Jasplakinolide attenuates isoflurane-mediated loss of neuritic processes and apoptosis in DIV4-7 neurons
Our results from figures 1 and 2 demonstrate that isoflurane causes p75NTR/RhoA activation resulting in cytoskeletal depolymerization and apoptosis, we thus tested whether actin cytoskeletal depolymerization contributes directly to apoptosis (fig. 3). Primary neuronal cultures were pre-treated (1 h) with Jasplakinolide (1 μM), a marine sponge cyclodepsipeptide that stabilizes the actin cytoskeleton and prevents depolymerization 22-26. Basally neurons displayed prominent drebrin immunofluorescence (fig. 3A). Isoflurane (fig. 3B) decreased (n = 4-6; # p = 0.0337 vs. basal) drebrin immunofluorescence, an effect significantly attenuated with Jasplakinolide pretreatment (fig. 3C, n = 4-6; *p=0.0144 vs. isoflurane). Quantitation is shown in fig. 3D. Basally neurons expressed minimal cl-Csp3 (fig. 3E). Isoflurane significantly increased cl-Csp3 (fig. 3F, n = 4-6; * p = 0.003 vs. basal), an effect attenuated with Jasplakinolide (fig. 3G, n = 4-6; # p = 0.001 vs. basal). Quantitation is shown in fig. 3H. These findings extend the notion that actin cytoskeletal depolymerization plays a central role in isoflurane-mediated neurotoxicity.
Fig. 3.
Jasplakinolide (Jasplakinolide) attenuates isoflurane-mediated reduction in dendritic filopodial spines and enhancement of apoptosis in DIV4-7 neurons. Primary neurons (4-7 days in vitro – DIV4-7) were exposed to 1.4% isoflurane for 4 h with and without pre-treatment with the actin cytoskeleton stabilizer Jasplakinolide (1 h, 1 μM) and incubated with antibodies for drebrin (neuronal Factin binding protein) (A-C, quantitation shown in panel D), the apoptotic marker cleaved caspase 3 (cl-Csp3) (E-G, quantitation shown in panel 3H), and the nuclear marker DAPI. Pretreatment with Jasplakinolide significantly (C, n = 4-6; * p = 0.0144 vs. isoflurane) attenuated isoflurane-mediated decreased (B, # p = 0.0337 vs. basal) in drebrin immunofluorescence and significantly (n = 4-6; * p = 0.001 vs. isoflurane) decreased cl-Csp3 expression (G). Drebrin (green pixels) along dendrites is normalized to DAPI (blue pixels) and cl-Csp3 (red pixels) is normalized to DAPI. Scale bar, 10 μm. Error bars, standard error of the mean (s.e.m.).
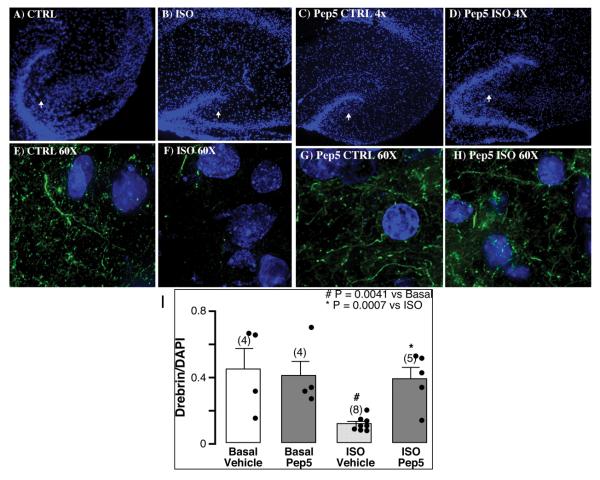
TAT-Pep5 decreases isoflurane-mediated reduction in drebrin immunofluorescence in hippocampal slices
We tested whether the neurotoxic effect of isoflurane on the actin cytoskeleton seen in primary neuronal cultures also occurs in intact hippocampal slices isolated from PND4-7 pups (fig. 4). Hippocampal slices at 4x magnification with DAPI stain are shown in fig. 4A (basal, CTRL), fig. 4B (isoflurane, ISO), fig. 4C (Pep5 CTRL), and fig. 4D (Pep5, ISO). Basally hippocampal slices at 60x magnification displayed normal drebrin immunofluorescence (fig. 4E). Isoflurane exposure (1.4%, 4 h) significantly (n = 4, # p = 0.0041 vs.CTRL) reduced drebrin immunofluorescence (fig. 4F). Pep5 (10 μM, 15 min) treatment on CTRL slices showed no change in drebrin expression (fig. 4G). TAT-Pep5 significantly attenuated isoflurane-mediated reduction in drebrin expression (fig. 4H, n = 4, * p = 0.0007 vs. isoflurane). Quantitation of the data is represented in I. The results demonstrate that the cytoskeletal destabilizing effects of isoflurane occur not only in isolated primary neurons in culture but also in intact hippocampal slices from developing pups.
Fig. 4.
TAT-Pep5 decreases isoflurane-mediated reduction in dendritic spines in hippocampal slices. Hippocampi were dissected from postnatal day (PND4-7) mouse pups and 400 μm slices were exposed to isoflurane with or without pretreatment with TAT-Pep5 (15 min; 10 μM). Images represented in 4X magnification are as follows: basal (CTRL, A), isoflurane (ISO, B), Pep5 CTRL (C), Pep5 ISO (D). 60X magnification images are as follows: CTRL (E), ISO (F), Pep5 CTRL (G), and Pep5 ISO (H). Isoflurane exposure significantly (n = 4, # p = 0.0041 vs. basal) reduced drebrin immunofluorescence in hippocampal slice cultures; TAT-Pep5 significantly (n = 4, * p = 0.0007 vs. isoflurane) attenuated the isoflurane-mediated reduction in drebrin expression. Quantitation of the data is represented in panel I. Drebrin expression (green pixels) was normalized to DAPI (blue pixels). Error bars, standard error of the mean (s.e.m.).
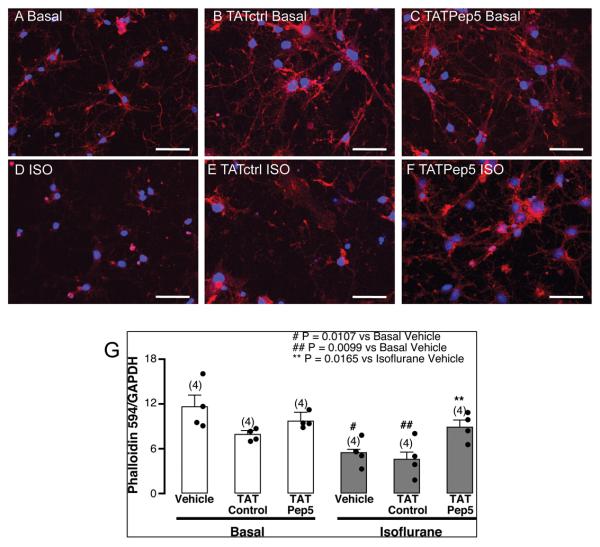
TAT-Pep5 decreases isoflurane-mediated reduction in phalloidin immunofluorescence in DIV4-7 neurons
DIV4-7 primary mouse neurons were treated with or without TATctrl peptide [TAT-Glu(E)-Pro(P)-Gln(Q)-Tyr(Y)-Glu(E)-Glu(E)-lle(I)-Pro(P)-lle(I)-Ala(A)-Cys(C)] (10 μM, 15 min) and with our without TAT-Pep5 (10 μM, 15 min) prior to isoflurane exposure (1.4%, 3 h) and stained with the actin binding toxin phalloidin (conjugated to Alexa-594) and the nuclear marker DAPI. Primary neurons under basal conditions (fig. 5A – no treatment; fig. 5B – TATctrl; fig. 5C – TAT-Pep5) exhibited normal phalloidin immunofluroscence. Exposure of DIV4-7 neurons to isoflurane resulted in a significant (n = 4; # p = 0.0107 vs. basal, p = 0.0094 vs. TATctrl basal, p = 0.0237 vs. TAT-Pep5 basal) reduction in phalloidin immunofluorescence (fig. 5D). Pre-treatment with TATctrl (fig. 5E) did not attenuate the neurotoxic effects from isoflurane (## p = 0.0099 vs. basal, p = 0.0209 vs. TATctrl basal, p = 0.0213 vs. TAT-Pep5 basal). However, isoflurane-mediated effects were significantly attenuated by TAT-Pep5 as shown in fig. 5F (** p = 0.0165 vs. isoflurane, p = 0.019 vs. TATctrl isoflurane). Quantitation of the data is shown in fig. 5G.
Fig. 5.
TAT-Pep5 decreases isoflurane-mediated reduction in phalloidin immunofluorescence. Primary neurons (4-7 days in vitro – DIV4-7) were exposed to 1.4% isoflurane for 3 h with or without pretreatment with TATctrl (10 μM, 15 min) and TAT-Pep5 (10 μM, 15 min) and stained with the actin binding toxin phalloidin (conjugated to Alexa-594) and the nuclear marker DAPI. Phalloidin (red pixels) is normalized to DAPI (blue pixels). A) basal, B) TATctrl basal, C) TAT-Pep5 basal, D) isoflurane, E) TATctrl isoflurane, F) TAT-Pep5 isoflurane. Exposure of DIV4-7 neurons to isoflurane significantly (n = 4; # p = 0.0107 vs. basal, p = 0.0094 vs. TATctrl basal, p = 0.0237 vs. TAT-Pep5 basal) decreased phalloidin immunofluorescence (D). Isoflurane-mediated effects were significantly attenuated by TAT-Pep5 (** p = 0.0165 vs. isoflurane, p = 0.019 vs. TATctrl isoflurane). Pretreatment with TATctrl did not attenuate the neurotoxic effects from isoflurane (## p = 0.0099 vs. basal, p = 0.0209 vs. TATctrl basal, p = 0.0213 vs. TAT-Pep5 basal). Scale bar, 50 μm. Quantitation of the data is represented in panel G. Error bars, standard error of the mean (s.e.m.).
Discussion
We have previously demonstrated that isoflurane exposure during the critical period of synaptogenesis leads to neuronal apoptosis that is mediated in part by preferential signaling of proBDNF-p75NTR 15. The mechanism by which p75NTR activation leads to neurotoxicity following exposure to anesthetic is not clear. What is known is that RhoA, a small GTPase and key regulator of the actin cytoskeleton 27,28, associates with and is activated by the p75NTR 20,29,30. Accumulating evidence has also linked RhoA and apoptosis 31-34. The present data clearly show that isoflurane exposure leads to increased RhoA activation, actin depolymerization and neuronal apoptosis. Inhibition of RhoA activation by TAT-Pep5 or downstream stabilization of the actin cytoskeleton with Jasplakinolide significantly attenuated neuronal death.
Previous work has shown that RhoA initiates cytoskeletal rearrangement through activation of Rho-associated kinase, a downstream serine/threonine kinase 35,36. To support our premise that RhoA plays a central role in isoflurane-mediated cytoskeletal rearrangement and apoptosis we used a specific inhibitor of p75NTR-mediated RhoA activation, TAT-Pep5 20. Pretreatment with TAT-Pep5 prior to isoflurane exposure significantly attenuated RhoA activation, cytoskeletal destabilization, and apoptosis. The link between RhoA and apoptosis previously established by other investigators 31-34 together with the data presented above strongly support the premise that RhoA activation plays a central role in isoflurane-mediated apoptosis. The attenuation of cytoskeletal depolymerization along with attenuation of apoptosis following RhoA inhibition suggested that cytoskeletal destabilization may play a significant role in isoflurane-mediated apoptosis.
Regulation of the actin cytoskeleton is critical for normal neuronal function, including synaptic spine morphogenesis, stability, and function. The actin cytoskeleton is directly involved in neurite arborization and has complex roles at both pre- and postsynaptic terminals 37. For example, at the presynaptic terminal, actin has been implicated in maintaining and regulating synaptic vesicle pools within the bouton as well as replenishing pools through endocytosis 38-40. Recently, two groups have shown that activity-driven induction of presynaptic boutons requires actin polymerization to convert immature non-functional boutons to active mature boutons capable of neurotransmitter release 41,42. At the postsynaptic neuron, actin is critical in anchoring, regulation of lateral trafficking, and assisting with exo-endocytosis of postsynaptic receptors 43,44. Actin is also highly concentrated within dendritic spines 45-47, and is critical for maintenance of spine morphology and plasticity 16,48. Furthermore, abnormal dendritic spine morphology due to alterations in actin assembly has been linked to cognitive and behavioral changes 49-52.
Given the central role of the actin cytoskeleton within neuronal synapses, any disruption in actin dynamics during the key period of synaptogenesis could potentially result in significant neuronal dysfunction. While our earlier data suggested that RhoA activation played a prominent role in isoflurane-mediated apoptosis, the role of actin depolymerization remained undetermined. To examine actin's role, we utilized Jasplakinolide, a cyclodepsipeptide isolated from a marine sponge that stabilizes the actin cytoskeleton and thus prevents depolymerization 22,53. Our data demonstrate that neurons pretreated with Jasplakinolide had significantly reduced isoflurane-mediated apoptosis. The data support the premise that actin depolymerization directly contributes to loss of neuritic processes and to neuronal apoptosis.
The means by which RhoA activation leads to actin depolymerization in the setting of isoflurane exposure is not known. However, a possible mediator of the actin depolymerization initiated by RhoA is Rho-associated kinase, a serine/threonine kinase 35,36. If Rho-associated kinase activation is, in fact, critical to isoflurane mediated actin depolymerization, then it might be possible to reduce isoflurane-induced neurotoxicity by specifically targeting Rho-associated kinase. This possibility will be evaluated in future studies.
Conclusion
In summary, the results demonstrate that isoflurane exposure leads to RhoA activation, cytoskeletal depolymerization and neuronal apoptosis. Inhibition of RhoA or stabilization of the actin cytoskeleton prevents these neurotoxic effects of isoflurane exposure during the critical period of synaptogenesis. These findings are consistent with our hypothesis that isoflurane-mediated apoptosis in developing neurons results from the cytoskeletal destabilizing effects of RhoA activation that is attendant with proBDNF activation of p75NTR. As such, the results provide a mechanistic framework upon which novel therapeutic approaches for the prevention of anesthetic neurotoxicity might be developed.
Summary Statement.
Isoflurane increased apoptosis in neonatal mouse neurons and led to a substantial loss of actin in neurons. This effect was mediated in part by RhoA activation. Inhibition of RhoA or stabilization of actin cytoskeleton prevented apoptosis.
ACKNOWLEDGMENTS
We are grateful for the assistance from the University of California, San Diego Cancer Center Digital Imaging Shared Resource, in particular James Feramisco, Ph.D. (Professor of Medicine, University of California, San Diego, La Jolla, California), Kersi Pestonjamasp, Ph.D. (Junior Faculty, School of Medicine, University of California, San Diego, La Jolla, California), Steve McMullen, B.A., J.D. (Technician, School of Medicine, University of California, San Diego, La Jolla, California). We are also grateful for the technical support from Michael Kidd, B.S. (Technician, Department of Anesthesiology, University of California, San Diego, La Jolla, California), Ana Moreno, B.S. (Technician, Department of Anesthesiology, University of California, San Diego, La Jolla, California), and Yue Hu (Laboratory Technician, Department of Anesthesiology, University of California, San Diego, La Jolla, California).
FUNDING. This work is supported by National Institutes of Health, Bethesda, Maryland, RO1 GM085179 (P. M. Patel), National Institutes of Health, Bethesda, Maryland, RO1 HL091071 (H. H. Patel), and Career Development Award-2 from the Department of Veterans Affairs, 3350 La Jolla Village Drive, San Diego, California (B. P. Head).
Footnotes
http://rsb.info.nih.gov/ij/, last accessed August 1, 2010.
REFERENCES
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jevtovic-Todorovic V, Wozniak DF, Benshoff ND, Olney JW. A comparative evaluation of the neurotoxic properties of ketamine and nitrous oxide. Brain Res. 2001;895:264–7. doi: 10.1016/s0006-8993(01)02079-0. [DOI] [PubMed] [Google Scholar]
- 3.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-Methyl-d-aspartate and γ-cminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–36. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 4.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 5.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 6.Clarke PG. Neuronal death during development in the isthmo-optic nucleus of the chick: Sustaining role of afferents from the tectum. J Comp Neurol. 1985;234:365–79. doi: 10.1002/cne.902340307. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 8.Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–71. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luikart BW, Nef S, Virmani T, Lush ME, Liu Y, Kavalali ET, Parada LF. TrkB has a cell-autonomous role in the establishment of hippocampal Schaffer collateral synapses. J Neurosci. 2005;25:3774–86. doi: 10.1523/JNEUROSCI.0041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 11.Lu B. Pro-region of neurotrophins: Role in synaptic modulation. Neuron. 2003;39:735–8. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- 12.Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–91. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 13.Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603–15. doi: 10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 15.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–25. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Davies AM. Neurotrophins: Neurotrophic modulation of neurite growth. Curr Biol. 2000;10:R198–200. doi: 10.1016/s0960-9822(00)00351-1. [DOI] [PubMed] [Google Scholar]
- 18.Schubert V, Dotti CG. Transmitting on actin: Synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–12. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: Turning on the switch. Genes Dev. 2002;16:1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–7. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- 21.Head BP, Patel HH, Tsutsumi YM, Hu Y, Mejia T, Mora RC, Insel PA, Roth DM, Drummond JC, Patel PM. Caveolin-1 expression is essential for Nmethyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. Faseb J. 2008;22:828–40. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- 22.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–71. [PubMed] [Google Scholar]
- 23.Cramer LP. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr Biol. 1999;9:1095–105. doi: 10.1016/s0960-9822(99)80478-3. [DOI] [PubMed] [Google Scholar]
- 24.Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–70. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 25.Hable WE, Miller NR, Kropf DL. Polarity establishment requires dynamic actin in fucoid zygotes. Protoplasma. 2003;221:193–204. doi: 10.1007/s00709-002-0081-0. [DOI] [PubMed] [Google Scholar]
- 26.Vallotton P, Gupton SL, Waterman-Storer CM, Danuser G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc Natl Acad Sci U S A. 2004;101:9660–5. doi: 10.1073/pnas.0300552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–80. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 28.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–93. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol. 2002;157:565–70. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- 32.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 33.Dubreuil CI, Winton MJ, McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol. 2003;162:233–43. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Gu X, Yuan X. Phenylalanine activates the mitochondria-mediated apoptosis through the RhoA/Rho-associated kinase pathway in cortical neurons. Eur J Neurosci. 2007;25:1341–8. doi: 10.1111/j.1460-9568.2007.05404.x. [DOI] [PubMed] [Google Scholar]
- 35.Katoh H, Aoki J, Ichikawa A, Negishi M. p160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem. 1998;273:2489–92. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- 36.Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26:431–41. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 37.Cingolani LA, Goda Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–56. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 38.Shupliakov O, Bloom O, Gustafsson JS, Kjaerulff O, Low P, Tomilin N, Pieribone VA, Greengard P, Brodin L. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci U S A. 2002;99:14476–81. doi: 10.1073/pnas.212381799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloom O, Evergren E, Tomilin N, Kjaerulff O, Low P, Brodin L, Pieribone VA, Greengard P, Shupliakov O. Colocalization of synapsin and actin during synaptic vesicle recycling. J Cell Biol. 2003;161:737–47. doi: 10.1083/jcb.200212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dillon C, Goda Y. The actin cytoskeleton: Integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 41.Yao J, Qi J, Chen G. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J Neurosci. 2006;26:8137–47. doi: 10.1523/JNEUROSCI.1183-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen W, Wu B, Zhang Z, Dou Y, Rao ZR, Chen YR, Duan S. Activity-induced rapid synaptic maturation mediated by presynaptic cdc42 signaling. Neuron. 2006;50:401–14. doi: 10.1016/j.neuron.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Kuriu T, Inoue A, Bito H, Sobue K, Okabe S. Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms. J Neurosci. 2006;26:7693–706. doi: 10.1523/JNEUROSCI.0522-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol. 2005;168:329–38. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–8. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- 46.Capani F, Martone ME, Deerinck TJ, Ellisman MH. Selective localization of high concentrations of F-actin in subpopulations of dendritic spines in rat central nervous system: A three-dimensional electron microscopic study. J Comp Neurol. 2001;435:156–70. doi: 10.1002/cne.1199. [DOI] [PubMed] [Google Scholar]
- 47.Yuste R, Bonhoeffer T. Genesis of dendritic spines: Insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- 48.Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Wisniewski KE, Segan SM, Miezejeski CM, Sersen EA, Rudelli RD. The Fra(X) syndrome: Neurological, electrophysiological, and neuropathological abnormalities. Am J Med Genet. 1991;38:476–80. doi: 10.1002/ajmg.1320380267. [DOI] [PubMed] [Google Scholar]
- 50.Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–44. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 51.Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: Emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–7. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- 53.Holzinger A. Jasplakinolide: An actin-specific reagent that promotes actin polymerization. Methods Mol Biol. 2009;586:71–87. doi: 10.1007/978-1-60761-376-3_4. [DOI] [PubMed] [Google Scholar]