Abstract
Interleukin-1β (IL-1β) is a potent mediator of inflammatory responses and plays a role in the differentiation of a number of lymphoid cells. In several inflammatory and autoimmune diseases, serum levels of IL-1β are elevated and correlate with disease development and severity. The central role of the IL-1 pathway in several diseases has been validated by inhibitors currently in clinical development or approved by the FDA. However, the need to effectively modulate IL-1β-mediated local inflammation with the systemic delivery of an efficacious, safe and convenient drug still exists. To meet these challenges, we developed XOMA 052 (gevokizumab), a potent anti-IL-1β neutralizing antibody that was designed in silico and humanized using Human Engineering™ technology. XOMA 052 has a 300 femtomolar binding affinity for human IL-1β and an in vitro potency in the low picomolar range. XOMA 052 binds to a unique IL-1β epitope where residues critical for binding have been identified. We have previously reported that XOMA 052 is efficacious in vivo in a diet-induced obesity mouse model thought to be driven by low levels of chronic inflammation. We report here that XOMA 052 also reduces acute inflammation in vivo, neutralizing the effect of exogenously administered human IL-1β and blocking peritonitis in a mouse model of acute gout. Based on its high potency, novel mechanism of action, long half-life and high affinity, XOMA 052 provides a new strategy for the treatment of a number of inflammatory, autoimmune and metabolic diseases in which the role of IL-1β is central to pathogenesis.
Key words: IL-1β, gevokizumab, gout, inflammation, autoimmune disease, affinity, therapeutic antibody
Introduction
Interleukin-1β (IL-1β) is a potent pleiotropic cytokine that affects the function of almost every cell type.1 Most importantly, by inducing growth and differentiation of immune competent lymphocytes, IL-1β is a dominant mediator of inflammatory responses.2 In this function, IL-1β plays a central role in protection from microbial pathogens and tissue injury repair.
IL-1β expression level and function are tightly regulated by a complex system of IL-1 family members and their receptors. The IL-1 family comprises at least 11 members, including IL-1α, IL-1β and the IL-1 Receptor antagonist (IL-1Ra). These mediators are produced by many cell types, including monocytes and macrophages. Both IL-1α and IL-1β are synthesized as precursors and, whereas IL-1β is secreted, IL-1α remains in the cytoplasm or is membrane-associated and released into circulation primarily during severe disease. IL-1β binds to the IL-1 Receptor type I (IL-1RI) expressed on all nucleated cells, which triggers the recruitment of the IL-1 Receptor accessory protein (IL-1RAcP) to form the active signaling complex. IL-1β also binds to a second receptor, IL-1 Receptor type II (IL-1RII), which is present in membrane-bound and soluble forms, both of which act as decoy receptors to downregulate the activity of IL-1β. The natural inhibitor IL-1Ra binds to both IL-1RI and IL-1RII, but does not allow the recruitment of IL-1RAcP.3
While IL-1β plays an important role in immunity, overexpression can have a deleterious effect on many cell types. Systemic effects from overexpression of IL-1β are the main cause of increased erythrocyte sedimentation rate (ESR), peripheral neutrophilia, thrombocytosis, pain hypersensitivity and anemia in a group of systemic inflammatory diseases that include systemic juvenile idiopathic arthritis (sJIA),4 neonatal onset multisystem inflammatory disease (NOMID),5 Muckle-Wells syndrome (MWS),6 pyogenic arthritis, pyoderma gangrenosum and acne syndrome (PAPA syndrome), Familial Mediterranean fever (FMF) and others.7 Excess IL-1β also causes joint bone degradation in rheumatoid arthritis (RA) patients8 and affects β cells in the pancreas, perturbing insulin production in models of Type 2 diabetes.9,10 Blockade of IL-1β was shown to improve glycemic control and β-cell function in a clinical trial of Type 2 diabetics.10 Recent studies in mice and with human cells have shown that IL-1β is essential for the development of TH17 cells,11–13 which are increasingly recognized as the key effectors responsible for organ-specific autoimmunity.14
Clinically, the role of the IL-1 pathway in disease has been validated by treatment with recombinant IL-1 receptor antagonist and other IL-1 pathway inhibitors. Although these inhibitors have shown efficacy in a number of diseases, there remains a need for new therapeutic options that are potent inhibitors and disease modifiers and that meet requirements for safety and convenience. To meet these needs, we generated the high affinity, IL-1β-specific therapeutic antibody XOMA 052, which is also known as gevokizumab.15 Designed for high potency and infrequent dosing, this antibody was generated from synthetic murine antibody sequences and constructed by rational design, utilizing XOMA's antibody technology platform. The antibody variable regions were humanized using Human Engineering™ technology16 and fused to human kappa light chain and γ-2 heavy chain constant regions. The Human Engineered™ IgG2 has 97% human sequence as compared to a Kabat consensus sequence. The antibody was characterized by a number of in vitro biophysical and functional assays, using either recombinant or naturally produced mature IL-1β protein. We have previously shown that XOMA 052 can reduce hyperglycemia and preserve β-cell function in the diet-induced obesity model of Type 2 diabetes,17 believed to be driven by low levels of chronic inflammation. In this report we further define the binding activity of XOMA 052 and show that it is efficacious in reducing acute inflammation in vivo, neutralizing exogenously administered IL-1β and blocking peritonitis in a mouse model of acute gout.
Results
Antibody discovery and Human Engineering™.
The first generation antibody was synthesized by rational design from murine antibody variable region sequences and produced as a chimeric antibody with mouse variable and human constant regions. Affinity maturation using site-directed mutagenesis yielded XMA005, which was subsequently humanized using Human Engineering™, an approach based on the conserved structure-function relationship among antibodies of different species.16 This Human Engineered™ antibody, XOMA 052, recapitulated the binding activity and potency of the parent antibody XMA005, and no back mutation or re-engineering of the final molecule was necessary.
XOMA 052 binding activity.
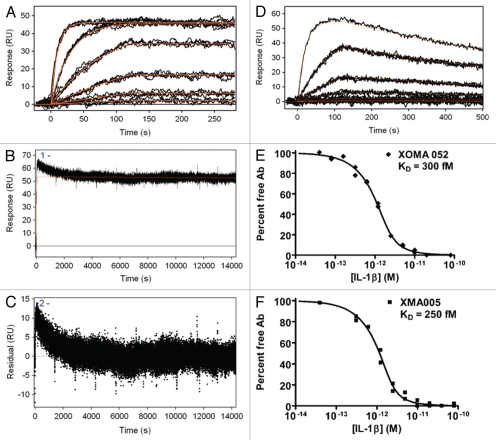
Previous studies that evaluated binding to other members of the IL-1 family, IL-1α and IL-1ra, demonstrated that XOMA 052 has high specificity for IL-1β.18 Investigation of the effect of XOMA 052 on the binding of IL-1β to its receptors revealed that neutralization of IL-1β activity occurs through a novel mechanism of action.18 To determine whether various rodent and primate disease models could be used to test the in vivo efficacy of XOMA 052, the ability of XOMA 052 to bind a number of species orthologs of IL-1β was measured by surface plasmon resonance (SPR). Figure 1 shows an overlay of the fitted curves and data for human (A–C) and mouse (D) IL-1β binding to XOMA 052 captured by immobilized Protein A/G on an SPR surface. While dissociation of mouse IL-1β is readily apparent within 10 minutes, this time was not sufficient to detect dissociation of human IL-1β. Additional experiments were therefore carried out with 4 h of dissociation time (B). In this experiment, the sensorgram appears heterogeneous, as evidenced by the trend in the residuals from the fit of the data (C). It is possible that capture of the antibody with Protein A/G results in heterogeneity either through dissociation of antibody from the capture surface or through heterogeneous contacts of the antibody with Protein A/G. In most cases these effects are negligible when measuring kinetics in the nanomolar (nM) to picomolar (pM) range, but become noticeable when measuring sub-pM interactions. (Subsequent experiments that used covalent immobilization through aldehyde coupling support this interpretation and are described below.) For this reason, the off-rate was estimated for the later, more stable portion of the sensorgram, which fit a 1:1 dissociation model. By this method, the affinity of XOMA 052 for binding mouse IL-1β was calculated to be 7 nM (D), which is well within the capabilities of the technology. In the case of the human IL-1β, however, the slow dissociation rate approaches or exceeds the limit of measurement by SPR, so the affinity was estimated to be ≤4 pM. To more accurately determine the affinity of XOMA 052 binding to IL-1β, the equilibrium binding constant (KD) was measured by the more sensitive kinetic exclusion assay, KinExA, which is a solution-based analysis useful for characterization of such high affinity interactions.19,20 Figure 1E and F show KinExA analyses that indicated a KD of 300 and 240 femtomolar (fM) for XOMA 052 and XMA005, respectively, demonstrating that the Human Engineering™ sequence modifications of the variable regions in XMA005 did not adversely affect the binding affinity of XOMA 052 for its target.
Figure 1.
Kinetic binding analysis of XOMA 052 and XMA005. (A) Injection of human IL-1β over XOMA 052 captured by protein A/G. Data were collected in triplicate for six IL-1β concentrations ranging from 23 pM to 57.8 nM. (B) Three injections of 57.8 nM human IL-1β were followed for 4 h of dissociation and fit using Scrubber2 software to estimate a dissociation rate (kd) of ≤6.3 × 10−6 sec−1. (C) The trend in the residuals from the fit of the data in the early part of dissociation profile is not seen in experiments using aldehyde-coupled antibody and is thus believed to result from instability due to capture. The value for the dissociation rate was determined from the later portion of the long dissociations. The association rate of 1.7 × 106 M−1s−1 was determined using a global fit of the association curves with a simple 1:1 Langmuir binding model with the dissociation rate fixed at the value determined from the long dissociations. The fit of the data is shown as a solid red line, yielding an affinity of ≤4 pM. (D) Injection of mouse IL-1β over XOMA 052 captured by protein A/G. Data were collected in triplicate for six IL-1β concentrations ranging from 23 pM to 300 nM, except for the highest concentration, which was a singlicate injection. Data were fit globally with a simple 1:1 Langmuir binding model, yielding an affinity of 7 nM. Analysis of XOMA 052 (E) and XMA005 (F) binding to human IL-1β by Kinetic Exclusion Assay (KinExA). This solution-based method yields equilibrium binding constants (KD) of 300 and 240 fM with 95% confidence intervals of 115 to 742 and 70 to 722 fM, for XOMA 052 and XMA005, respectively.
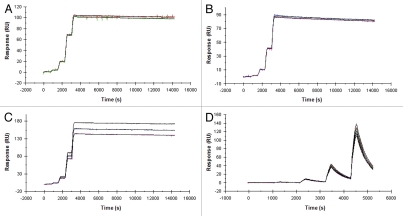
The ability of XOMA 052 to bind additional species orthologs of IL-1β was measured by SPR using a kinetic titration approach.21 To avoid effects related to antibody capture, XOMA 052 was immobilized directly onto the sensor surface. Previous work (not shown) indicated that amine coupling of XOMA 052 leads to heterogeneous kinetics, possibly due to influences of lysine residues on the binding activity of the antibody. For this reason, the antibody was immobilized through carbohydrates using an aldehyde coupling approach. In addition to high-affinity binding to human IL-1β (Fig. 2A), XOMA 052 binds to rhesus (Fig. 2B), rat (Fig. 2C), and rabbit (data not shown) orthologs with affinities of ≤10 pM (Table 1). As seen in the previous SPR measurement, XOMA 052 binds to mouse IL-1β with lower affinity, determined to be 3 nM by this method (Fig. 2D and Table 1). Although this affinity is at least three orders of magnitude lower than that for the human homolog, the single-digit nM binding affinity to the murine IL-1β is similar to that of many therapeutic products, and is sufficiently high to test XOMA 052 efficacy in mouse disease models.
Figure 2.
XOMA 052 cross-reacts with four species orthologs of IL-1β. Kinetic titration analysis of XOMA 052 binding to IL-1β from human (A), rhesus (B), rat (C) and mouse (D). Five concentrations of recombinant human, rhesus, rat and mouse IL-1β were injected in serial over XOMA 052 immobilized on a Biacore 2000 biosensor surface by aldehyde coupling. KD was determined by fitting data to a 1:1 Langmuir model using kinetic titration analysis with BIAevaluation software.
Table 1.
Comparative binding affinities and kinetics of XOMA 052 binding to IL-1β from different species determined by Biacore
| Species | ka (M−1s−1) | kd (s−1) | KD (pM) |
| Human | 1.7 × 106 | ≤6.3 × 10−6 | ≤4 ± 2* |
| Rhesus | 8.5 × 105 | ≤6.6 × 10−6 | ≤8 ± 2* |
| Rat | 1.5 × 106 | ≤2.8 × 10−6 | ≤2 ± 1* |
| Mouse | 7.7 × 105 | 2.4 × 10−3 | 3000 ± 100 |
The kinetics of the interaction between XOMA 052 and IL-1β from these three species are at the limit of measurement by Biacore, and therefore the KD values in this table represent upper limits of KD (i.e., lower limits of affinity). Error values reflect the range derived from replicate kinetic titration experiments.
Epitope mapping of XOMA 052.
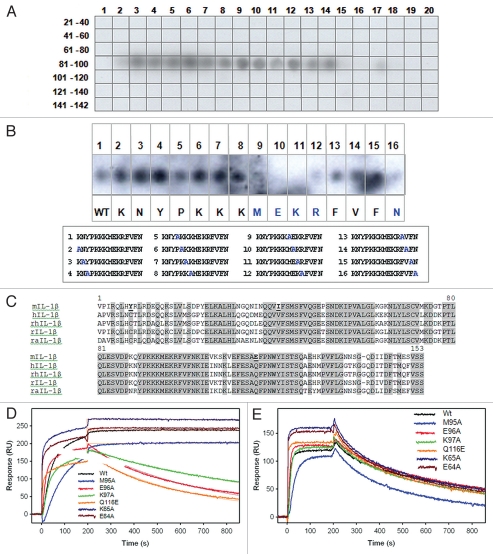
To identify the region of IL-1β that is bound by XOMA 052, we utilized a combination of PepSpot™ peptide arrays, sequence comparison and site-directed mutagenesis approaches. XOMA 052 can bind denatured (both reduced and non-reduced) recombinant human IL-1β in western blot analysis (data not shown), suggesting that the XOMA 052 epitope of human IL-1β might be linear or include a linear component. To map the binding site, XOMA 052 was hybridized to an IL-1β PepSpot™ membrane, displaying overlapping 12-mer peptides spanning the entire protein. The results showed that XOMA 052 specifically bound to a number of spots that cover the region from residues 83 to 105 of the mature protein (Fig. 3A). This region is larger than expected for a linear epitope that generally ranges between 4–8 residues, suggesting that the XOMA 052 epitope might be more complex. Because of the high affinity of XOMA 052 for its target, it is possible that a linear portion of the full discontinuous epitope could still be bound by XOMA 052 with an affinity that is sufficient to allow detection by western blot. To determine which residues contribute to binding, additional peptides, each containing a single alanine substitution, were re-probed by XOMA 052. Substitution of amino acids M95, E96 and K97 abolished binding to XOMA 052, while substituting R98 and N102 strongly reduced binding (Fig. 3B).
Figure 3.
XOMA 052 epitope mapping. (A) The IL-1β PepSpot™ Peptide Array membrane probed with XOMA 052 reveals that XOMA 052 binds to peptide spots corresponding to amino acids 83–105 of the mature protein. (B) Alanine substituted peptides hybridized with XOMA 052. Sequences of the 16 peptides with the alanine substitution (in blue) are shown in the box below. Peptides 9–12 and 16 showed little or no binding by XOMA 052 (WT, wild type). (C) Sequence alignment of mature forms of mouse (m), human (h), rhesus (rh), rat (r) and rabbit (ra) IL-1β are shown. Residues that are identical in human, rhesus, rat and rabbit and differ in mouse are shown in bold and underlined. (D) Supernatants from wild type and six mutants of IL-1β (E64A, K65A, M95A, E96A, K97A and Q116E) were injected over XOMA 052 immobilized on a ProteOn XPR sensor chip. The fits of the off-rate data are shown as red lines. Mutants E96A, K97A and Q116E showed off-rates increased by 1,000-fold. (E) Sensorgrams of wild type and IL-1β mutants binding to sRI show that the mutant proteins were expressed and folded properly.
Species cross-reactivity data (Figs. 1 and 2 and Table 1) suggest that the epitope bound by XOMA 052 is in a region of IL-1β that is not completely conserved between mouse and other tested orthologs (human, rat, rhesus and rabbit). Figure 3C shows an alignment of the mouse, human, rhesus, rat and rabbit IL-1β protein sequences. Residues that are conserved among human, rhesus, rat and rabbit IL-1β, but which differ in the mouse ortholog, are C8, V41 and Q116. We examined the contribution of these residues to XOMA 052 binding, as well as some of the amino acid residues identified by PepSpot, using site-directed mutagenesis of the full length protein combined with SPR analysis. The dissociation rates of all the mutants tested are summarized in Table 2.
Table 2.
Effect of single amino acid substitutions of human IL-1β on binding to sRI and XOMA 052
| IL-1β mutant | sRI binding (RU)* | XOMA 052 kd (sec−1)** |
| wt | 130 ± 26 | ≤1 × 10−6 |
| R4A† | 121 ± 12 | ≤1 × 10−6 |
| R4D† | 7 ± 5 | ≤1 × 10−6 |
| L6A | 118 ± 2 | ≤1 × 10−6 |
| C8Y | 114 ± 7 | ≤1 × 10−6 |
| V40A | 134 | ≤1 × 10−6 |
| V41I | 132 ± 7 | ≤1 × 10−6 |
| E50A | 131 | ≤1 × 10−6 |
| E64A‡ | 126 | ≤1 × 10−5 |
| K65A‡ | 122 | ≤1 × 10−5 |
| L67A‡ | 124 ± 10 | ≤1 × 10−5 |
| Y68A‡ | 130 ± 3 | ≤1 × 10−5 |
| K93A | 65 ± 20 | ≤1 × 10−6 |
| M95A | 110 ± 6 | ≤1 × 10−6 |
| E96A | 135 ± 1 | 3 × 10−3 |
| K97A | 132 ± 1 | 2 × 10−3 |
| Q116E | 145 ± 2 | 2 × 10−3 |
| F150S | 113 ± 13 | ≤1 × 10−6 |
Mean and range of multiple measurements for those mutants measured more than one time.
Dissociation rates ≤1 × 10−5 approach the limit of measurement in this experiment, which followed dissociation for only 10 min. The curves fit as 10−5 sec−1 could be distinguished qualitatively from those fit at 10−6 sec−1, but the difference cannot be quantitatively determined from these measurements.
This residue is located in the binding interface with sRI.
These residues belong to the same loop. The slight increase in off-rates most likely results from a destabilization of the IL-1β structure because a similar decrease was observed with control anti-IL-1β antibodies with non-overlapping epitopes to XOMA 052 (data not shown).
Two additional residues of note are P51 and S150, which while not completely conserved in other species, are different in mouse from the other species tested. For example, residue 150 is serine (a polar, uncharged amino acid) in the mouse sequence and phelylalanine or proline (basic non-polar amino acids), in other species. Because of its position at the C-terminus, a proline at this position is unlikely to cause broader structural change in the molecule. Residue 51, on the other hand, is non-conserved across species, with a proline (basic, non-polar) in mouse and threonine (polar, uncharged) or glutamate (acidic) in the other species. Because of its location in the folded protein, the presence of a proline at this position in the mouse sequence could cause a shift in the geometry or folding of the loops, which in theory could contribute to the reduced affinity of XOMA 052 for the cytokine. However, because the amino acid change in the other species is also non-conservative, it is unlikely to participate directly in the binding interaction with the antibody. For these reasons we generate and tested S150F, but not a mutation at residue 51.
In total, 12 alanine substitution mutants of human IL-1β were generated and expressed transiently in HEK 293E cells (R4A, L6A, V40A, E50A, E64A, K65A, L67A, Y68A, K93A, M95A, E96A, K97A). The culture supernatant from each transfection was tested for binding to recombinant IL-1 sRI and XOMA 052 in parallel by SPR. Substitution of either E96 or K97 with alanine increases the off-rate from XOMA 052 by at least 1,000-fold (Fig. 3D). Binding to sRI verified that these mutant proteins were expressed and properly folded (Fig. 3E). Four alanine substitutions of residues that belong to the same loop (E64A, K65A, L67A, Y68A) impacted the off-rate from XOMA 052 10-fold (Table 2). This slight decrease in off-rates most likely results from a destabilization of the IL-1β structure because a similar decrease was observed with control anti-IL-1β antibodies that bind to epitopes that do not overlap that of XOMA 052 (data not shown).
One basic residue located in the binding interface with the receptor was substituted to aspartate (R4D); not surprisingly, substitution with an acidic strongly affected binding to sRI, but it had no effect on binding of XOMA 052.
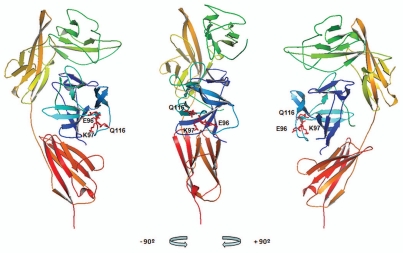
Four additional mutants of human IL-1β were constructed by substituting the residues at positions 8, 41, 116 and 150 with the amino acid found at the equivalent position of the mouse ortholog. The single mutations C8Y, V41I and F150S had no effect on dissociation rates as measured by SPR, but Q116E increased the dissociation rate from XOMA 052 at least 1,000-fold (Fig. 3D and Table 2) while having no effect on the dissociation from IL-1 sRI (Fig. 3E). Interestingly, the increased dissociation rate of Q116E recapitulates the dissociation rate seen with mouse IL-1β. The positions in the IL-1β crystal structure of the three residues identified by these analyses as critical for XOMA 052 binding are illustrated in Figure 4.22 The clustering of three non-contiguous IL-1β residues critical for XOMA 052 binding on the surface of the cytokine suggests that they may form part of a conformational epitope. However, the possibility that these residues play a structural role in forming the epitope rather than making direct contacts with XOMA 052 cannot be excluded, so additional studies are under way to unambiguously identify residues involved in the direct interaction between the two molecules.
Figure 4.
XOMA 052 epitope as predicted by PepSpot™ and alanine scan analyses. Figure shows the ribbon representation of the structure for the human IL-1β/IL-1RI complex22 using Pymol visualization software (DeLano Scientific LLC, San Carlos, CA). Receptor domains I, II and III are depicted in green, yellow and orange respectively and IL-1β depicted in blue. The side chains of E96, K97 and Q116 identified as critical for binding to XOMA 052 are shown in red. Center: front view, left: a 90° rotation view to the left, right: a 90° rotation view to the right.
XOMA 052 activity in MRC5 and whole blood bioassays.
To test the inhibitory activity of XOMA 052, two bioassays based on the ability of IL-1β to induce cytokine expression following activation of the IL-1 pathway were used. The ability of XOMA 052 to inhibit the expression of IL-6 was measured in the IL-1β-treated MRC-5 human lung fibroblast cell line and compared to that of the murine parental antibody XMA005 and the recombinant form of IL-1Ra, anakinra (Fig. 5A). In this assay, XOMA 052 completely blocked the IL-1β-induced expression of IL-6 with an IC50 of 4.9 pM. The potency of XOMA 052 was equivalent to that of the parental antibody XMA005, with an IC50 of 4.4 pM, demonstrating that Human Engineering™ did not alter the target inhibitory activity of the parental murine antibody. This potency is ∼10-fold more potent than that of anakinra, which has an IC50 of 45 pM. The IL-1β neutralizing activity of XOMA 052 also was assessed in a human whole blood bio-assay where the IL-1β-mediated expression of IL-8 was measured (Fig. 5B). Similar to what was observed in the MRC-5 IL-6 release bioassay, the IL-1β neutralizing activity of XOMA 052 (IC50 = 28 pM) was 20-fold more potent than anakinra (IC50 = 603 pM).
Figure 5.
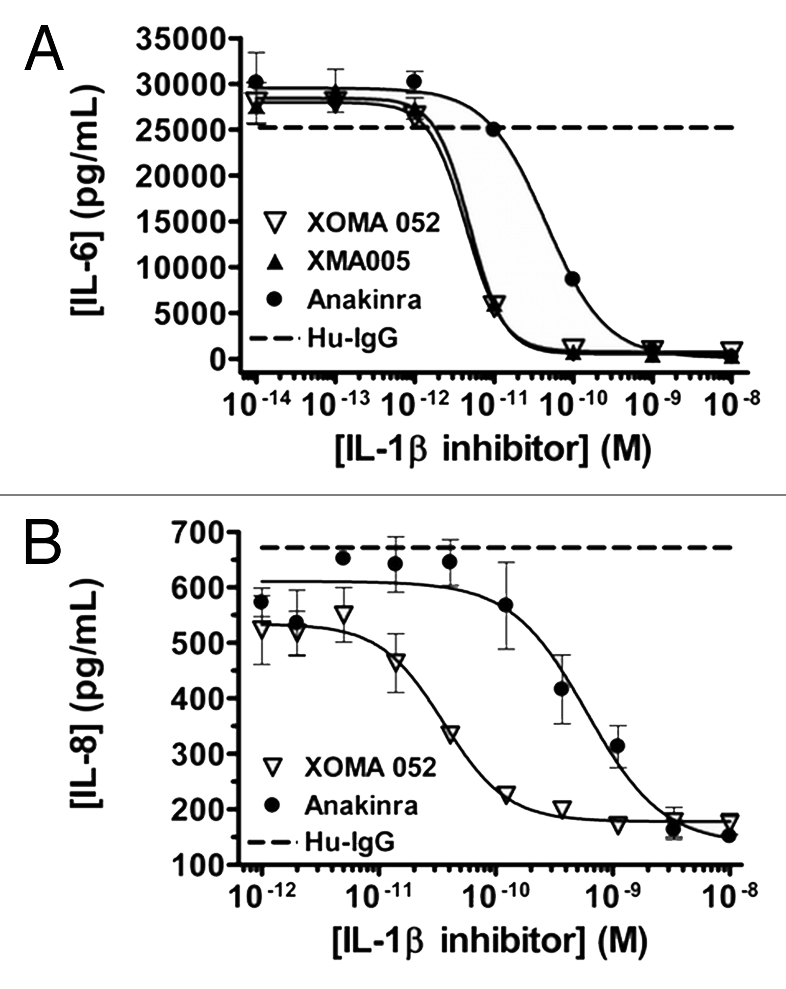
XOMA 052 neutralizes human IL-1β activity in vitro. (A) Inhibition of IL-1β-induced IL-6 release in MRC5 human fibroblast cells. Cells were stimulated with 5.8 pM of IL-1β in the presence of XMA005, XOMA 052, anakinra or a control IgG2 Ab. XMA005 and XOMA 052 had an IC50 of 4.9 and 4.4 pM respectively, while anakinra had an IC50 of 45 pM. (B) Inhibition of IL-1β induced IL-8 expression in a human whole blood assay. Whole blood was stimulated with 100 pM of IL-1β in the presence of XOMA 052, anakinra or a control IgG2 Ab. XOMA 052 has an IC50 of 28 pM, while recombinant human IL-1Ra anakinra has an IC50 of 603 pM.
XOMA 052 in vivo activity (CBA and gout).
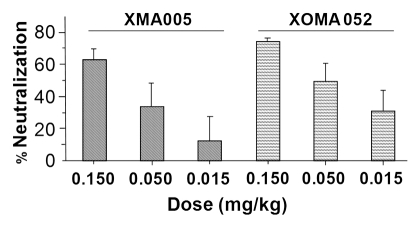
To assess whether XOMA 052 could neutralize human IL-1β systemically, an in vivo cytokine biomarker assay (CBA) was used. In this assay, mice were pre-treated with XOMA 052, XMA005 or control IgG, then challenged with an exogenous dose of human IL-1β, and the induction of murine IL-6 was measured in the serum. XOMA 052 and XMA005 blocked the ability of human IL-1β to induce systemic IL-6 in the mouse, with 60–70% inhibition at 0.15 mg/kg antibody (Fig. 6). In a previous experiment, this dose of XOMA 052 did not block exogenously administered murine IL-1β, and a higher dose (15 mg/kg) was required.17 Thus, while the affinity of XOMA 052 for mouse IL-1β is over 1,000-fold lower than for human IL-1β, a 100-fold higher dose is sufficient to neutralize its activity in vivo. This suggests that XOMA 052 could have significant efficacy in a murine disease model where IL-1β has a key role in the induction and maintenance of pathology.
Figure 6.
XOMA 052 inhibits IL-6 expression in IL-1β treated mice. Mice (n = 8 per treatment group) were injected with XMA005 parent, XOMA 052 or IgG control and treated 24 h later with 1 µg/kg of recombinant human IL-1β as described in Materials and Methods. Two hours later mice were sacrificed and neutralization activity of the antibodies was calculated by measuring the levels of IL-6 in the mouse serum. Percent neutralization was calculated using the mean of the IgG control treatment group as 100%. Both antibodies inhibited IL-6 stimulation in a dose-dependent manner with comparable potency. Values are mean percent neutralization, and error bars show standard error of the mean. The difference between XMA005 and XOMA 052 is not significant as measured by unpaired two-tailed t-test (p = 0.17, 0.41 and 0.36 for 0.15, 0.05 and 0.015 mg/kg doses, respectively).
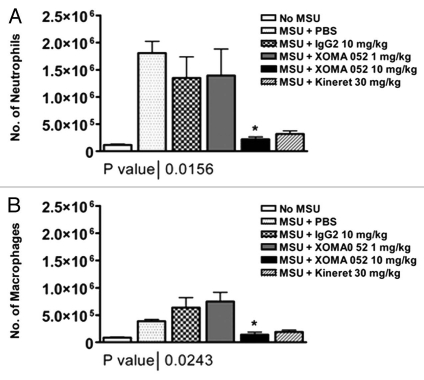
We tested XOMA 052 in a mouse model of acute gout, monosodium urate (MSU) crystal-induced acute peritonitis. In this model, injection of MSU crystals leads to a rapid influx of inflammatory cells, in an IL-1-dependent manner.23 Due to its lower affinity for mouse IL-1β, XOMA 052 was administered at a relatively high dose of 10 mg/kg. At this dose, XOMA 052 blocked infiltration of both neutrophils (Fig. 7A) and macrophages (Fig. 7B) in this murine model, and peritonitis induced by the MSU crystals was reduced by 84% relative to isotype control (p < 0.05, unpaired t-test).
Figure 7.
XOMA 052 blocks inflammation in a model of acute gout. (A) Number of infiltrating neutrophils in the peritoneal lavage, 6 h after MSU injection. (B) Number of infiltrating macrophages in the peritoneal lavage, 6 h after MSU injection. n = 6 mice/group. *p < 0.05 for XOMA 052 10 mg/kg vs. IgG2 control or anakinra vs. PBS using an unpaired t-test.
Discussion
IL-1β is a potent mediator of inflammation that functions at the initiation of the inflammatory cascade. This pathway must maintain a delicate balance between beneficial and disease-related effects, as evidenced by the role that IL-1β plays in the development of many inflammatory, autoimmune, metabolic and oncological diseases.1 IL-1β is believed to act locally, rather than systemically, and is very potent, requiring less than 5% of receptor occupancy to induce maximum response.7,24 Effectively modulating an early, locally acting, potent and short-lived inflammatory cytokine with a potent inhibitor has been a common challenge for all existing IL-1 pathway inhibitors. The challenge is even greater when trying to address and meet patients' needs for a safe, efficacious and convenient drug.
Several inhibitors of the IL-1 pathway have been developed and have provided important proof of concept to illustrate that this pathway has great potential for the development of new therapeutic drugs. While providing proof of concept in many diseases, these inhibitors require frequent or high dosing regimens to achieve and maintain efficacy, which is possibly due to their mechanism of inhibition together with their pharmacokinetic properties.7 This is particularly true for diseases like rheumatoid arthritis, where a therapeutic agent, in order to exert its inhibitory effect, must penetrate the synovial compartment and maintain a steady-state of local efficacious levels.
To overcome these limitations, we sought to design and develop a “best-in-class” anti-IL-1β therapeutic antibody, XOMA 052. This high affinity antibody specifically inhibits IL-1β activity with a unique mechanism of action.18 Such specificity for IL-1β alone will have the added advantage of sparing both IL-1α, which could provide a safety margin for protection against potential infections and IL-1Ra, which is the natural antagonist of the IL-1 signaling pathway. Indeed, inactivation of IL-1Ra would work against the very purpose of the therapeutic drug.
Additionally, because circulating levels of IL-1β in disease are in the pM range,7,25 inhibiting the IL-1β pathway by blocking IL-1β-specific activity is predicted to be a better strategy than blocking the activating receptor, IL-1RI, which is ubiquitously expressed on all nucleated cells and could lead to increased clearance of antibody from circulation. In addition to specifically inhibiting IL-1β, XOMA 052 has desirable pharmacokinetic properties. In humans, humanized antibodies generally have a half-life of 14–21 days and our initial Phase 1 studies in Type 2 diabetes have shown that XOMA 052 exceeds this range with a half-life of approximately 23 days.26
In vitro characterization studies have shown that two of the residues of IL-1β that are important for XOMA 052 binding (E96, K97) are located in loop G of the mature IL-1β protein. The crystal structure of IL-1β complexed to its receptor shows that loop G is adjacent to the region that contacts the receptor,22 and part of loop G has been shown to be important for receptor binding.27 The third residue important for XOMA 052 binding, (Q116), while not a part of loop G, is located near this loop in the folded protein, as shown in crystal structure of IL-1β. XOMA 052 is the first reported example of a therapeutic antibody binding to this region. Because the epitope of XOMA 052 is proximal to, but does not overlap the receptor/ligand interface, binding by XOMA 052 may interfere with assembly of the active signaling complex in a manner different from simple competitive binding. This is consistent with the novel mechanism of action of XOMA 052 for neutralization of IL-1β previously described.18
XOMA 052 has an in vitro potency that is >10-fold higher than anakinra, and an in vitro binding affinity for IL-1β of 300 fM. The antibody binding affinity for the mouse IL-1β is 3 nM, which is sufficiently high to allow testing XOMA 052 efficacy in a variety of disease models in mice. It was therefore not necessary to develop a surrogate antibody or to rely exclusively on the ability of XOMA 052 to neutralize the effects of exogenous human IL-1β injected systemically in mice. We have recently reported that specific inhibition of IL-1β with XOMA 052 can treat the chronic inflammation of diet-induced obesity in a mouse model of Type 2 diabetes.17
Recent studies in humans have shown that inhibition of the IL-1 pathway can be an effective therapy for acute gout.28–30 In the present study we have shown that XOMA 052 is functionally active in a murine model of acute gout, blocking both neutrophil and macrophage influx triggered by MSU crystals. Production of IL-1 in response to MSU crystals has been reported to occur in resident macrophages, rather that infiltrating monocytes or neutrophils.23 Thus we envision that recurrent accumulation of MSU in an affected joint would lead to recurrent production of IL-1β. XOMA 052 with its long half-life would be able to neutralize recurrent flares. We show in this report that there was no significant difference between treatment with 10 mg/kg XOMA 052 and 30 mg/kg anakinra in a mouse model of acute gout, thus confirming its in vivo efficacy in a new class of auto-inflammatory diseases that result from inappropriate activation of the innate immune system.31
Gout is one of several diseases that can now be classified as auto-inflammatory, involving activation of the “inflammasome,” which results in processing of pro-IL-1β to active IL-1β. Included in these diseases are NOMID, MWS, PAPA syndrome and FMF.32 Interestingly, a recent study described the role of cholesterol crystals in activating the inflammasome, leading to atherogenesis.33 Thus, diseases previously considered unrelated could now potentially be treated by targeting a common mechanism. For example, while the role of IL-1β in type 1 diabetes has long been acknowledged,34 recognition of its role in development of type 2 diabetes is a more recent development.10 In addition, IL-1β has long been implicated in the cause and pathogenesis of autoimmune diseases such as psoriasis, multiple sclerosis, asthma, inflammatory bowel disease and rheumatoid arthritis.35 Although anakinra has only shown modest effects in rheumatoid arthritis,36,37 we hypothesize that XOMA 052 could have increased efficacy due to its higher affinity for IL-1β and longer half-life. Indeed, in the collagen-induced arthritis mouse model, XOMA 052 was effective in preventing and treating joint inflammation, at lower and less frequent doses than anakinra.38 Furthermore, the role of chronic inflammation is increasingly recognized in cardio-metabolic diseases, including diabetes and atherosclerosis, even in the absence of measurable increases in serum levels of IL-1β. Results for another anti-IL-1β antibody that inhibited IL-1β-mediated joint inflammation in preclinical models and was associated with treatment effects and clinical improvement in a subset of RA patients with weekly dosing in a small proof of concept study have been reported.39 This study further validates the approach of targeting IL-1β with specific antibodies for the treatment of autoimmune diseases.
As XOMA 052 is more potent against human IL-1β than the murine homolog,17 its activity in mouse models of several classes of inflammation driven diseases suggests that this antibody could be a highly effective treatment for a wide variety of human inflammatory diseases and conditions. Because of its unique biophysical, pharmacokinetic and functional properties, as well as its unique mechanism of action, XOMA 052 may offer advantages compared with other inhibitors of the IL-1 pathway. This antibody has potential as a novel approach to addressing the need for a potent inhibitor while also acting as an efficacious and safe therapeutic that can be administered at low and infrequent doses. XOMA 052 is currently in Phase 2 clinical trials in Type 2 diabetes, and is being evaluated as a treatment for a number of other IL-1β-mediated diseases.
Material and Methods
Human Engineering™.
Human Engineering™ does not require modeling; rather, it is based on the alignment of the parental non-human variable region sequences to either Kabat or germline consensus human sequences. Briefly, surface exposed variable region amino acids are assigned a “low,” “moderate” or “high” risk that if changed to human would adversely affect the antibody binding activity. The non-human residues with “low” and “moderate” risk assignment are candidates for being changed to a corresponding human residue.16 For the Human Engineering™ of the lead antibody candidate, the heavy and light variable region sequences of XMA005 parental antibody were humanized based on consensus sequences from the Kabat database of human antibody sequences. For the light chain, nine low risk and two moderate risk residues were modified to match a human VK1 sequence template; for the heavy chain, nine low risk and three moderate risk residues were modified to match a human VH2 sequence template. Human Engineered− light and heavy chain variable region sequences were cloned into modular expression vectors containing human κ and γ-2 constant region sequences respectively. Four humanized antibody variants were generated. The antibody variant with the highest human content, 97% based on V-regions humanized to Kabat human consensus sequences, XOMA 052, was chosen as the lead for therapeutic development.
Expression and purification of recombinant antibodies.
Parental and Human Engineered™ antibody variants were transiently expressed in HEK293E cells. Briefly, a total of 40 µg of heavy chain (HC) and light chain (LC) vector (1:2 HC to LC) was transfected into 40 ml of HEK293E at a density of 8 × 105 cells/ml. Cells were incubated for seven days at 37°C at which time supernatant was harvested and batch purified using Protein A-Sepharose CL-4B (GE Amersham, Uppsala, Sweden). Protein A-Sepharose suspension was added to the cell supernatant and the mixture incubated at 4°C for 2 h. Protein A-Sepharose beads were separated from the supernatant, washed with phosphate-buffered saline (PBS) and antibody eluted with 200 µl 0.2 M glycine-HCl, pH 2.5. Eluted antibody was neutralized with 1 M Tris-HCl, pH 9 and dialyzed against PBS overnight.
Binding affinity determination.
Recombinant IL-1β was obtained from the following sources: human, Peprotech Inc., Rocky Hill, NJ or R&D Systems, Minneapolis, MN; rhesus, rat and mouse, R&D Systems. Binding kinetics of four orthologs of recombinant IL-1β, including human, rhesus, rat and mouse to immobilized XOMA 052 were measured using a surface plasmon resonance (SPR) method employing two immobilization approaches on a Biacore® 2000. All experiments were performed at 25°C using running buffer containing 10 mM HEPES, pH 7.4, 150 mM NaCl, 0.005% P20 and 3 mM EDTA, (HBS-EP, Biacore, Piscataway, NJ). In the first approach, the antibody was captured using Protein A/G immobilized on a CM-5 sensor surface. Protein A/G was immobilized onto flow cells 1 and 2 using amine-coupling chemistry using a standard procedure. XOMA 052 was captured on flow cell 2 at density of 200 RU. Recombinant mouse or human IL-1β was diluted in running buffer to six concentrations (23 pM–57.8 nM) and injected in triplicate for 2 min at 20 µL/min over the captured antibody and the reference flow cells. For the mouse ortholog, an additional injection of 300 nM IL-1β was added. Dissociation was measured for 10 min. Control experiments (not shown) verified that this system is not mass transport-limited at this flow rate. In a separate experiment, three injections of 57.8 nM IL-1β were followed for a dissociation time of 4 h. The surfaces were regenerated between cycles with one 30 sec injection each of 10 mM Glycine pH 2.0 (Biacore) and running buffer at 100 µL/m. The sensorgrams were double-referenced against the reference flow cell and a buffer injection and fit globally to a 1:1 binding model to generate kinetic rate constants using Scrubber2 software (Biologic Software, Campbell, Australia). The second approach utilized a kinetic titration protocol21 over XOMA 052 immobilized by aldehyde coupling to a CM-5 sensor chip. Five concentrations of recombinant human, rhesus, rat and mouse IL-1β (R&D Systems) were injected in a series over XOMA 052 and a reference surface, and regenerated at the end of each cycle. For human and rhesus IL-1β, each cycle was repeated once, for a total of two replicate injections. Rat IL-1β data included three and mouse IL-1β five, replicate cycles. The binding responses were fit using a simple 1:1 Langmuir binding model with BIAevaluation software using kinetic titration analysis to yield on-rate (k-on) and off-rate (k-off) parameters. The kinetic parameters were subsequently used to calculate the equilibrium dissociation constant (KD). Due to the extremely slow dissociation rates, the kinetics of the interaction between XOMA 052 and IL-1β from human, rhesus and rat species approach the limit of measurement by Biacore®, and therefore the equilibrium binding affinities of XOMA 052 and its parent XMA005 binding to human IL-1β were determined accurately by a solution equilibrium method. Antibodies were incubated with increasing concentrations of ligand (0.04–80 pM) and free antibody was measured using KinExA™ (Sapidyne, Boise, ID). The equilibrium binding constants (KD) were derived from fitting the curve of a plot of % free antibody versus IL-1β concentration to a 1:1 binding model using KinExA software (Version 2.4; Sapidyne).
Epitope mapping.
Mapping of the IL-1β epitope bound by the XOMA 052 paratope was carried out using a combination of PepSpot™ peptide array (JPT Peptide Technologies, Berlin, Germany), alanine mutagenesis and comparative binding with IL-1β from different species. A series of 12-mer peptides spanning the entire IL-1β amino-acid sequence, each with a one amino acid offset from the next, was synthesized directly on a membrane. The membrane was probed with XOMA 052 at a concentration of 2 µg/ml for 2 h at room temperature. Binding of XOMA 052 was detected using a secondary horseradish peroxidase-conjugated goat anti-human antibody and visualized using enhanced chemiluminescence (ECL) substrate (PerkinElmer, Waltham, MA). Each amino acid in the peptide found by PepSpot™ analysis to bind XOMA 052 was substituted individually with alanine and the resulting peptides were re-probed by immunoblotting with XOMA 052.
Vectors encoding mutants of full-length mature IL-1β were constructed using oligonucleotide-mediated site-directed mutagenesis (Quickchange II XL Site-Directed Mutagenesis, Stratagene). The following single amino acid substitutions were generated: R4A, R4D, L6A, C8Y, V40A, V41I, E50A, E64A, K65A, L67A, Y68A, K93A, M95A, E96A, K97A, Q116E and F150S. All mutants were sequence-confirmed and transfected transiently into HEK293-EBNA cells (Invitrogen cat # R620-07) using Lipofectamine (Lipofectamine™ 2000, Invitrogen) and tested for production of secreted, active mature IL-1β protein. HEK293-EBNA cells were maintained in Hyclone SFM4Transfx-293™ medium containing 1% FBS + 250 ug/ml G418 in a 37°C incubator with 5% CO2 on a shaking platform. Prior to transfection HEK293-EBNA cells were diluted to 8 × 105 cells/ml in 20 ml of growth medium, and then incubated 48 h with 20 µg plasmid DNA + 20 µg Lipofectamine 2000 (Invitrogen). Supernatants were collected and cleared by centrifugation followed by filtration through a 0.22 micron filter. Supernatants were tested by SPR using the ProteOn™ XPR36 (Bio-Rad Laboratories) for binding to biotinylated XOMA 052 and IL-1 Receptor I (sRI) (R&D Systems, Minneapolis, MN) captured on a NeutrAvidin coated chip (NLC chip cat # 176-5021). Samples were diluted 10-fold in HBS-EP buffer and injected for 200 seconds at 30 µL/min over captured XOMA 052 and IL-1 RI. Dissociation was followed for 10 min and the curves were fit with Scrubber2.
MRC5 assay.
MRC-5 human lung fibroblast cells (ATCC, Manassas, VA) were used to assess IL-1β inhibitory activity as described.40 Briefly, MRC-5 cells were seeded in a 96-well plate at 5,000 cells per well in MEM complete growth medium with 10% fetal bovine serum. After an overnight incubation at 37°C with 6.5% CO2, supernatants were removed and replaced with growth medium containing control antibodies, XOMA 052 or recombinant human IL-1ra anakinra (Amgen, Thousand Oaks, CA) at concentrations ranging from 10 fM to 10 nM. Recombinant human IL-1β (Roche Applied Sciences, Indianapolis, IN) was added to a final concentration of 5.8 pM. As a negative control, rhIL-1β treated cells were incubated with 10 nM anti-Keyhole Limpet Hemocyanin (KLH)-specific isotype control antibody (clone KLH8.G2, XOMA). Following 20-h incubation at 37°C with 6.5% CO2, supernatants were assayed in triplicate for human IL-6 by ELISA (Quantikine human IL-6 ELISA, R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Whole blood assay.
Whole blood collected from healthy donors was distributed at 200 µl/well into a round-bottom 96-well plate. XOMA 052 or anakinra was added to final concentrations ranging from 1 pM to 10 nM. Recombinant human IL-1β (Roche Applied Sciences, Indianapolis, IN) was then added to a final concentration of 100 pM. KLH8.G2 antibody was used as the isotype control.
The assay plate was incubated at 37°C with 6% CO2 for 6 h. After incubation, 50 µl/well of 2.5% Triton X-100 (Sigma-Aldrich, Saint Louis, MO) was added to each well and mixed thoroughly to generate whole blood lysates with a final detergent concentration of 0.5%. The sample plate was then centrifuged for 5 min at 2,000 rpm to pellet debris and cleared lysates were transferred to a polypropylene, V-bottom 96-well plate and stored overnight at −80°C. The following morning, lysates were quickly thawed at 37°C, centrifuged for 5 min at 2,000 rpm to ensure lysate clarity, diluted and assayed in triplicate for human IL-8 by ELISA (Human IL-8 Quantikine, R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The experiment was repeated three times, using the same donor for each experiment.
Cytokine biomarker assay.
Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were injected intraperitoneally with IL-1β neutralizing antibody or control IgG (Jackson Immuno Research Laboratories, West Grove, PA) at 0, 0.015, 0.05 or 0.15 mg/kg (n = 8 mice per treatment group). Twenty-four hours after antibody injection, mice were injected subcutaneously with recombinant human IL-1β (PeproTech Inc., Rocky Hill, NJ) at a dose of 1 µg/kg. Two hours post rhIL-1β injection (peak IL-6 response time), mice were euthanized and blood collected via cardiac puncture. Serum mouse IL-6 levels were measured using a Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
Monosodium urate (MSU) crystal-induced acute peritonitis.
Studies were performed at Bio-Quant (San Diego, CA). Peritonitis was induced as described23 by injecting 0.5 mg of MSU crystals into the peritoneal space of Balb/c mice. Mice were treated 2 h earlier with intraperitoneal injection of isotype control antibody or XOMA 052. For comparison, one group of mice received IL-1Ra (anakinra) at the same time as MSU injection. After 6 h, peritoneal lavage was performed and the lavage fluid was centrifuged to collect cells. Cells were counted and a fraction was used for cytospin and leukocyte differential counts. Peritonitis was measured by calculating the number of neutrophils in the lavage. The number of neutrophils is determined by multiplying the total cell count in the lavage by the percentage of neutrophils in the differential count. The same method was used to calculate the number of macrophages in the lavage fluid.
Acknowledgements
The authors wish to thank Seema Kantak and Mark White for critically reading the manuscript, Gang Chen for contributions to discovery and characterization, and Steve Neben and Matt Gross for early work on functional assays.
Abbreviations
- IL-1
interleukin-1
- IL-1Ra
IL-1 receptor antagonist
- IL-1RAcP
IL-1 receptor accessory protein
- IL-1RI
IL-1 receptor type I
- IL-1RII
IL-1 receptor type II
- ESR
erythrocyte sedimentation rate
- sJIA
systemic juvenile idiopathic arthritis
- NOMID
neonatal onset multisystem inflammatory disease
- MWS
Muckle-Wells syndrome
- PAPA syndrome
pyogenic arthritis, pyoderma gangrenosum and acne syndrome
- FMF
Familial Mediterranean fever
- RA
rheumatoid arthritis
- CBA
cytokine biomarker assay
- MSU
monosodium urate
- SPR
surface plasmon resonance
- HC
heavy chain
- LC
light chain
- KLH
keyhole limpet hemocyanin
Authors' Contributions
Authors' contributions to this work are as follows: A.O. designed and performed research, analyzed data and wrote the paper; P.L., E.P., K.A., J.C. and A.H. designed and performed experiments and analyzed data; M.H. and M.H.F. designed experiments and analyzed data; H.I. and M.K.R. designed and performed experiments, analyzed data and contributed portions of the paper, L.M. designed experiments, analyzed data and wrote portions of the paper. L.M., A.H.H. and M.K.R. managed and performed antibody discovery and engineering to generate XOMA 052.
References
- 1.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 2.Lichtman AH, Chin J, Schmidt JA, Abbas AK. Role of interleukin 1 in the activation of T lymphocytes. Proc Natl Acad Sci USA. 1988;85:9699–9703. doi: 10.1073/pnas.85.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 4.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovell DJ, Bowyer SL, Solinger AM. Interleukin-1 blockade by anakinra improves clinical symptoms in patients with neonatal-onset multisystem inflammatory disease. Arthritis Rheum. 2005;52:1283–1286. doi: 10.1002/art.20953. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–612. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA. The many worlds of reducing interleukin-1. Arthritis Rheum. 2005;52:1960–1967. doi: 10.1002/art.21107. [DOI] [PubMed] [Google Scholar]
- 8.Abramson SB, Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 2002;41:972–980. doi: 10.1093/rheumatology/41.9.972. [DOI] [PubMed] [Google Scholar]
- 9.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 11.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 13.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 14.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Masat L, Haak-Frendscho M, Chen G, Horwitz AH, Roell MK. IL-1β binding antibodies and fragments thereof. 2009. May 12, US Patent 7,531,116. [Google Scholar]
- 16.Studnicka GM, Soares S, Better M, Williams RE, Nadell R, Horwitz AH. Human-engineered monoclonal antibodies retain full specific binding activity by preserving non-CDR complementarity-modulating residues. Protein Eng. 1994;7:805–814. doi: 10.1093/protein/7.6.805. [DOI] [PubMed] [Google Scholar]
- 17.Owyang AM, Maedler K, Gross L, Yin J, Esposito L, Shu L, et al. XOMA 052, an anti-IL-1beta monoclonal antibody, improves glucose control and beta-cell function in the diet-induced obesity mouse model. Endocrinology. 2010;151:2515–2527. doi: 10.1210/en.2009-1124. [DOI] [PubMed] [Google Scholar]
- 18.Roell MK, Issafras H, Bauer RJ, Michelson KS, Mendoza N, Vanegas SI, et al. A kinetic approach to pathway attenuation using XOMA 052, a regulatory therapeutic antibody that modulates interleukin-1 beta (IL-1β) activity. J Biol Chem. 2010 doi: 10.1074/jbc.M110.115790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drake AW, Myszka DG, Klakamp SL. Characterizing High Affinity Antibody/Antigen Complexes. In: Shire SJGW, Bechtold-Peters K, Andya J, editors. Current Trends in Monoclonal Antibody Development and Manufacturing. Springer: AAPS Press; 2010. pp. 179–192. [DOI] [Google Scholar]
- 20.Rathanaswami P, Roalstad S, Roskos L, Su QJ, Lackie S, Babcook J. Demonstration of an in vivo generated sub-picomolar affinity fully human monoclonal antibody to interleukin-8. Biochem Biophys Res Commun. 2005;334:1004–1013. doi: 10.1016/j.bbrc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson R, Katsamba PS, Nordin H, Pol E, Myszka DG. Analyzing a kinetic titration series using affinity biosensors. Anal Biochem. 2006;349:136–147. doi: 10.1016/j.ab.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Vigers GP, Anderson LJ, Caffes P, Brandhuber BJ. Crystal structure of the type-I interleukin-1 receptor complexed with interleukin-1beta. Nature. 1997;386:190–194. doi: 10.1038/386190a0. [DOI] [PubMed] [Google Scholar]
- 23.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 24.Gallis B, Prickett KS, Jackson J, Slack J, Schooley K, Sims JE, et al. IL-1 induces rapid phosphorylation of the IL-1 receptor. J Immunol. 1989;143:3235–3240. [PubMed] [Google Scholar]
- 25.Punzi L, Pozzuoli A, Pianon M, Bertazzolo N, Oliviero F, Scapinelli R. Pro-inflammatory interleukins in the synovial fluid of rheumatoid arthritis associated with joint hypermobility. Rheumatology (Oxford) 2001;40:202–204. doi: 10.1093/rheumatology/40.2.202. [DOI] [PubMed] [Google Scholar]
- 26.Donath M. XOMA 052, an anti-IL1b antibody, in a double-blind, placebo-controlled, dose excalation study of the safety and pharmacokinetics in patients with type 2 diabetes mellitus—A new approach to therapy. Diabetologia. 2008;51:1–7. [Google Scholar]
- 27.Grutter MG, van Oostrum J, Priestle JP, Edelmann E, Joss U, Feige U, et al. A mutational analysis of receptor binding sites of interleukin-1beta: differences in binding of human interleukin-1beta muteins to human and mouse receptors. Protein Eng. 1994;7:663–671. doi: 10.1093/protein/7.5.663. [DOI] [PubMed] [Google Scholar]
- 28.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, Bookbinder S, Biedermann S, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68:1613–1617. doi: 10.1136/ard.2009.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.So A, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62:3064–3076. doi: 10.1002/art.27600. [DOI] [PubMed] [Google Scholar]
- 31.Mills KH, Dunne A. Immune modulation: IL-1, master mediator or initiator of inflammation. Nat Med. 2009;15:1363–1364. doi: 10.1038/nm1209-1363. [DOI] [PubMed] [Google Scholar]
- 32.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 33.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandrup-Poulsen T, Pickersgill L, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:158–166. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- 35.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 36.Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–624. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 38.Owyang AM, Vanegas S, Gross L, Masat E, Pongo P, Larsen J, et al. Kantak efficacy of XOMA 052 anti-IL-1beta antibody in the dba/1 mouse collagen-induced arthritis model. The European League Against Rheumatism. Paris, France: Ann Rheum Dis. 2008:591. [Google Scholar]
- 39.Alten R, Gram H, Joosten LA, van den Berg WB, Sieper J, Wassenberg S, et al. The human anti-IL-1beta monoclonal antibody ACZ885 is effective in joint inflammation models in mice and in a proof-of-concept study in patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10:67. doi: 10.1186/ar2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinarello CA, editor. Cytokines and their cellular receptors, Current Protocols in Immunology. Vol. 6. New York: Wiley; 2000. pp. 1–7. [Google Scholar]