Abstract
A 19-year-old man developed rapidly progressive muscle weakness and dysesthesia in the extremities, and dyspnea after a flu-like episode. Nerve conduction studies showed reduced motor nerve conduction velocities with conduction block, and sensory nerve action potentials could not be evoked. The patient was diagnosed as having Guillain-Barré syndrome (GBS), and was treated with 2 cycles of intravenous immunoglobulin (IVIg) therapy and was assisted by mechanical ventilation. During the recovery course of the illness, he experienced several attacks of psychomotor agitation from the 37th hospital day, and generalized tonic convulsive seizures suddenly developed on the 42nd hospital day. Brain MRI showed high-intensity lesions in the bilateral thalamus and medial temporal lobes. The convulsions were controlled by continuous thiopental infusion (until the 50th hospital day) and mechanical ventilation (until the 84th hospital day). Intravenous methylprednisolone pulse therapy (1,000 mg/day) for 3 days followed by dexamethasone (16 mg/day) was added. After relief of convulsive seizures, prominent orolingual dyskinesia appeared, and on MRI marked atrophy of the bilateral medial temporal lobes was seen. Anti-N-methyl-D-aspartate receptor (NMDAR) antibodies in serum and cerebrospinal fluid were positive on the 92nd hospital day. Anti-NMDAR encephalitis usually affects young females but a small number of male cases with this disease have been reported. Our male patient was unique in having GBS, a post-infectious autoimmune disease, as a preceding disease, suggesting that anti-NMDAR encephalitis itself is caused by a parainfectious autoimmune mechanism.
Key Words: Anti-N-methyl-D-aspartate receptor encephalitis, Guillain-Barré syndrome, Parainfectious autoimmune disorder, Male gender
Introduction
Recently, a unique limbic encephalitis that predominantly affects young females and shows various manifestations, including initial psychosis, and subsequent central hypoventilation, intractable seizures, dysautonomia and prominent orofacial dyskinesia, has been noted [1, 2]. In patients with this disorder a new anti-neural antibody for the NR1/NR2 heteromers of the N-methyl-D-aspartate receptor (NMDAR) has been identified as a disease-specific hallmark [3], recently adding that the NR1 is possibly a main epitope [2]. Thus, this disorder is now called anti-NMDAR encephalitis. Although it is now widely accepted that the presence of ovarian teratoma is an important predisposing factor for the development of anti-NMDAR encephalitis [4, 5], the mechanisms that initiate the disease are still incompletely understood.
We here report on a young male patient with anti-NMDAR encephalitis which developed during the recovery course of severe Guillain-Barré syndrome (GBS), and propose that a post-infectious autoimmune mechanism may play an important role in the pathogenesis of anti-NMDAR encephalitis.
Case Report
A 19-year-old man with no history of systemic disease was hospitalized due to muscle weakness and dysesthesia in the extremities following cough and nasal discharge 2 weeks earlier. Neurological examination showed muscle weakness and sensory disturbance in the distal parts of the extremities, and all deep tendon reflexes were absent. Cerebrospinal fluid (CSF) at 5 days after onset of weakness contained a normal cell count, but the concentration of total protein was elevated (52.8 mg/dl). The patient was diagnosed as having GBS and treated with intravenous immunoglobulin (IVIg) therapy (0.4 g/kg/day) for 5 days. However, the symptoms worsened gradually, he was not able to raise his upper limbs and to walk from the 5th hospital day. Furthermore, dysarthria, dysphagia, and dyspnea appeared gradually from the 9th hospital day. He was transferred to our hospital on the 12th hospital day. On the day of transfer, nerve conduction studies showed reduced motor nerve conduction velocities (right median nerve: 11.3 m/s, normal value >55 m/s, right tibial nerve: 36.6 m/s, normal value >45 m/s) with conduction block (fig. 1), and sensory nerve action potentials could not be evoked in both nerves. The F-wave of the right tibial nerve was not evoked. There was neither anti-GM1 IgG antibody nor anti-GQ1b IgG antibody in the serum. Although mechanical ventilation was necessary from the day of transfer, muscle weakness in the extremities and respiratory function gradually improved after the second course of IVIg therapy from the 22nd hospital day for 3 days, and the patient was trained for weaning from mechanical ventilation. However, he started to experience occasional psychomotor agitation from the 37th hospital day, and generalized tonic convulsive seizures suddenly developed following paralytic ileus and tachycardia on the 42nd hospital day. Since the convulsive seizures did not respond to intravenous administration of diazepam, phenytoin, or midazolam, continuous thiopental infusion was used and mechanical ventilation was continued. CSF showed a slightly increased cell count (8/μl, mononuclear cells 7), a highly elevated level of total protein (446 mg/dl), and normal glucose concentration. Brain MRI showed high-intensity lesions in the bilateral thalamus and medial temporal lobes on diffusion and FLAIR images (fig. 2a), and the electroencephalogram showed diffuse spike and wave complexes. He was diagnosed as having some type of autoimmune encephalitis, and intravenous methylprednisolone pulse therapy (1,000 mg/day) for 3 days followed by dexamethasone (16 mg/day) was administered, and used until the 70th hospital day with a gradual dose reduction. Although the generalized convulsive seizures disappeared and the infusion of thiopental was ceased on the 52nd hospital day, orolingual dyskinesia gradually developed. On the brain MRI on the 64th hospital day, the abnormal high-intensity lesions in the bilateral thalamus were reduced, while marked atrophy of the bilateral medial temporal lobes was seen (fig. 2b). Respiratory failure and autonomic dysfunction that included disturbed bowel movement and abnormal cardiovascular responses gradually improved, and he was released from mechanical ventilation on the 84th hospital day. However, the patient remained unresponsive to verbal commands and severe orolingual dyskinesia persisted until he was transferred to a local hospital on the 193rd hospital day.
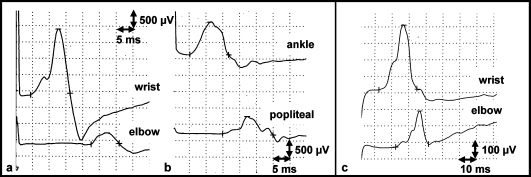
Fig. 1.
Motor nerve conduction study of the right median nerve and the right tibial nerve. a Right median nerve on the 12th hospital day. b Right tibial nerve on the 12th hospital day. These studies showed reduced motor nerve conduction velocities (MCV) with conduction block (median nerve 11.3 m/s, tibial nerve 36.6 m/s). The sizes of the compound muscle action potential of the right median nerve were 2.0 mV at the wrist and 0.33 mV at the elbow, and those of the right tibial nerve were 1.0 mV at the ankle and 0.57 mV at the popliteal fossa, respectively. c Motor nerve conduction study of the right median nerve on the 94th hospital day, showing improvement of the MCV (23.2 m/s) and severity of the conduction block (the sizes of the compound muscle action potential were 0.404 mV at the wrist and 0.225 mV at the elbow). MCV of right tibial nerve on the 94th day was not evoked.
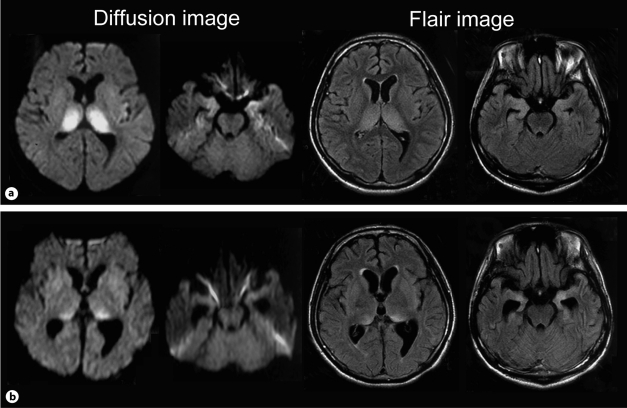
Fig. 2.
Brain MRI findings. a Images on the 42nd hospital day showed high-intensity lesions in the bilateral thalamus and bilateral medial temporal lobes. b Images on the 64th hospital day revealed improved abnormal high-intensity lesions in the bilateral thalamus but progressive atrophy of the bilateral medial temporal lobes.
During investigation of the causes of the encephalitis, there were no laboratory data suggesting viral encephalitis, including herpes simplex, influenza, varicella zoster, cytomegalovirus, measles, and human herpes virus-6. Urological examination was normal and no detectable neoplasm was seen on either chest or abdominal CT. Two years after the onset of seizures, his condition was very similar to when he was discharged from our hospital, and repeated examinations showed no appearance of a tumor.
Immunochemical Analysis of Anti-NMDAR Antibody in Serum and CSF
Detection of anti-NMDAR antibody on the same samples was carried out as follows [6]: cDNA encoding NR1 and NR2B was ligated into the expression vectors and transfected into human embryonic kidney (HEK) 293 cells in the media containing 10 μM MK-801 using Lipofectamine (Invitrogen). Twelve hours after transfection, HEK-293 cells were fixed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4) for 20 min. After non-specific binding was blocked with 10% goat serum in PBS, these cells were incubated with patient sera (1:40) or CSF (1:2) overnight at 4°C and then with FITC-conjugated rabbit anti-human IgG (BD Biosciences) for 30 min at room temperature. SlowFade gold anti-fade reagent (Molecular Probes) was applied to the slides and the staining was observed under a fluorescence microscope.
Both serum and CSF obtained from the 92nd hospital day specifically reacted with HEK-293 cells expressing heteromers of NR1/NR2B (fig. 3).
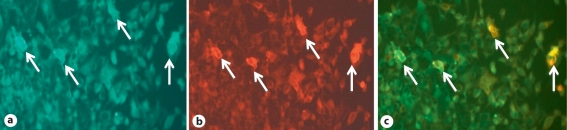
Fig. 3.
Immunohistochemical demonstration of antibodies against NMDAR. a CSF of the patient showing positive immunoreactivity against heteromers of NR1 and NR2B subunits of NMDAR. b Anti-rabbit IgG showing positive immunoreactivity against NR1 subunit of NMDAR. c Merge image. Arrows indicate positively stained HEK cells. Immunofluorescence staining (×200).
Discussion
In our case, encephalitis was shown by abnormal intensity of the bilateral thalamus and medial temporal lobes on brain MRI at an early stage. However, the former thalamic lesions seemed to be edema due to status epilepticus, and the main lesion of this encephalitis was assumed to be in the bilateral medial temporal lobes, because the thalamic lesions rapidly resolved after treatment, whereas the bilateral medial temporal lobes showed progressive atrophy. Moreover, his clinical manifestations consisted of psychiatric symptoms, intractable seizures, dysautonomia and involuntary movements, and anti-NMDAR antibody was demonstrated in both serum and CSF. He was finally diagnosed as having anti-NMDAR encephalitis.
Anti-NMDAR encephalitis usually affects young females, and an early series of patients with this disease was found to have a high association of ovarian teratoma [3, 4]. Thus, the pathogenetic significance of ovarian teratoma in this encephalitis was investigated and it was proposed that, since the ovarian teratomas obtained from diseased patients showed mature- and immature-appearing neurons with expression of NR2B and/or NR2A [3, 4], ectopically expressed NMDARs in ovarian teratoma contribute to the production of antibodies to NMDARs [3]. Thus, early removal of ovarian teratoma has been recommended for patients with this disease [7]. However, a recent study on a large number of patients with anti-NMDAR encephalitis has shown that this ovarian tumor could not be found in about 40 to 80% of adult patients with the disease [1, 2, 8], although careful follow-up examinations on the detection of tumors are always required in these patients. Increased recognition of this unique encephalitis has also disclosed that children and adolescents also encounter it [9, 10], although the frequency of associated ovarian teratoma was much lower in children than adults. Additionally, a small number of male cases with anti-NMDAR encephalitis [11] were reported, indicating that other causes besides ovarian teratoma can produce encephalitis.
In the pathogenesis of anti-NMDAR encephalitis, the antibody immune response has been shown to be more relevant than cytotoxic T-cell mechanisms [12] and the vast majority of patients with this type of disease have a history of prodromal flu-like symptoms. It was, therefore, suggested that the preceding flu-like illness leads to the triggering of abnormal antibody production targeting NMDARs [12]. In this situation the presence of ovarian teratoma with a high expression of NMDAR epitopes may predispose or exaggerate the production of anti-NMDAR antibodies; these NMDAR antibodies then cause a specific, titer-dependent, and reversible decrease in NMDAR surface density and synaptic localization, especially in the hippocampus [13], resulting in learning, memory, and other behavioral deficits seen in patients with anti-NMDAR-encephalitis. Thus, this encephalitis seems to be causally related to a parainfectious autoimmune mechanism. Immunosuppressive therapy, including corticosteroid, plasma exchange and IVIg, has been used for the treatment of this disease [1]. Recently, rituximab, an anti-CD20 monoclonal antibody, is expected to accelerate the recovery of patients with this type of disease [14].
Our young male patient with anti-NMDAR encephalitis lacked testicular or mediastinal teratoma, which was previously reported to be of paraneoplastic origin [11]. On the other hand, his clinical course was characterized by a preceding attack of GBS: this disease is a representative post-infectious peripheral demyelinating neuropathy with underlying autoimmune abnormalities [15]. Although a causative relationship between GBS and anti-NMDAR encephalitis has not been studied, it is likely that GBS-related abnormal immune reactions secondarily caused another autoimmune state with anti-NMDAR encephalitis. Although serial examinations of anti-NMDAR antibodies in both serum and CSF might be useful in clarifying the underlying immune condition of this patient, there were no available samples. This case report has provided the clinical evidence that a parainfectious autoimmune reaction is an important pathogenetic mechanism in the development of anti-NMDAR encephalitis.
Acknowledgements
This work was supported by a grant from the Neuroimmunological Disease Division, the Ministry of Public Health, Labor and Welfare, Japan.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Dalmau J, Gleichman A, Hughes EG, Rossi JE, Peng X, Lai M, Dessain S, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorders of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, Suzuki K, Lynch DR, Suzuki N, Hara T, Dalmau J. Anti-NMDA receptor encephalitis in Japan. Long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tachibana N, Shirakawa T, Ishii K, Takahashi Y, Tanaka K, Arima K, Yoshida T, Ikeda S. Expression of various glutamate receptors including N-methyl-D-aspartate receptor (NMDAR) in an ovarian teratoma removed from a young woman with anti-NMDAR encephalitis. Intern Med. 2010;49:2167–2173. doi: 10.2169/internalmedicine.49.4069. [DOI] [PubMed] [Google Scholar]
- 7.Seki M, Suzuki S, Iizuka T, Shimizu T, Nihei Y, Suzuki N, Dalmau J. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2010;79:324–326. doi: 10.1136/jnnp.2007.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamei S, Kuzuhara S, Ishihara M, Morita A, Taira N, Togo M, Matsui M, Ogawa M, Hisanaga K, Mizutani T, Kuno S. Nationwide survey of acute juvenile female non-herpetic encephalitis in Japan: relationship to anti-N-methyl-D-aspartate receptor encephalitis. Intern Med. 2009;48:673–679. doi: 10.2169/internalmedicine.48.1898. [DOI] [PubMed] [Google Scholar]
- 9.Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, Campen CJ, Moss H, Peter N, Gleichman AJ, Glaser CA, Lynch DR, Rosenfeld MR, Dalmau J. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niehusmann P, Dalmau J, Rudlowski C, Vincent A, Elger CE, Rossi JE, Bien CG. Diagnostic value of N-methyl-D-aspartate receptor antibodies in women with new-onset epilepsy. Arch Neurol. 2009;66:458–464. doi: 10.1001/archneurol.2009.5. [DOI] [PubMed] [Google Scholar]
- 11.Eker A, Saka E, Dalmau J, Kurne A, Bilen C, Ozen H, Ertoy D, Ogurz KK, Elibol B. Testicular teratoma and anti- N-methyl-D-aspartate receptor-associated encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:1082–1083. doi: 10.1136/jnnp.2008.147611. [DOI] [PubMed] [Google Scholar]
- 12.Tzn E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737–743. doi: 10.1007/s00401-009-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons D, Lynch DR, Dalmau J, Balice-Gordon RJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishiura H, Matsuda S, Higashihara M, Hasegawa M, Hida A, Hanajima R, Yamamoto T, Shimizu J, Dalmau J, Tsuji S. Response of anti-NMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology. 2008;71:1921–1922. doi: 10.1212/01.wnl.0000336648.43562.59. [DOI] [PubMed] [Google Scholar]
- 15.Griffin JW, Sheikh K. The Guillain-Barré syndrome. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4th ed. Philadelphia: Elssevier and Saunders; 2005. pp. 2197–2220. [Google Scholar]