Abstract
Prior studies, both in vitro and in vivo, have suggested that cutaneous porphyrin photosensitization requires the generation of superoxide anion (.O2-) and various other reactive oxygen metabolites. No unifying concept has emerged, however, that unequivocally demonstrates the source of generation of these species. Since xanthine oxidase is known to generate .O2- in reperfused ischemic tissue and in certain inflammatory disorders, we attempted to assess its role in porphyrin photosensitization. C3H mice were rendered photosensitive by the intraperitoneal administration of dihematoporphyrin ether (DHE) (5 mg/kg) followed by irradiation with visible light. Murine ear swelling was used as a marker of the acute photosensitization response and involvement of oxygen radicals was evaluated using electron spin resonance (ESR) spectroscopy. The administration of allopurinol, a potent inhibitor of xanthine oxidase, afforded 90% protection against DHE-mediated acute photosensitivity in vivo. Furthermore, xanthine oxidase activity was twofold higher in the skin of photosensitized mice than in unirradiated animals. ESR spectra of 5,5-dimethyl-1-pyrroline N-oxide-trapped radicals from the skin of photosensitized mice verified the presence of .O2- and .OH, while neither of these species was detected in the skin of control mice or mice receiving allopurinol. The administration of a soybean trypsin inhibitor or verapamil before irradiation also partially blocked the photosensitivity response, suggesting that calcium-dependent proteases play a role in the activation of xanthine oxidase in this photodynamic process. These data provide in vivo evidence for the involvement of .O2- in DHE-mediated cutaneous photosensitization and suggest that these radicals are generated through the activation of the xanthine oxidase pathway. The administration of allopurinol and calcium channel blockers may thus offer new approaches for the treatment of cutaneous porphyrin photosensitization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Khalidi U. A., Chaglassian T. H. The species distribution of xanthine oxidase. Biochem J. 1965 Oct;97(1):318–320. doi: 10.1042/bj0970318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar M., Mukhtar H., Bickers D. R. Differential role of reactive oxygen intermediates in photofrin-I- and photofrin-II-mediated photoenhancement of lipid peroxidation in epidermal microsomal membranes. J Invest Dermatol. 1988 May;90(5):652–657. doi: 10.1111/1523-1747.ep12560814. [DOI] [PubMed] [Google Scholar]
- Athar M., Mukhtar H., Elmets C. A., Zaim M. T., Lloyd J. R., Bickers D. R. In situ evidence for the involvement of superoxide anions in cutaneous porphyrin photosensitization. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1054–1059. doi: 10.1016/s0006-291x(88)80472-8. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. The apparent production of superoxide and hydroxyl radicals by hematoporphyrin and light as seen by spin-trapping. FEBS Lett. 1980 Nov 17;121(1):161–164. doi: 10.1016/0014-5793(80)81288-9. [DOI] [PubMed] [Google Scholar]
- Chambers D. E., Parks D. A., Patterson G., Roy R., McCord J. M., Yoshida S., Parmley L. F., Downey J. M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985 Feb;17(2):145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Corey E. J., Mehrotra M. M., Khan A. U. Water induced dismutation of superoxide anion generates singlet molecular oxygen. Biochem Biophys Res Commun. 1987 Jun 15;145(2):842–846. doi: 10.1016/0006-291x(87)91041-2. [DOI] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Badwey J. A., Robinson J. M., Karnovsky M. J., Karnovsky M. L. Studies on the mechanism of superoxide release from human neutrophils stimulated with arachidonate. J Biol Chem. 1984 Oct 10;259(19):11851–11857. [PubMed] [Google Scholar]
- Das M., Mukhtar H., Greenspan E. R., Bickers D. R. Photoenhancement of lipid peroxidation associated with the generation of reactive oxygen species in hepatic microsomes of hematoporphyrin derivative-treated rats. Cancer Res. 1985 Dec;45(12 Pt 1):6328–6330. [PubMed] [Google Scholar]
- Dixit R., Mukhtar H., Bickers D. R. Destruction of microsomal cytochrome P-450 by reactive oxygen species generated during photosensitization of hematoporphyrin derivative. Photochem Photobiol. 1983 Feb;37(2):173–176. doi: 10.1111/j.1751-1097.1983.tb04454.x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photosensitizers: therapy and detection of malignant tumors. Photochem Photobiol. 1987 Jun;45(6):879–889. doi: 10.1111/j.1751-1097.1987.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Elmets C. A., Bergstresser P. R., Tigelaar R. E., Wood P. J., Streilein J. W. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J Exp Med. 1983 Sep 1;158(3):781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Gibson S. L., Hilf R. Photosensitization of mitochondrial cytochrome c oxidase by hematoporphyrin derivative and related porphyrins in vitro and in vivo. Cancer Res. 1983 Sep;43(9):4191–4197. [PubMed] [Google Scholar]
- Girotti A. W. Mechanisms of photosensitization. Photochem Photobiol. 1983 Dec;38(6):745–751. doi: 10.1111/j.1751-1097.1983.tb03610.x. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Rozen M. G., Quintavalla J. C., Amoruso M. A. Decrease in mouse lung and liver glutathione peroxidase activity and potentiation of the lethal effects of ozone and paraquat by the superoxide dismutase inhibitor diethyldithiocarbamate. Biochem Pharmacol. 1979;28(1):27–30. doi: 10.1016/0006-2952(79)90265-x. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Razum N. J. Acute skin response in albino mice following porphyrin photosensitization under oxic and anoxic conditions. Photochem Photobiol. 1984 Oct;40(4):435–439. doi: 10.1111/j.1751-1097.1984.tb04614.x. [DOI] [PubMed] [Google Scholar]
- Hawkins C. W., Bickers D. R., Mukhtar H., Elmets C. A. Cutaneous porphyrin photosensitization: murine ear swelling as a marker of the acute response. J Invest Dermatol. 1986 Jun;86(6):638–642. doi: 10.1111/1523-1747.ep12275644. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Fingar V. H. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987 Jun 15;47(12):3110–3114. [PubMed] [Google Scholar]
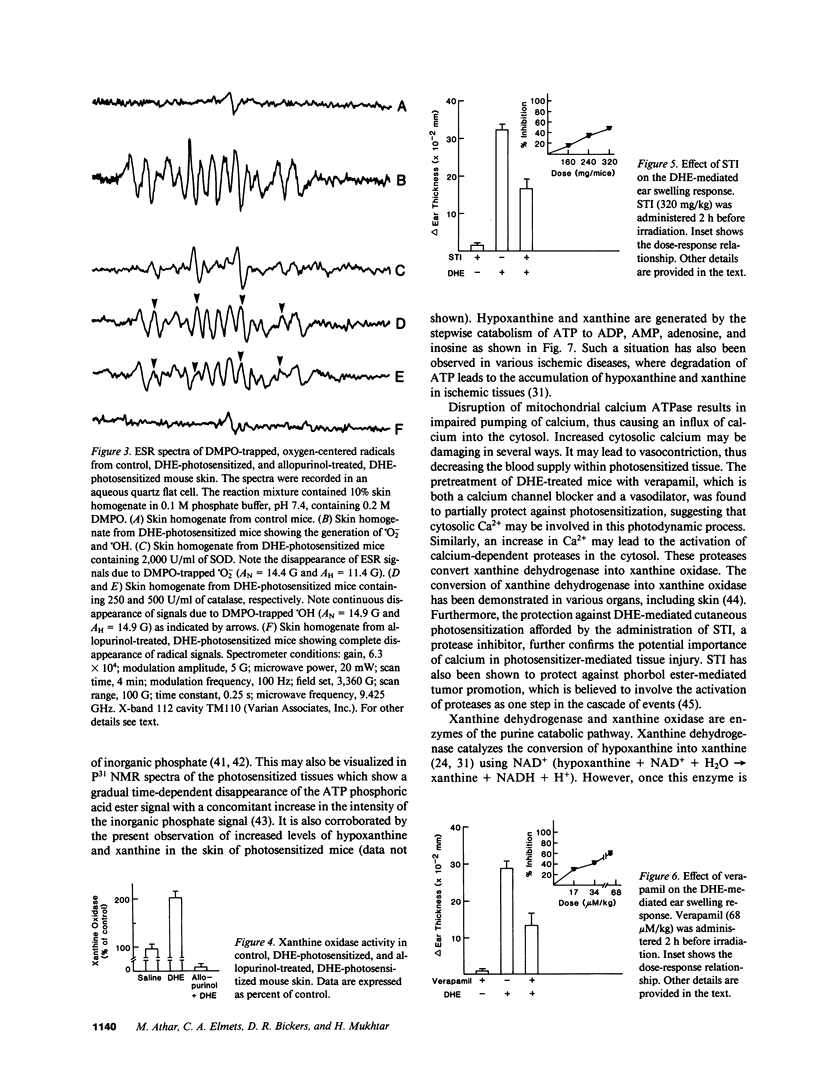
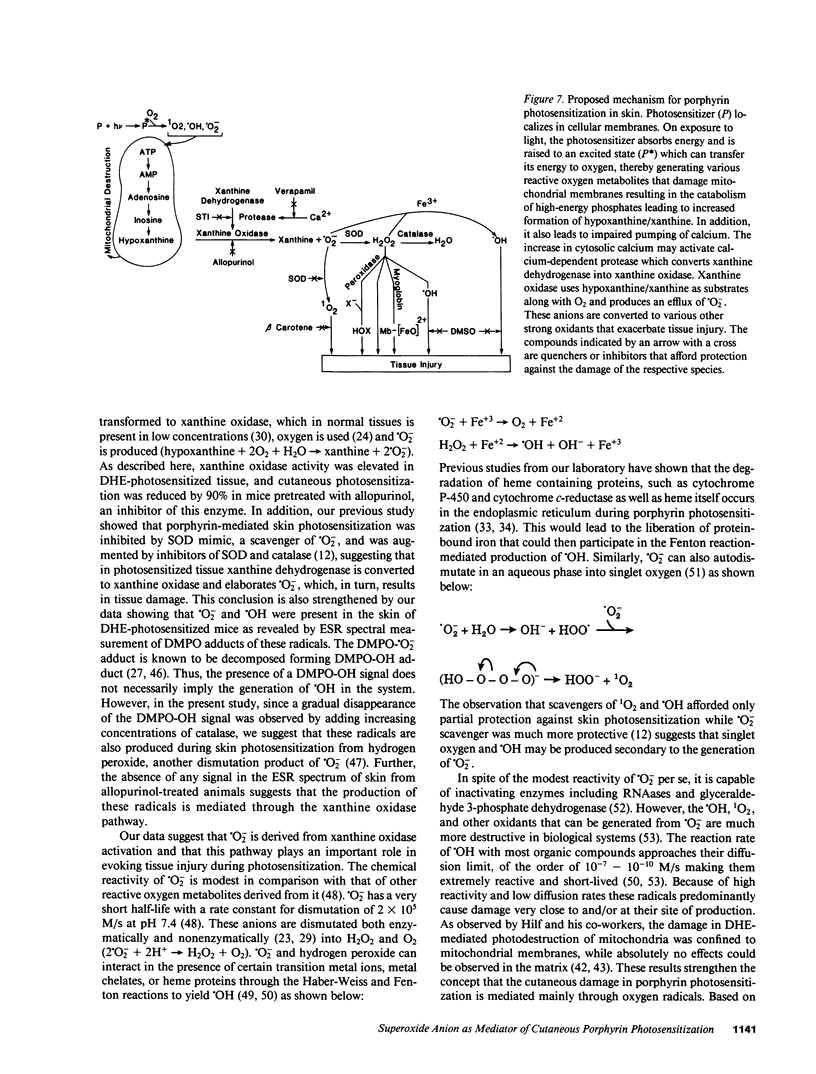
- Hilf R., Gibson S. L., Penney D. P., Ceckler T. L., Bryant R. G. Early biochemical responses to photodynamic therapy monitored by NMR spectroscopy. Photochem Photobiol. 1987 Nov;46(5):809–817. doi: 10.1111/j.1751-1097.1987.tb04852.x. [DOI] [PubMed] [Google Scholar]
- Hilf R., Murant R. S., Narayanan U., Gibson S. L. Relationship of mitochondrial function and cellular adenosine triphosphate levels to hematoporphyrin derivative-induced photosensitization in R3230AC mammary tumors. Cancer Res. 1986 Jan;46(1):211–217. [PubMed] [Google Scholar]
- Hilton B. D., Misra R., Zweier J. L. Magnetic resonance studies of fredericamycin A: evidence for O2-dependent free-radical formation. Biochemistry. 1986 Sep 23;25(19):5533–5539. doi: 10.1021/bi00367a028. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Sohnle P. G. Generation of free radical intermediates from foreign compounds by neutrophil-derived oxidants. J Clin Invest. 1985 May;75(5):1618–1622. doi: 10.1172/JCI111868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D. Hematoporphyrin and HPD: photophysics, photochemistry and phototherapy. Photochem Photobiol. 1984 Jun;39(6):851–859. doi: 10.1111/j.1751-1097.1984.tb08871.x. [DOI] [PubMed] [Google Scholar]
- Kessel D. Photosensitization with derivatives of haematoporphyrin. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Jun;49(6):901–907. doi: 10.1080/09553008514553131. [DOI] [PubMed] [Google Scholar]
- Kessel D. Proposed structure of the tumor-localizing fraction of HPD (hematoporphyrin derivative). Photochem Photobiol. 1986 Aug;44(2):193–196. doi: 10.1111/j.1751-1097.1986.tb03585.x. [DOI] [PubMed] [Google Scholar]
- Kessel D. Sites of photosensitization by derivatives of hematoporphyrin. Photochem Photobiol. 1986 Oct;44(4):489–493. doi: 10.1111/j.1751-1097.1986.tb04697.x. [DOI] [PubMed] [Google Scholar]
- Krall J., Bagley A. C., Mullenbach G. T., Hallewell R. A., Lynch R. E. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J Biol Chem. 1988 Feb 5;263(4):1910–1914. [PubMed] [Google Scholar]
- Lavelle F., Michelson A. M., Dimitrijevic L. Biological protection by superoxide dismutase. Biochem Biophys Res Commun. 1973 Nov 16;55(2):350–357. doi: 10.1016/0006-291x(73)91094-2. [DOI] [PubMed] [Google Scholar]
- McBurney A., Gibson T. Reverse phase partition HPLC for determination of plasma purines and pyrimidines in subjects with gout and renal failure. Clin Chim Acta. 1980 Mar 14;102(1):19–28. doi: 10.1016/0009-8981(80)90429-5. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide. J Biol Chem. 1987 Jan 25;262(3):1098–1104. [PubMed] [Google Scholar]
- Myers C. L., Weiss S. J., Kirsh M. M., Shlafer M. Involvement of hydrogen peroxide and hydroxyl radical in the 'oxygen paradox': reduction of creatine kinase release by catalase, allopurinol or deferoxamine, but not by superoxide dismutase. J Mol Cell Cardiol. 1985 Jul;17(7):675–684. doi: 10.1016/s0022-2828(85)80067-5. [DOI] [PubMed] [Google Scholar]
- Reiners J. J., Jr, Pence B. C., Barcus M. C., Cantu A. R. 12-O-tetradecanoylphorbol-13-acetate-dependent induction of xanthine dehydrogenase and conversion to xanthine oxidase in murine epidermis. Cancer Res. 1987 Apr 1;47(7):1775–1779. [PubMed] [Google Scholar]
- Salet C., Moreno G., Vinzens F. Effects of photodynamic action on energy coupling of Ca2+ uptake in liver mitochondria. Biochem Biophys Res Commun. 1983 Aug 30;115(1):76–81. doi: 10.1016/0006-291x(83)90970-1. [DOI] [PubMed] [Google Scholar]
- Sorenson J. R. Copper chelates as possible active forms of the antiarthritic agents. J Med Chem. 1976 Jan;19(1):135–148. doi: 10.1021/jm00223a024. [DOI] [PubMed] [Google Scholar]
- Spector T., Johns D. G. Stoichiometric inhibition of reduced xanthine oxidase by hydroxypyrazolo [3,4-d]pyrimidines. J Biol Chem. 1970 Oct 10;245(19):5079–5085. [PubMed] [Google Scholar]
- Thomas J. P., Hall R. D., Girotti A. W. Singlet oxygen intermediacy in the photodynamic action of membrane-bound hematoporphyrin derivative. Cancer Lett. 1987 Jun;35(3):295–302. doi: 10.1016/0304-3835(87)90131-5. [DOI] [PubMed] [Google Scholar]
- Torinuki W., Miura T., Seiji M. Lysosome destruction and lipoperoxide formation due to active oxygen generated from haematoporphyrin and UV irradiation. Br J Dermatol. 1980 Jan;102(1):17–27. doi: 10.1111/j.1365-2133.1980.tb05667.x. [DOI] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Grootveld M., Moorhouse C. P., Hutchison D. C., Baum H. The specificity of thiourea, dimethylthiourea and dimethyl sulphoxide as scavengers of hydroxyl radicals. Their protection of alpha 1-antiproteinase against inactivation by hypochlorous acid. Biochem J. 1987 May 1;243(3):867–870. doi: 10.1042/bj2430867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Gianni L., Muindi J., Myers C. E. Differences in O2 reduction by the iron complexes of adriamycin and daunomycin: the importance of the sidechain hydroxyl group. Biochim Biophys Acta. 1986;884(2):326–336. doi: 10.1016/0304-4165(86)90181-9. [DOI] [PubMed] [Google Scholar]
- Zweier J. L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–1357. [PubMed] [Google Scholar]
- Zweier J. L., Rayburn B. K., Flaherty J. T., Weisfeldt M. L. Recombinant superoxide dismutase reduces oxygen free radical concentrations in reperfused myocardium. J Clin Invest. 1987 Dec;80(6):1728–1734. doi: 10.1172/JCI113264. [DOI] [PMC free article] [PubMed] [Google Scholar]