Abstract
A single-chain triplebody (sctb) 33-ds16-ds19 comprising two distal single-chain Fv fragments (scFvs) specific for the lymphoid antigen CD19 and the myeloid antigen CD33 flanking a central scFv specific for CD16, which is the low affinity Fc-receptor (FcγRIII) present on natural killer cells and macrophages, was produced and its properties were investigated. CD33 and CD19 in combination are present on acute leukemiablasts with mixed lineage phenotype, but not on normal human hematopoietic cells. For comparison, two bispecific scFvs (bsscFvs), ds19-ds16 and 33-ds16, with monovalent binding to CD19 and CD33, respectively, were also studied. The sctb 33-ds16-ds19 specifically interacted with all three antigens. On the antigen double-positive cell line BV-173, the sctb bound with 2-fold greater avidity than bsscFv ds19-ds16 (KD = 21 vs. 42 nM) and with 1.4-fold greater avidity than bsscFv 33-ds16 (KD = 29 nM). All three fusion proteins had similar affinity for CD16 and sufficient thermic stability in human serum. In antibody-dependent cellular cytotoxicity (ADCC) reactions with human mononuclear cells as effectors, the sctb promoted lysis of BV-173 cells at 23-fold lower concentrations than bsscFv ds19-ds16 and at 1.4-fold lower concentrations than bsscFv 33-ds16. The sctb also mediated potent ADCC of the antigen double-positive mixed lineage leukemia cell line SEM, and the half-maximal concentration EC50 for BV-173 cells was 7 pM. Therefore, CD19 and CD33 are present on the surface of these leukemic cell lines such that they can be connected by a single sctb molecule, permitting the recruitment of NK cells via CD16 and tumor cell lysis.
Key words: leukemia, natural killer cells, antibody-derivatives, dual targeting, sctb
Introduction
A major advantage of antibodies in cancer therapy over chemotherapeutic agents and radiation derives from their antigen specificity. All antibody-based products currently approved as anti-cancer drugs target one single class of antigen on tumor cells. While this allows for selective targeting of antigen-positive cells, it does not achieve discrimination between antigen-positive tumor cells and antigen-positive normal cells, unless the antigen is strongly overexpressed on the cancer cell or the cancer cell is more sensitive to the antibody than normal cells. Indeed, antigens particularly suited for antibody therapy are frequently overexpressed on cancer cells, such as Her2/neu on mammary carcinoma cells, the epidermal growth factor receptor (EGF-R) and epithelial cell adhesion molecule (EpCAM) on a variety of carcinomas and the high molecular weight melanoma associated antigen (HMW-MAA) on melanomas and some acute childhood leukemias.1–7 However, many attractive antigens are also expressed on normal cells, and antibodies that target these antigens may cause undesired side effects by depleting valuable normal cells. An example is the interference of CD52-specific alemtuzumab (Campath-1H; Genzyme Corp.)8 with vital leukocyte defenses against infectious agents.
An important objective for the development of anti-cancer antibody agents is therefore to additionally increase the specificity of cancer cell targeting over that of normal cells. One approach is the development of dual-targeting agents. These are recombinant proteins with two antigen binding domains for two different antigens on a tumor cell. In some cases, it is possible to identify a pair of target antigens that is exclusively present on cancer cells, while normal cells carry only one of these markers. In other cases, double-positive cancer cells carry the pair in greater combined density than double-positive normal cells. The expectation is that in both cases a dual-targeting agent may bind with greater probability to the cancer cell and over time may achieve a preferential elimination of cancer cells in vivo.
The concept is attractive, but only a few cases of successful dual-targeting have been reported. One example are dual-targeting immunotoxins, such as the protein DT-19-22, which carries a truncated diphtheria toxin (DT) fused in tandem to two single-chain variable fragments (scFv) specific for the lymphoid antigens CD19 and CD22.9 The underlying expectation was that this agent may bind with increased probability to malignant B lymphoid cells and eliminate them because many of these cells, in particular hairy cell leukemias, express one or both of these antigens in greater density than normal B-lineage cells.10 This molecule eliminated antigen double-positive cells more effectively than the control molecules DT-19-19 and DT-22-22, suggesting that it may have bound to one each of CD19 and CD22 on the same cancer cell. Therefore, preferential targeting of antigen double-positive tumor cells over normal cells may be possible with this design, but direct evidence for a preferential elimination of tumor cells over simultaneously present antigen single-positive normal cells has not been reported.
Our team has investigated the merits of dual-targeting by designing and testing single-chain triplebodies (sctbs) that rely on effector cells for the elimination of cancer cells rather than toxin-components.11–13 Sctbs carry three scFv antigen binding domains in tandem in a single polypeptide chain. The two distal domains bind antigens on a cancer cell and the central domain recruits an effector cell. The prototype sctb 19-16-19 provided the proof-of-concept but did not yet aim for dual-targeting. It carried two scFv binding sites for CD19 flanking a central site for CD16, the low-affinity Fcγ RIII receptor for IgG that is present on natural killer cells (NK) and macrophages.11 This protein mediated strongly redirected lysis of human leukemia-derived cell lines and primary leukemic cells by NK-effectors at approximately 25-fold lower concentrations than the control molecule 19-16, a bispecific single chain Fv (bsscFv) with one binding site each for CD19 and CD16.14
Another prototype in the same format was the sctb 33-16-33 with distal binding sites for CD33, a validated target for antibody-derivatives against acute myelogenous leukemia (AML).13 This triplebody was also not designed for dual-targeting, but it mediated effective redirected lysis of AML-derived cell lines and primary AML cells by mononuclear cells (MNCs) as effectors. This agent was approximately 20-fold more potent than the corresponding control bsscFv 33-16, indicating that one triplebody molecule was capable of simultaneous binding to two CD33 molecules on the same tumor cell, as well as one CD16.13 The first dual-targeting triplebody was 123-16-33 with one binding site each for the AML tumor antigens CD123 and CD33, and one for CD16.12 This molecule was capable of simultaneous binding to one each of CD123 and CD33 on the same tumor cell plus CD16, and this simultaneous binding was responsible for a strongly enhanced redirected lysis.12 Therefore, there is currently published evidence for four different triplebodies (19-16-19; 33-16-33; 123-16-123; and 123-16-33), suggesting that one single sctb can simultaneously bind two target antigens on the same tumor cell and CD16, and thereby achieve enhanced redirected lysis over the corresponding bsscFvs.
However, CD33 and CD123 are also present on normal myeloid cells, and there is no evidence yet that this agent will lead to a preferential elimination of double-positive AML cells over simultaneously present double-positive normal cells. To further assess the benefits of dual targeting, we have therefore investigated a different situation in this study, in which a pair of target antigens is exclusively present on tumor cells but not on related normal cells. If such a triplebody were also capable of simultaneous binding to one each of these targets on the same tumor cell and to CD16, then one would expect to find a preferential elimination of the tumor cell because the agent should bind to double-positive tumor cells with bivalent avidity, but only with monovalent affinity to single-positive normal cells. We identified a suitable pair of antigens to test this hypothesis, produced the corresponding triplebody and performed the initial steps towards validation of the hypothesis.
The pair of antigens selected was CD19 and CD33. CD19 is an attractive target for antibody-derived cancer therapeutics because it is expressed by the blasts of most B-cell precursor acute lymphoblastic leukemias (BCP-ALLs) of children and on most B-cell malignancies of adults.15,16 It is a clinically validated target for antibody-derived anti-cancer agents because a bsscFv CD19-CD3 that recruits cytotoxic T cells via CD3 for the lysis of tumor cells has recently demonstrated positive results in clinical trials. This agent blinatumomab produced significant clinical responses for non-Hodgkin lymphoma (NHL) and pediatric B-ALL.17,18 Similarly, CD33 is a clinically validated target for antibody-derived agents against AML.19,20 The anti-CD33 immunotoxin gemtuzumab ozogamicin (Mylotarg™; Pfizer) was approved in the United States as a treatment for relapsed and refractory AML in patients over 60 years of age. Although the agent produced toxicities, it brought clear therapeutic benefit for some patients.19
CD19 and CD33 are present on the same tumor cells for patients with mixed lineage leukemia (MLL) or bi-phenotypic leukemia. These are mostly childhood leukemias, but also occur as secondary therapy-related AML in adults.21 Approximately 80% of acute childhood leukemias are BCP-ALL with favorable prognosis; the remainder are high-risk leukemias with unfavorable prognosis.22 The majority of the high-risk cases carry reciprocal chromosomal translocations to the MLL-gene on chromosome 11q23.21,23 Fusion genes are generated at the translocation breakpoint, which specify chimeric proteins carrying some domains coded by each of the translocation partner genes. These fusion proteins are aberrant regulators of gene expression, initiating the clonal expansion of the cell in which the translocation occurred and the leukemogenic process. They also cause the joint expression of B lymphoid and myeloid lineage markers, including the lymphoid markers CD19 and CD22, as well as the myeloid markers CD13, CD15, CD33, CD65 and CD66c.21,24 Simultaneous expression of these markers does not occur on normal human hematopoietic cells, hence the designation “mixed lineage” to characterize this unusual set of diseases. These leukemias are a distinct disease entity differing from both ALL and AML in their gene expression profiles, disease progression, responsiveness to therapy and prognosis.25–27 Among the myeloid markers of MLL cells, we have chosen CD33 as a target because (a) it is a clinically validated target for AML, (b) it has a more restricted tissue distribution, being expressed exclusively on hematopoietic cells21 and (c) it is the only myeloid marker of this set that showed statistically significant correlation with poor treatment outcome.28
We investigated whether a dual-targeting triplebody 33-ds16-ds19 designed in the same format as those discussed above11–13 would also be able to bind simultaneously to both antigens on the same MLL cell, and whether this would mediate significant redirected lysis by effectors recruited via CD16. Our results suggest that this was indeed the case. While this information is valid and new, it does not yet answer the question of whether triplebodies will lyse double-positive leukemic cells preferentially over normal, antigen single-positive cells that are also present in vivo. The experiments required to answer this question will require substantial additional work that is in progress, but the agents and their properties described here suggest that triplebodies could achieve this result.
Results
Construction, expression and purification of the scFv triplebody 33-ds16-ds19.
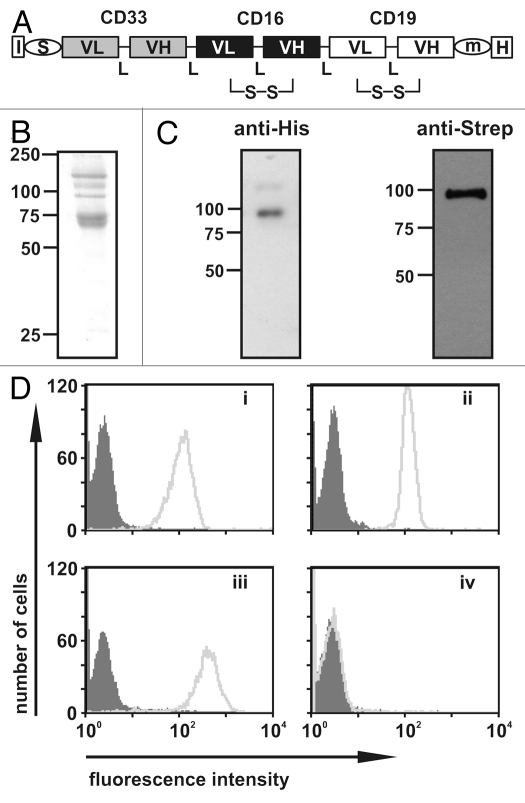
The sctb 33-ds16-ds19 was constructed from scFv components with specificity for CD19, CD16 and CD33 that have been previously described (Fig. 1A).14,29 The sctb and the control bsscFvs ds19-ds16 and 33-ds16 were produced in stably transfected human HEK 293T cells and enriched from culture supernatants by affinity chromatography. Yields of the enriched proteins were in the range of 1–3 mg per liter. The electrophoretic mobility corresponded to a Mr of ∼90 kDa, which was close to the calculated value of 89 kDa (Fig. 1B), and no degradation products were observed (Fig. 1C). High molecular weight impurities still remained in the preparation after enrichment by a single chromatographic step (Fig. 1B), but no high molecular weight aggregates including the triplebody were observed by electrophoresis in non-denaturing polyacrylamide gels, followed by immunoblotting (Sup. Fig. 1). The preparations were therefore of sufficient purity to permit a meaningful initial evaluation of biochemical properties and biological activities.
Figure 1.
Design, purification and antigen binding of sctb 33-ds16-ds19. (A) Block-structure of the sctb. I, secretion leader sequence from the murine IgkappaL chain; VL, VH, V regions of Ig L- and H-chains; L, 20 amino acid flexible linker (Gly4Ser)4; S, m, H, cDNA coding for a strep, c-myc or a hexahistidine tag; S-S, stabilizing disulfide bond. (B) Integrity and purity of the sctb after affinity chromatography with Ni-NTA agarose beads as evaluated by reducing SDS-PAGE and staining with Coomassie blue. (C) Western blot analysis using anti-his or anti-Strep antibodies for detection. (D) FACS analysis of specific binding of the sctb to CD19-positive SEM cells (i), CD33-positive THP-1 cells (ii), CD16-transfected CHO cells (iii) and CD33-, CD16- and CD19-negative CEM cells (iv). (grey peak: signal from control sctb; light grey peak: signal from sctb).
Binding characteristics of the sctb.
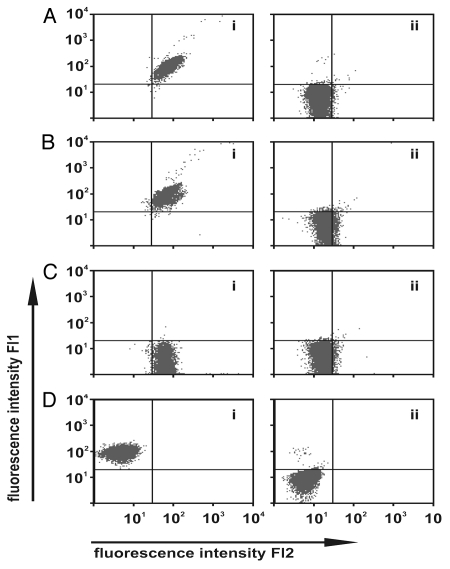
The sctb was capable of reacting with CD19, CD16 and CD33 on separate cell lines that each expressed one of these antigens (Fig. 1D). To investigate the possibility that the protein preparation may have consisted of two subpopulations, with each capable of binding only one of these antigens, the ability to simultaneously bind more than one antigen was analyzed. Recombinant fusion proteins comprising the extracellular domain of CD16 or CD33 and green fluorescent protein (GFP) or red fluorescent protein (RFP), termed CD16ex-GFP and CD33ex-RFP, were used to test the hypothesis. Cells from the CD33-negative, CD19-positive SEM sub-line were incubated first with the sctb and then with both CD16ex-GFP and CD33ex-RFP simultaneously. Strong cell-bound green and red fluorescence signals resulted, indicating that the sctb could bind simultaneously to both cell-associated CD19 and fluid-phase CD16ex and CD33ex (Fig. 2Ai). Incubation of the CD7-negative SEM cells with the similarly constructed control sctb 7-ds16-7 showed no fluorescence signal (Fig. 2Aii). The fluorescence signal was reduced to baseline levels in competition-binding experiments when excess amounts of the parental CD19 monoclonal antibody (mAb) 4G7, the parental CD16 mAb 3G8 or the parental CD33-scFv were added, whereas the signal was not decreased after addition of a non-relevant IgG1 isotype control antibody or a control scFv (Fig. 2B–D).
Figure 2.
Simultaneous and specific antigen binding of the sctb. (A) Simultaneous binding to CD19, CD33 and CD16. SEM cells were incubated with the sctb and simultaneous binding was revealed by addition of two fusion proteins consisting of the extracellular domain of CD16 plus GFP (CD16ex-GFP) and CD33 plus RFP (CD33ex-RFP) (i). SEM cells incubated with a control sctb, 7-ds16-7, which did not bind to the CD7-negative SEM cells, showed no cell-bound fluorescence signal after subsequent staining with CD16ex-GFP or CD33ex-RFP (ii). (B) The fluorescence signal produced by CD16ex-GFP and CD33ex-RFP was blocked by pre-incubation of SEM cells with a 50-fold molar excess of the intact CD19 mAb 4G7 (ii), whereas incubation with an isotype-matched control antibody produced no competition effect. (i) SEM cells were incubated with the sctb and one of the GFP- or RFP-labeled fusion proteins. (C) After pre-incubating the CD16ex-GFP fusion protein with the CD16 mAb 3G8 the signal was reduced to background level (ii). No blocking occurred by addition of a non relevant isotype matched antibody (i). (D) After pre-incubation of the CD33ex-RFP fusion protein with the CD33 scFv, binding was blocked (ii). No reduction occurred with a non-relevant control scFv (i).
Two experiments were done to evaluate whether insertion of a second scFv component into the sctb would result in a gain of avidity for CD19- and CD33-double-positive cells. First, the equilibrium binding constants (KD) of the sctb and bsscFv molecules were determined from binding curves recorded by calibrated flow cytometry (Table 1). The sctb had an overall KD of 21.5 ± 1.5 nM for antigen double-positive BV-173 cells, while the affinities of the bsscFvs were approximately 2-fold lower for CD19 (KD = 42.4 ± 5.7 nM11) and 1.4-fold lower for CD33 (KD = 29 ± 1.9 nM13) on these cells. The avidity of the sctb for CD19- and CD33-double-positive BV-173 cells was thus approximately 2-fold higher than the affinity of the monovalent CD19-specific scFv contained in ds19-ds16, and approximately 1.4-fold higher than the affinity of the CD33-specific scFv contained in the bsscFv 33-ds16, which indicated both scFv components were functional and contributed to the overall avidity of the sctb for double-positive cells. In contrast, the affinity of the CD16-specific scFv was almost unmodified. No difference was observed between scFv carried in a bsscFv or in the sctb, where it was engaged at both ends. The KD value of the sctb for CD16 was 49 ± 5.2 nM, and for the CD16-binding sites carried in ds19-ds16 and 33-ds16 the values were 57.6 ± 8.9 and 35 ± 2.2 nM,13 respectively. These latter values deviated by less than 15% from the value for the sctb, and thus remained largely unchanged, with the deviations possibly due to minor distortions of the CD16-scFv in the individual context of these molecules or their sequence-dependent ability to mediate synapse-formation with NK-cells. The key difference is that the affinity of these molecules for CD16 remained practically constant, whereas the avidity changed about 2-fold for the double-positive cells due to the addition of the extra scFv in the triplebody. These changes are of the same order of magnitude as those previously observed for other sctbs.11–13
Table 1.
Equilibrium binding constants (KD), surface retention half-life and stability in human serum of sctb 33-ds16-ds19 and the bsscFvs ds19-ds16 and 33-ds16
| Tested on cells for binding to | 19 + 33 | 19 | 33 | 16 | ||||
| KD [nM]* | ||||||||
| sctb ds19-ds16-33 | 21 ± 1.5 | 49 ± 5.2 | ||||||
| bsscFv ds19-ds16 | 42 ± 5.7a | 57 ± 8.9a | ||||||
| bsscFv 33-ds16 | 29 ± 1.9b | 35 ± 2.2b | ||||||
| Surface retention t1/2 [min]** | ||||||||
| sctb ds19-ds16-33 | 16 ± 2 | n.d. | ||||||
| bsscFv ds[CD19xCD16] | 7 ± 2 | 9 ± 5 | ||||||
| bsscFv [CD33xdsCD16] | 17 ± 3 | 9 ± 9 | ||||||
| Stable in hu serum 37°C t1/2 [hrs]*** | ||||||||
| sctb ds19-ds16-33 | 86 ± 4 | 198 ± 2 | 186 ± 2 | |||||
Tested on BV-173 cells and CHO 16-10 cells, respectively
Tested on BV-173 cells and CHO 16-10 cells, respectively
CD19-positive SEM, CD33-positive THP1 and CD16-positive CHO cells; a 11; b 13; hu, human.
In a second experiment, the cell surface retention of all three molecules, the sctb and bsscFvs ds19-ds16 and 33-ds16, on intact CD19- CD33-double-positive BV-173 cells at 37°C was measured. The retention half-lifes (t1/2) of ds19-ds16 and the sctb were 7 min and 16 min, respectively (Table 1), indicating that the addition of the extra scFv domain contributed to doubling the retention half-life. Surprisingly, bsscFv 33-ds16 had a retention half-life of 17 min, which was similar to that of the sctb. Both bsscFvs showed a similar retention time of 9 min via their CD16 binding domain on CD16-transfected CHO cells, indicating that the long retention time of 33-ds16 on BV-173 cells was not due to the formation of aggregates.
The results allow us to rule out the alternative, which is that a fraction of the sctb population bound with monovalent affinity to CD19 and another fraction with monovalent affinity to CD33 on the double-positive cells. In this case, no change in molar avidity for the sctb relative to the bsscFvs should have been seen.
Thermostability in human serum in vitro.
Antibody-derived proteins with potential as therapeutics must be thermostable in human serum. To evaluate the proteolytic sensitivity and possible thermic denaturation (heat inactivation), the sctb was incubated in human serum at 37°C and binding to CD19, CD33 and CD16 on viable antigen single-positive cells was measured by FACS-analysis. Half-life values were determined (Table 1), and the values for binding to the different antigens at times between 86 and 198 h were recorded. The results indicated that each of the three scFv domains underwent thermic denaturation with an individual, sequence-specific half-life. However, the magnitude of all values was enough to assure that the sctb had overall thermic stability that was sufficient for preclinical development.
Measurement of ability to mediate ADCC.
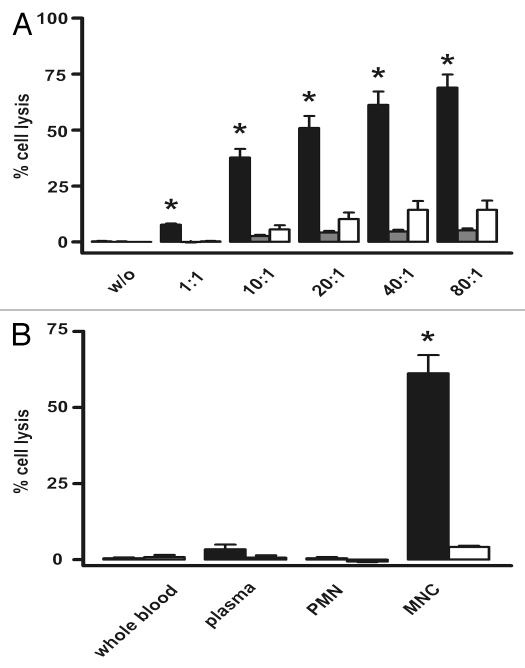
The ability of the molecules to mediate ADCC of human leukemic cells with MNCs from healthy, unrelated human donors as effector cells was determined using CD19- and CD33-double-positive pro-B ALL derived cell line SEM and the BV-173 cell line with pre-B phenotype. To investigate which fraction of blood leukocytes contained the relevant effector cells for this lytic effect, whole blood from healthy, unrelated donors was fractionated into plasma, mononuclear-(MNC) and polymorphonuclear cells (PMN; neutrophilic granulocytes), and plasma, respectively. The different fractions were then used in 51Cr-release assays against BV-173 cells (Fig. 3B). The sctb triggered significant tumor cell lysis only in combination with MNCs, suggesting that CD16-positive NK-cells were the sole relevant effector cells because CD16-positive macrophages and mast cells were not contained in this fraction, and monocytes are CD16-negative.30 Cell mediated cytotoxicity thus resided exclusively in the MNC fraction and plasma-mediated killing, due for some intact Igs to complement-activation, was not observed.
Figure 3.
The sctb mediates potent lysis of CD19- and CD33-double-positive BV-173 cells with enriched MNCs from healthy human donors as effector cells. (A) At the concentration of 1 nM the sctb induced significant lysis over a broad range of E:T ratios and the extent of specific lysis increased with increasing E:T ratios (black bars, sctb; grey bars, control sctb, white bars, no ab). Data are presented as mean percentage lysis ± standard error of the mean (SEM) obtained with MNCs from at least four different healthy donors. * Statistically significant p values ≤ 0.05. (B) Whole blood was fractionated into PMN, MNC and complement-containing plasma to identify the relevant effector population for the sctb. Black bars (left) indicate sctb at a concentration of 1 nM, white bars indicate no ab. * Statistically significant (p ≤ 0.05) killing. Data acquired with MNCs from four different donors.
In another recent study of the sctb 33-ds16-33, our team demonstrated that, within the MNC fraction, NK cells were the main CD16-positive cell type responsible for the cytolytic effect, and that T cells and NKT cells did not contribute.13 By analogy, we conclude that the same was probably also true for the sctb 33-ds16-ds19. ADCC reactions were performed with freshly prepared unstimulated MNCs. The sctb 33-ds16-ds19 induced target cell lysis by ADCC over a broad range (80:1 to 1:1) of effector-to-target cell (E:T) ratios (Fig. 3A). The extent of lysis steadily increased with the E:T ratio, which demonstrated that lysis of the tumor cells depended on the presence of effector cells. The control sctb with specificity for CD7 induced no significant lysis, which suggests that the effect was antigen-specific.
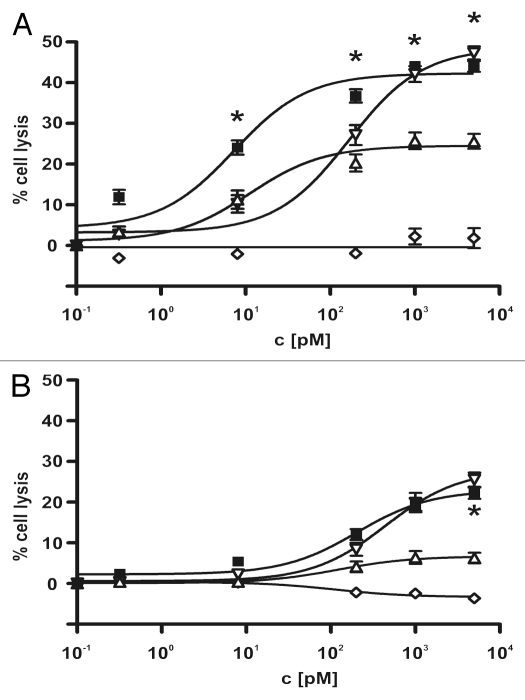
To investigate whether the increased avidity of the sctb compared with the bsscFvs that had single specificities for CD19 and CD33 was correlated with an enhanced cytotoxic potential, the ability of the recombinant molecules to mediate ADCC was evaluated in parallel for two different CD19- CD33-double-positive leukemic cells. The cell lines SEM and BV-173, which represent different B lymphoid malignancies and reflect different stages of B cell maturation, were used for the experiments. SEM cells are derived from a pro B-ALL with translocation t(4;11) to the MLL-locus, BV-173 from a Philadelphia-positive (Ph'-positive) CML at blast crisis with a pre-B ALL phenotype. Ph'-positive ALL often have poor prognosis and frequently co-express myeloid markers.21,31 Both leukemic cells were lysed in a dose-dependent manner by the sctb and the bsscFvs, whereas a control molecule with specificity for CD7 and CD16 was ineffective (Fig. 4). For BV-173 cells, the sctb was far more effective than the bsscFvs, and the dose-response curve was shifted to lower concentrations by about 25-fold relative to the curve for the ds19-ds16. The curve was shifted by about 1.5-fold relative to the curve for the 33-ds16 bsscFv (Fig. 4), but the sctb reached a higher maximum lysis than the bsscFv 33-ds16. These differences are also reflected by the corresponding numerical EC50 values listed in Table 2. For SEM cells, the difference between the sctb and the bsscFvs was less pronounced and not statistically significant, although a small left-shift of the dose-response curve for the sctb relative to the ds19-ds16 bsscFv was detected. The differences between the two cell lines are probably due at least in part to higher surface densities of both CD19 and CD33 on BV-173 than on SEM cells (Table 3). However, these cells reflect different stages of B lymphoid maturation (pre-B vs. pro-B), and the leukemia initiating cells were immortalized by different translocations (t 4–11 vs. t 9–22). The cell lines therefore were likely to display intrinsic differences in their susceptibility to NK cell-mediated lysis.
Figure 4.
Dose-dependent induction of ADCC of different leukemia-derived cell lines by the sctb and bsscFv controls. The CD19- CD33-double-positive leukemic cell lines BV-173 (A) and SEM (B) were used as targets to compare the lytic efficacy of the scFv fusion proteins at a constant E:T cell ratio of 40:1. The sctb (filled square), the bsscFv ds19-ds16 (open inverted triangle) and the bsscFv 33-ds16 (open triangle) triggered ADCC in a dose-dependent manner. The non-relevant control bsscFv (open diamond) induced no significant killing. Data points represent mean percentage of lysis ± SEM obtained with isolated MNCs from seven different healthy donors for BV-173 and eight for SEM cells. * Statistically significant differences between killing induced by sctb and bsscFv 33-ds16.
Table 2.
EC50 for ADCC by sctb 33-ds16-ds19 and bsscFvs ds19-ds16, 33-ds16a
| Target cells | sctb (max) | bsscFv ds19-ds16 (max) | bsscFv 33xds16 (max) | Fold difference |
| BV-173 | 7.2 ± 2 (42.2) | 167.2 ± 1 (48.5) | 11.2 ± 2 (24.5) | 23.2/1.5 |
| SEM | 203.7 ± 2 (22.9) | 449.6 ± 1 (27.9) | 119.5 ± 1 (6.6) | 2.2/- |
EC50 values were calculated from the dose-response curves (Fig. 4). Values in pM (picoMol/L). (max), maximum lysis in [%] achieved for this fusion protein; fold-difference, first number is the quotient of values listed in column 3 over column 2; number after the slash, quotient of numbers from column 4 over column 2.
Table 3.
Antigen densities of CD19 and CD33 on two mixed lineage leukemia-derived cell linesa
| Antigen density [molecules/cell] | BV-173 | SEM |
| CD19 | 109,000 | 89,000 |
| CD33 | 12,400 | 2,300 |
densities measured by calibrated cytofluorimetry using the commercial QuiFiKit® (Dako) as described in Materials and Methods and in reference 10.
For both cell lines, the EC50 values for the 33-ds16-ds19 were in the picomolar range, which indicated a very high specific activity. For the leukemic cell line BV-173, the sctb obtained an equal level of ADCC-activity at 22.9-fold lower concentrations than ds19-ds16 and at a 1.5-fold lower concentration than 33-ds16. These findings indicated that the gain in avidity of the sctb translated into a significant and strong gain in ADCC-activity.
Discussion
The key findings of this study are that tumor antigens CD19 and CD33 are arranged on the surface of CD33-positive ALL cells such that they can be connected successfully by sctbs, and that sctbs addressing this combination of antigens mediate a more effective elimination of the cancer cells by CD16-positive NK cells than the control bsscFvs 33-ds16 and ds19-ds16. This result was not obvious because of our limited knowledge of the surface architecture of human leukocytes. The prevailing paradigm, the “protein-island model,” states that integral transmembrane proteins are clustered into islands, and that certain proteins, such as the T cell antigen receptor (TCR) and the signal transducer LAT on human T cells, are normally located in separate islands that can merge upon appropriate signals and activation.32,33 Therefore, CD19 and CD33 on MLL cells might conceivably have segregated into separate islands located far enough apart that they could not be successfully bridged by a single sctb molecule.
Molecular modeling had previously predicted the two distal antigen-combining sites of a sctb in our standard format to be located at most 20 nm apart.13 The data presented here suggest that CD19 and CD33 on MLL cells are either located in the same islands or that the islands are sufficiently mobile to allow the two proteins to come within a 20 nm distance often enough to permit their connection by the sctb, which resulted in the 23-fold gain in ADCC activity reported here for the triplebody over the bsscFv ds19-ds16.
The main elements of evidence supporting this conclusion are: (1) The sctb was capable of simultaneous binding to CD19 on SEM cells and to the fluid phase fusion proteins CD33ex-RFP and CD16ex-GFP (Fig. 2); (2) The avidity of the sctb for binding to CD33- plus CD19-double-positive cells with a KD of 21 nM (Table 1) was increased 2-fold over the monovalent affinity of the bsscFv ds19-ds16 (42 nM) and 1.4-fold over 33-ds16 (29 nM). These effects would not have been expected if the triplebody were capable of binding tumor cells with only one of its two distal scFvs at any given time, but not with both simultaneously. In the latter case, the avidity of the whole triplebody should not have been greater than the monovalent affinity of the strongest binding control bsscFv and (3) the dose-response curve for the sctb in ADCC experiments with BV-173 cells was shifted to lower concentrations by about an order of magnitude relative to both bsscFvs (Fig. 4A). This result would not have been expected if the triplebody had contributed to specific killing only by monovalent binding to either CD19 or CD33, but not to both simultaneously.
The increase in surface retention half-life from 7 min for the bsscFv ds19-ds16 to 16 min for the sctb (Table 1) lends additional support to the conclusion that simultaneous binding of both scFvs specific for the tumor antigens did occur. The shift of the dose-response curve towards lower concentrations was less pronounced for SEM than for BV-173 cells (Fig. 4), which may be due in part to the fact that the SEM cells used here carried only 2,300 molecules of CD33 per cell and 89,000 CD19 molecules/cell (Table 3). The probability of dual targeting was therefore lower for SEM than for BV-173 cells, which carried 12,400 molecules of CD33 and 109,000 of CD19 per cell. The maximum level of lysis obtained with bsscFv 33-ds16 was lower for SEM than for BV-173 (Fig. 4), probably also due to the differences in CD33 density between these two cell lines (Table 3). Similarly, the maximum degree of lysis achieved by bsscFv ds19-ds16 for both lines was greater than the level achieved by 33-ds16 (Fig. 4), possibly due again to the greater CD19- than CD33-densities of both lines.
We can offer no satisfactory explanation for the observation that the gain in specific cytolytic activity was greater by about an order of magnitude than the gain in avidity for the sctb compared with the bsscFv (Tables 1 and 2). However, similar observations have been made consistently for three different sctbs, 19-16-19, 33-16-33 and 123-16-33, and the numerical values were so close in all cases that the data are taken to reinforce each other.11–13 The sctb 19-16-19 had an approximately 3-fold greater avidity than bsscFv ds19-ds16 and an EC50 lower by about 25-fold.11 Similar numbers were obtained for the comparison between sctb 33-16-33 and bsscFv 33-ds16,13 and those reported here for the comparison between sctb 33-ds16-ds19 and bsscFv ds19-ds16, with the exception that here the difference in avidity was only 2-fold and the drop in EC50 23-fold (Table 1).
Avidity is probably a key parameter contributing to the specific cytolytic potential of scFv-fusion proteins, but other hidden parameters must provide an additional important contribution. One possibility is that triplebodies may permit the formation of a particularly effective synapse between effector and target cell, more effective than what is reflected by the simple numerical increase in binding activity. Signals emitted into the cell by engagement of two different target molecules by an sctb may contribute more strongly to the elimination of the tumor cell than the signals triggered by bsscFvs, which have only monovalent binding to the target.
Development of sctbs for clinical applications should proceed with caution because scFv-domains and tandem bsscFvs have a tendency to form high molecular weight aggregates. Aggregates must be avoided for all human therapeutics intended for intravenous injection. However, after electrophoresis in non-denaturing polyacrylamide gels followed by immunoblotting, no high molecular weight aggregates comprising the sctb were detected in our preparations (Sup. Fig. 1), although the preparations still contained high molecular weight contaminants (Fig. 1). Another triplebody extensively studied by our team, 19-16-19,11 has been analyzed by size exclusion chromatography (SEC) and far less than 5% of the total protein eluted at a mobility greater than the monomers. For further preclinical development of any triplebody, a more detailed search for aggregates is clearly required.
The available data suggest that the triplebodies studied so far by our team may have had a lower tendency to form aggregates than anticipated based on existing knowledge about monomeric scFvs and tandem bsscFvs. The reason may be either that the triplebody format is less prone for aggregate-formation than the monomeric and bispecific formats or the disulfide stabilization contained in our scFv components prevented aggregation or a combination of both. We consider it unlikely that aggregates significantly altered the biological properties of the molecules reported here and interfered with our ability to correctly interpret the data.
Aggregate formation is an intrinsic protein property governed by the primary amino acid sequence, and can therefore occur at any concentration. However, aggregation most often occurs when the protein therapeutics are formulated at very high concentrations. Some of the antibodies in current clinical use are formulated at concentrations between 30 and 150 mg/ml, close to 1 mM, and at these concentrations aggregation becomes a major concern. However, in our study the triplebodies were used in nanomolar concentrations and aggregation is less likely to have distorted the data and their interpretation at these concentrations. Before further developing triplebodies for clinical use, it will be necessary to carefully scrutinize them both experimentally and by computer-assisted methods for the propensity to form aggregates. Computer algorithms, such as AggreSolve™ (Lonza), are sufficiently powerful to reliably pinpoint amino acid residues contributing to aggregate-formation, and experimental analysis by physico-chemical methods is an absolute requirement for clinical development, at a minimum by SEC. However, the observations reported here and the conclusions drawn are unlikely to have been gravely distorted by hidden aggregation.
Dual-targeting triplebodies offer the promise of enhanced specificity of lysis of antigen double-positive over single-positive target cells, as in the case reported here of results for CD19- and CD33-double-positive mixed lineage leukemia cells over healthy hematopoietic cells that express only one of these two antigens. Preferential lysis of double-positive over single-positive cells has not yet been demonstrated, but efforts to study this ability in a rigorous manner are underway. If preferential lysis could be achieved by dual targeting, then this would be important progress. The relevant new finding of the present study is that CD19 and CD33 are arranged on the surface of mixed-lineage leukemia cells in such a manner that they can be connected by a single molecule of our dual-targeting sctb and that this allows for more effective ADCC lysis by NK-cells than the effects achieved by the bispecific agents 33-ds16 and ds16-ds19. Dual targeting triplebodies in the format presented here therefore are attractive candidates for further preclinical and clinical development.
Material and Methods
Cell lines and hybridomas.
Chinese hamster ovary (CHO) cells, stably transfected with a human CD16a cDNA expression vector, were from Dr. J. van de Winkel (University Medical Centre, Utrecht, The Netherlands). The 4G7 hybridoma CD19, mIgG1,34 was from Dr. R. Levy (Stanford University, Palo Alto, CA). The hybridoma 3G8 FcgRIII, CD16, mIgG1,30 was from the American Type Cell Culture Collection (ATCC, Manassas, VA). CHO cells, the hybridomas, the AML derived cell line THP1 (German Collection of Microorganisms and Cell Cultures, DSMZ, Braunschweig, Germany), the T-ALL derived cell line CEM (ATCC), the pro-B ALL-derived cell line SEM,35 and the pre-B phenotypic cell line BV-173 (DSMZ36) were cultured in Roswell Park Memorial Institute 1640 Glutamax-I medium (Invitrogen, Karlsruhe, Germany) containing 10% fetal calf serum (FCS; Invitrogen) respectively 20% FCS for BV-173, 100 units/ml penicillin (Invitrogen) and 100 µg/ml streptomycin (Invitrogen). Human embryonal kidney (HEK) 293T cells (ATCC) were maintained in DMEM Glutamax-I medium (Invitrogen) supplemented with 10% FCS, penicillin and streptomycin at 100 units/ml and 100 µg/ml, respectively.
Bacterial strains and plasmids.
Escherichia coli strain XL-1 blue (Stratagene, Amsterdam, The Netherlands) was used as host for the amplification of the plasmids and cloning. For construction and eukaryotic expression the vector pSecTag2HygroC (Invitrogen) was employed.
Construction of recombinant antibody fragments.
To generate the expression plasmid for the sctb 33-ds16-ds19, the cDNA coding for the CD33 scFv was excised from the vector pAK400-CD33-K132,29 and cloned as an SfiI cassette into SfiI digested pSecTag2HygroC-Strep-ds19-ds16-ds19,11 generating the plasmid pSecTag2HygroC-CD33-dsCD16-dsCD19. To confirm correct construction, the final constructs were sequenced37 on an Applied Biosystems automated DNA sequencer (ABI Prism 310 Genetic Analyzer; Perkin-Elmer, Ueberlingen, Germany).
Expression and purification of recombinant fusion proteins.
For expression of the bsscFvs ds19-ds16,14 and 33-ds16,13 the sctb 33-ds16-ds19 and the CD7-specific control bsscFv 7-ds16,11 HEK 293T cells were transfected with the respective expression plasmid using the calcium phosphate technique including chloroquine.37 Stable production cell lines for each construct were obtained after transfection with the plasmids linearized with the restriction enzyme FspI. Positive clones were selected in the presence of 200 µg/ml Hygromycin B (Roth, Karlsruhe, Germany) and single cell clones were isolated by limiting dilution. Supernatants were analyzed for the presence of antibody fragments by flow cytometry. Culture supernatants containing the recombinant protein were collected at four time points over a period of one week and dialyzed at 4°C against a buffer containing 50 mM NaH2PO4, 300 mM NaCl and 10 mM imidazole at pH 8.0. The recombinant His-tagged proteins were purified by affinity chromatography with nickel-nitrilotriacetic acid (Ni-NTA) agarose beads (Qiagen) and finally dialyzed against phosphate buffered saline (PBS). Fusion proteins with green fluorescent protein (GFP) or with the red fluorescent protein (RFP) were transiently expressed in HEK 293T cells and purified as described in reference 11.
Sodium dodecyl sulfate (SDS)-PAGE and western blot analysis.
Reducing SDS-PAGE was performed by standard procedures.37 In western blot experiments, the recombinant proteins were detected either with a horseradish peroxidase (HRP)-conjugated antibody specific for the Strep-tag (IBA, Goettingen, Germany) or with an unconjugated penta-His antibody (Qiagen, Hilden, Germany), and a secondary HRP-coupled goat anti-mouse IgG antibody (Dianova, Hamburg, Germany). Western blots were developed using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Freiburg, Germany).
Flow cytometric analysis.
Immunofluorescence analysis was performed on a FACSCalibur™ instrument using CellQuest software (Becton Dickinson, Heidelberg, Germany) as described in reference 11. For each sample, 1 × 104 events were collected and whole cells were analyzed using appropriate scatter gates to exclude cellular debris and aggregates. The recombinant antibody derivatives were detected using a penta-His antibody and a phycoerythrin (PE)-conjugated goat anti-mouse IgG antibody (DAKO Diagnostica GmbH, Hamburg, Germany), unless otherwise stated.
KD values were determined as described in reference 11 and 38. The highest mean fluorescence value was set to 100% and all data points were normalized. The experiments were repeated six to seven times and mean values are reported. The KD values were calculated using a nonlinear regression curve fit. Cell surface antigen densities were determined by calibrated cyto-fluorimetry using QuiFiKit® (Dako) according to published procedures.10
Cell surface retention assay.
Cell surface retention assays were performed by published procedures, under conditions preventing the internalization of antigens.39 Briefly, 4 × 106 SEM cells were incubated for 1 h on ice, with 10 µg/ml of either the sctb 33-ds16-ds19 or the bsscFvs ds19-ds16 or 33-ds16. Cells were washed twice with 12 ml of cold FACS buffer (0.15 M sodium chloride, 10 mM sodium phosphate, 1% bovine serum albumin, 0.1% sodium azide, pH 7.2) and then collected by centrifugation. The cells were resuspended in 4 ml FACS buffer and incubated at 37°C. At different time points the cells were washed again to remove dissociated molecules and resuspended in FACS buffer. Aliquots of 0.5 × 106 cells were removed, placed on ice for 5 min and washed again. Molecules retained on the cell surface were then detected by FACS, stained as described above. All data points were normalized to the time point t0. Experiments were performed three times and mean values are reported. Half-life values for cell surface retention were obtained by fitting the data to a single-phase exponential decay.
Measurement of thermic stability in human serum.
The sctb 33-ds16-ds19 was incubated in human serum at sub-saturating concentrations of 2.5 µg/ml in a total volume of 350 µl at 37°C. Residual binding activity was determined at various time points by flow cytometry. The time point t0 was set to 100% and all data were normalized to this value. The experiments were performed four times and mean values are reported. The half-life values for each of the binding sites were calculated from a one-phase exponential decay curve fit.
Isolation of mononuclear cells (MNCs) and polymorph nuclear cells (PMNs) from unrelated healthy human donors.
Citrate buffered peripheral blood samples, drawn from healthy volunteers, were obtained after receiving informed consent, and with the approval of the Ethics Committee of the University of Erlangen-Nuremberg. MNCs or PMNs were enriched by Lymphoflot (Biotest, Dreieich, Germany) Ficoll density centrifugation in Leukosep tubes (Greiner, Frickenhausen, Germany) according to manufacturer's instructions and suspended in Roswell Park Memorial Institute 1640 Glutamax-I medium (Invitrogen) containing 10% FCS and penicillin and streptomycin at 100 units/ml and 100 µg/ml, respectively. Viability was verified by Trypan blue exclusion and exceeded 95%.
ADCC reactions.
ADCC assays, using MNCs from healthy donors as effector cells at different or constant effector-to-target (E:T) ratio, were performed in triplicate using a 3 h 51Cr release assay as described in reference 40. Dose response curves were recorded using several equimolar 5-fold or 25-fold serial dilutions of the respective antibody fragments at a constant E:T ratio of 40:1. E:T ratio dependence of lysis was determined at a constant concentration of 1 nM of the antibody fragments. Background lysis induced by MNCs alone was subtracted from each data point for the dose response curves and EC50 values (concentration of fusion protein producing 50% of maximum specific lysis) were calculated by using a nonlinear regression curve fit (variable slope). The experiments were repeated seven to eight times and mean values are reported.
Graphical and statistical analysis.
Graphical and statistical analyses were performed using Graph Pad Prism Software (Graph Pad Software Inc., San Diego, CA). Group data are reported as means ± standard error of the mean (SEM). Differences between groups were analyzed using unpaired (or, where appropriate) paired Student's t-test. p values = 0.05 were considered significant.
Acknowledgements
We thank Dr. R. Levy for the 4G7 hybridoma, Dr. J.G. van de Winkel for the CD16 transfected CHO cells, B. Bock for excellent technical assistance and for support in the laboratory. Lange is gratefully acknowledged for administrative assistance. The German José-Carreras Leukemia-Foundation for financial support, grant No. D°CLS F07/03.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- AML
acute myeloid leukemia
- B-CLL
B-cell chronic lymphocytic leukemia
- B-ALL
B-cell acute lymphoblastic leukemia
- BCP-ALL
B-cell precursor ALL
- CML
chronic myeloid leukemia
- MLL
mixed lineage leukemia
- LSC
leukemia stem cell
- scFv
single-chain fragment variable
- CHO
Chinese hamster ovary cells
- bsab
bispecific antibody
- bsscFv
bispecific single-chain Fv
- sctb
single-chain triplebody
- MNC
mononuclear cell
- PMN
polymorph-nuclear cells
- ds
disulfide stabilized
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- FI
fluorescence intensity
Financial Support
This research was supported by grants from the DFG (Deutsche Forschungsgemeinschaft; German Research Community) to G.H.F, W.H. (SFB 643 project C6) and A.M. (SFB 643 project C8), a student fellowship from Bayerische Eliteförderung (Bavarian Scholarship Foundation) to C.S., postdoctoral/Ph.D., fellowships from the German José-Carreras Leukemia-Foundation to M.S. and I.S. (D°CLS F07/03), a research grant No. 2007.049.1 from the Wilhelm Sander Foundation, Neustadt, Germany to G.H.F. and B.S., and support from the Stiftung Deutsche Krebshilfe (project No. 108242), the Beitlich Foundation, Tübingen, and the Association ‘Kaminkehrer helfen krebskranken Kindern’ (Chimney Sweeps support children with cancer) to G.H.F. Part of this work was funded by an intramural grant from the ELAN fond and the Training Grant GK592 from the German Research Community (DFG) for H.S.
Supplementary Material
References
- 1.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–296. doi: 10.1615/critrevimmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 2.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg R. The biology of cancer. New York: Garland Science; 2007. [Google Scholar]
- 5.Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122–128. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Wuchter C, Harbott J, Schoch C, Schnittger S, Borkhardt A, Karawajew L, et al. Detection of acute leukemia cells with mixed lineage leukemia (MLL) gene rearrangements by flow cytometry using monoclonal antibody 7.1. Leukemia. 2000;14:1232–1238. doi: 10.1038/sj.leu.2401840. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117:2051–2058. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faderl S, Ferrajoli A, Wierda W, O'Brien S, Lerner S, Keating MJ. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence. Cancer. 116:360–2365. doi: 10.1002/cncr.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallera DA, Todhunter DA, Kuroki DW, Shu Y, Sicheneder A, Chen H. A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B-cell leukemia/lymphoma. Clin Cancer Res. 2005;11:3879–3888. doi: 10.1158/1078-0432.CCR-04-2290. [DOI] [PubMed] [Google Scholar]
- 10.Olejniczak SH, Stewart CC, Donohue K, Czuczman MS. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol Invest. 2006;35:93–114. doi: 10.1080/08820130500496878. [DOI] [PubMed] [Google Scholar]
- 11.Kellner C, Bruenke J, Stieglmaier J, Schwemmlein M, Schwenkert M, Singer H, et al. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother. 2008;31:871–884. doi: 10.1097/CJI.0b013e318186c8b4. [DOI] [PubMed] [Google Scholar]
- 12.Kugler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, et al. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol. 2010;150:574–586. doi: 10.1111/j.1365-2141.2010.08300.x. [DOI] [PubMed] [Google Scholar]
- 13.Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33:599–608. doi: 10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
- 14.Bruenke J, Barbin K, Kunert S, Lang P, Pfeiffer M, Stieglmaier K, et al. Effective lysis of lymphoma cells with a stabilised bispecific single-chain Fv antibody against CD19 and FcgammaRIII (CD16) Br J Haematol. 2005;130:218–228. doi: 10.1111/j.1365-2141.2005.05414.x. [DOI] [PubMed] [Google Scholar]
- 15.Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, Catovsky D. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51:364–369. doi: 10.1136/jcp.51.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henze G, von Stackelberg A. Relapsed acute lymphoblastic leukemia. In: Ching-Hon Pui., editor. Childhood Leukemias. Cambridge UK: Cambridge University Press; 2006. pp. 473–486. [Google Scholar]
- 17.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 18.Topp M, Goekbuget N, Kufer P, Zugmaier G, Degenhard E, Neumann S, et al. Treatment with anti-CD19 BiTE antibody blinatumomab (MT103/MEDI-538) is able to eliminate minimal residual disease (MRD) in patients with B-precursor acute lmphoblastic leukemia (ALL): first results of an ongoing phase II study (ASH Annual Meeting Abstract) Blood. 2008;112:1926. [Google Scholar]
- 19.Stasi R. Gemtuzumab ozogamicin: an anti-CD33 immunoconjugate for the treatment of acute myeloid leukaemia. Expert Opin Biol Ther. 2008;8:527–540. doi: 10.1517/14712598.8.4.527. [DOI] [PubMed] [Google Scholar]
- 20.Zwaan CM, Reinhardt D, Zimmerman M, Hasle H, Stary J, Stark B, et al. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: results of a phase II study. Br J Haematol. 2010;148:768–776. doi: 10.1111/j.1365-2141.2009.08011.x. [DOI] [PubMed] [Google Scholar]
- 21.Hrusak O, Porwit-MacDonald A. Antigen expression patterns reflecting genotype of acute leukemias. Leukemia. 2002;16:1233–1258. doi: 10.1038/sj.leu.2402504. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong SA, Look AT. Molecular genetics of acute lymphoblastic leukemia. J Clin Oncol. 2005;23:6306–6315. doi: 10.1200/JCO.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 23.Pui CH. Childhood Leukemias. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 24.Ziemin-van der Poel SMNR, Gill HJ, Espinosa R, Patel Y, Harden A, Rubinelli P, et al. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci USA. 1991;88:10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 26.Guenther MG, Lawton LN, Rozovskaia T, Frampton GM, Levine SS, Volkert TL, et al. Aberrant chromatin at genes encoding stem cell regulators in human mixed-lineage leukemia. Genes Dev. 2008;22:3403–3408. doi: 10.1101/gad.1741408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper DP, Aplan PD. Chromosomal rearrangements leading to MLL gene fusions: clinical and biological aspects. Cancer Res. 2008;68:10024–10027. doi: 10.1158/0008-5472.CAN-08-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejstrikova E, Kalina T, Trka J, Stary J, Hrusak O. Correlation of CD33 with poorer prognosis in childhood ALL implicates a potential of anti-CD33 frontline therapy. Leukemia. 2005;19:1092–1094. doi: 10.1038/sj.leu.2403737. [DOI] [PubMed] [Google Scholar]
- 29.Schwemmlein M, Peipp M, Barbin K, Saul D, Stockmeyer B, Repp R, et al. A CD33-specific single-chain immunotoxin mediates potent apoptosis of cultured human myeloid leukaemia cells. Br J Haematol. 2006;133:141–151. doi: 10.1111/j.1365-2141.2005.05869.x. [DOI] [PubMed] [Google Scholar]
- 30.Fleit HB, Wright SD, Unkeless JC. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci USA. 1982;79:3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pui CH, Chessells JM, Camitta B, Baruchel A, Biondi A, Boyett JM, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003;17:700–706. doi: 10.1038/sj.leu.2402883. [DOI] [PubMed] [Google Scholar]
- 32.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci USA. 2006;103:18992–18997. doi: 10.1073/pnas.0609009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeker TC, Miller RA, Link MP, Bindl J, Warnke R, Levy R. A unique human B lymphocyte antigen defined by a monoclonal antibody. Hybridoma. 1984;3:305–320. doi: 10.1089/hyb.1984.3.305. [DOI] [PubMed] [Google Scholar]
- 35.Greil J, Gramatzki M, Burger R, Marschalek R, Peltner M, Trautmann U, et al. The acute lymphoblastic leukaemia cell line SEM with t(4;11) chromosomal rearrangement is biphenotypic and responsive to interleukin-7. Br J Haematol. 1994;86:275–283. doi: 10.1111/j.1365-2141.1994.tb04726.x. [DOI] [PubMed] [Google Scholar]
- 36.Pegoraro L, Matera L, Ritz J, Levis A, Palumbo A, Biagini G. Establishment of a Ph1-positive human cell line (BV173) J Natl Cancer Inst. 1983;70:447–453. [PubMed] [Google Scholar]
- 37.Sambrook J, Russel DW. Molecular Cloning: A Laboratory Manual. NY: Cold Spring Harbor; 2001. [Google Scholar]
- 38.Benedict CA, MacKrell AJ, Anderson WF. Determination of the binding affinity of an anti-CD34 single-chain antibody using a novel, flow cytometry based assay. J Immunol Methods. 1997;201:223–231. doi: 10.1016/s0022-1759(96)00227-x. [DOI] [PubMed] [Google Scholar]
- 39.Adams GP, Schier R, McCall AM, Crawford RS, Wolf EJ, Weiner LM, et al. Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu. Br J Cancer. 1998;77:1405–1412. doi: 10.1038/bjc.1998.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elsasser D, Valerius T, Repp R, Weiner GJ, Deo Y, Kalden JR, et al. HLA class II as potential target antigen on malignant B cells for therapy with bispecific antibodies in combination with granulocyte colony-stimulating factor. Blood. 1996;87:3803–3812. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.