Abstract
A 51-year-old man was referred to the Department of Cardiology in our hospital due to severe congestive heart failure and ventricular arrhythmias in March 2008. He had repeated ventricular tachycardia for years and the left ventricular ejection fraction (EF) was 11% on admission. A myocardial biopsy revealed that over 50% cardiomyocytes were replaced by fibrosis. Due to the typical acromegalic features, he was referred to the endocrinology department and diagnosed as acromegaly. He was treated with octreotide for 8 months followed by trans-sphenoidal surgery. The plasma levels of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) decreased by octreotide and normalised by surgery after which the cardiac function improved drastically. The current case demonstrates that cardiac dysfunction in acromegaly could be recovered by normalisation of GH and IGF-1 even in the presence of severe fibrosis in the myocardium.
Background
Acromegaly is associated with an increased morbidity and mortality especially due to cardiovascular diseases.1 While other diseases, such as hypertension or diabetes mellitus, are often present in acromegaly, the cardiac dysfunction is supposed to be independent of the coexisting diseases in acromegaly, at least in part.2 3 Acromegalic cardiomyopathy occasionally leads to congestive heart failure.4–8 In some cases, the cardiac dysfunction was resistant to treatment7 suggesting that only the early stage of acromegalic cardiomyopathy without severe fibrosis is reversible.
Case presentation
A 51-year-old man was referred to our hospital due to severe congestive heart failure and ventricular arrhythmias in March 2008. He had been suffering from heart failure and ventricular tachycardia for years despite receiving the conventional treatment (ie, furosemide, lisinopril hydrate, pimobendan, warfarin potassium, carvedilol, metildigoxin and amiodarone hydrochloride).
Investigations
His height was 161 cm and body weight was 83 kg. Blood pressure was 110/60 mm Hg. The fasting blood glucose was 107 mg/dl and HbA1c was 6.3%. The chest x-ray showed cardiomegaly with a cardiothoracic ratio of 0.67 (figure 1A). ECG, as shown in video 1, revealed a diffused hypokinetic left ventricle with markedly dilated left ventricle dimension (end-diastole: 95 mm) and reduced EF (0.11). The coronary angiogram was normal. A myocardial biopsy revealed that over 50% of cardiomyocytes were replaced by fibrosis (figure 2). An implantable cardioverter-defibrillator was implanted. Due to the typical facial appearance and thickening of hands, he was referred to the endocrinology department. The plasma level of GH was 8.2 ng/ml and IGF-1 was 504 ng/ml. MRI demonstrated a macroadenoma in the pituitary. Thus, he was diagnosed as acromegaly.
Figure 1.
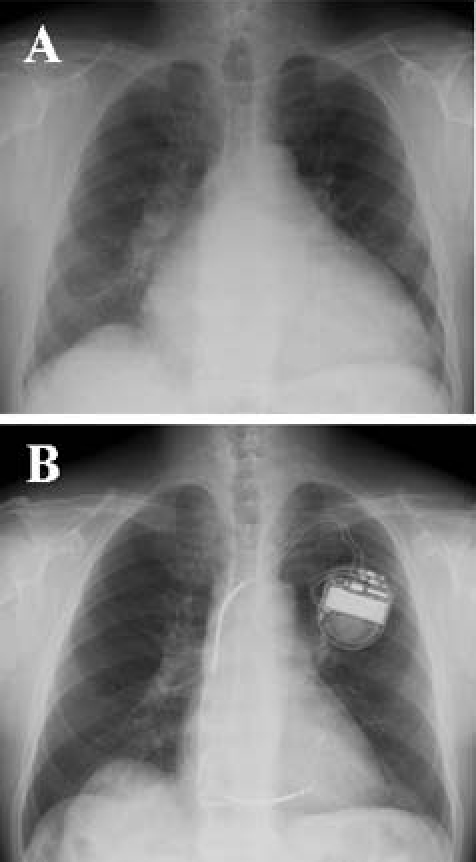
Chest x-ray before (A) and after (B) treatment.
Figure 2.
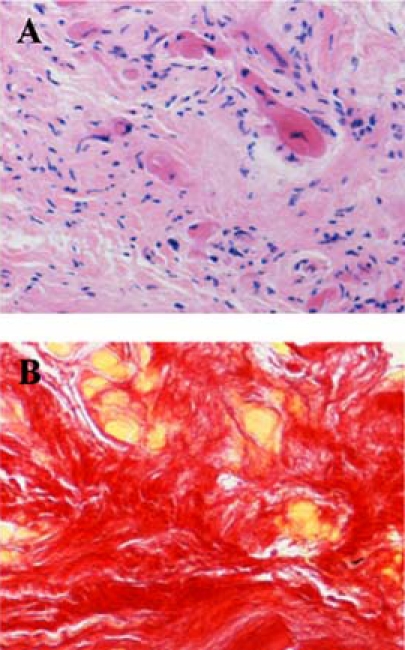
Histology of cardiac biopsy specimens before treatment. (A) H&E staining, (B) picrosirius-red stainings in which myocytes were stained in yellow while fibrosis was stained in red.
Video 1a.
ECGs before (A; May 2008) and after (B; October 2009) treatment.
Video 1b.
Treatment
He was treated with octreotide long-acting-release (LAR) for 8 months. Thereafter, he underwent trans-sphenoidal surgery in January 2009 (figure 3).
Figure 3.
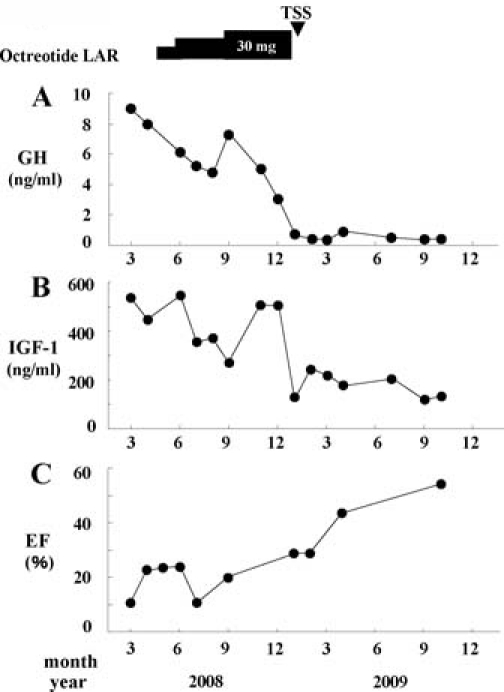
Time course changes in blood levels of growth hormone (GH) (A) and insulin-like growth factor-1 (IGF-1) (B) as well as ejection fraction (EF) (C). LAR, long-acting-release; TSS, trans-sphenoidal surgery.
Outcome and follow-up
The plasma levels of GH and IGF-1 were decreased with octreotide LAR, but basal plasma levels of GH were always more than 4 ng/ml and plasma IGF-1 levels were above the appropriate age range (figure 3). After surgery, both plasma levels of GH and IGF-1 further decreased. Basal plasma levels of GH were mostly less than 0.5 ng/ml and IGF-1 levels were within normal ranges (figure 3). With the treatment of LAR, the cardiac function improved partially (cardiothoracic ratio=0.58; EF=0.20 in September 2008). After surgery, the cardiac function improved drastically (cardiothoracic ratio=0.47; EF=0.55 in April 2009; figures 1B and 2B). Frequency of ventricular arrhythmias decreased and pimobendan was tapered off.
Discussion
It is reported that heart failure due to acromegalic cardiomyopathy was improved after normalisation of plasma GH and IGF-1 levels in some cases,4–6 although there were also other cases in which the cardiac dysfunction was irreversible.7 In the former cases, the fibrosis of the cardiomyocytes was moderate.4 5 Furthermore, while myocardial damage such as infiltration of inflammatory cells disappeared after treatment, the interstitial fibrosis remained unchanged in both cases.4 5 Based on these data, it has been suggested that acromegalic cardiomyopathy with severe fibrosis has a poor prognosis. However, the criteria for the cure of acromegaly with medicine or surgery could differ among studies, and it is not clear whether plasma GH and IGF-1 levels were normalised completely in the cases in which cardiac dysfunction was not improved. Direct effects of GH and IGF-1 on cardiomyocytes as well as increases in circulating plasma volume have been implicated in the pathogenesis of the acromegalic cardiomyopathy.8 9 While it is shown that there were no differences in cardiomyocyte histology between acromegalic and idiopathic cardiomyopathy,10 the residual cardiomyocytes were reportedly reconstituted after normalisation of plasma GH and IGF-I levels in the case of acromegaly.4 5 Although we have not conducted a biopsy after treatment, drastic changes in cardiac function were probably due to the improvement in function of the residual cardiomyocytes in our case. It should be also emphasised that such drastic changes only occurred after surgery when plasma GH and IGF-I levels decreased to normal levels. Thus, it is also demonstrated that normalisation of plasma GH and IGF-1 levels was mandatory to improve acromegalic cardiomyopathy as has been suggested.11 Since effective treatment of acromegaly reportedly did not necessarily improve long-term survival,12 a careful follow-up of cardiac function is needed in our case.
Learning points.
-
▶
Acromegalic cardiomyopathy could be reversed even if severe fibrosis exists in the myocardium.
-
▶
The normalisation of plasma GH and IGF-1 levels was mandatory to improve cardiac function in acromegalic cardiomyopathy.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Orme SM, McNally RJ, Cartwright RA, et al. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab 1998;83:2730–4 [DOI] [PubMed] [Google Scholar]
- 2.López-Velasco R, Escobar-Morreale HF, Vega B, et al. Cardiac involvement in acromegaly: specific myocardiopathy or consequence of systemic hypertension? J Clin Endocrinol Metab 1997;82:1047–53 [DOI] [PubMed] [Google Scholar]
- 3.Colao A, Baldelli R, Marzullo P, et al. Systemic hypertension and impaired glucose tolerance are independently correlated to the severity of the acromegalic cardiomyopathy. J Clin Endocrinol Metab 2000;85:193–9 [DOI] [PubMed] [Google Scholar]
- 4.Legrand V, Beckers A, Pham VT, et al. Dramatic improvement of severe dilated cardiomyopathy in an acromegalic patient after treatment with octreotide and trans-sphenoidal surgery. Eur Heart J 1994;15:1286–9 [DOI] [PubMed] [Google Scholar]
- 5.Nishiki M, Murakami Y, Sohmiya M, et al. Histopathological improvement of acromegalic cardiomyopathy by intermittent subcutaneous infusion of octreotide. Endocr J 1997;44:655–60 [DOI] [PubMed] [Google Scholar]
- 6.Akaza I, Tsuchiya K, Akaza M, et al. Improvement of congestive heart failure after octreotide and transsphenoidal surgery in a patient with acromegaly. Intern Med 2009;48:697–700 [DOI] [PubMed] [Google Scholar]
- 7.Hayward RP, Emanuel RW, Nabarro JD. Acromegalic heart disease: influence of treatment of the acromegaly on the heart. Q J Med 1987;62:41–58 [PubMed] [Google Scholar]
- 8.Saccà L, Cittadini A, Fazio S. Growth hormone and the heart. Endocr Rev 1994;15:555–73 [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Hiroe M, Hirata Y, et al. Insulin-like growth factor-I induces hypertrophy with enhanced expression of muscle specific genes in cultured rat cardiomyocytes. Circulation 1993;87:1715–21 [DOI] [PubMed] [Google Scholar]
- 10.van den Heuvel PA, Elbers HR, Plokker HW, et al. Myocardial involvement in acromegaly. Int J Cardiol 1984;6:550–3 [DOI] [PubMed] [Google Scholar]
- 11.Colao A, Cuocolo A, Marzullo P, et al. Is the acromegalic cardiomyopathy reversible? Effect of 5-year normalization of growth hormone and insulin-like growth factor I levels on cardiac performance. J Clin Endocrinol Metab 2001;86:1551–7 [DOI] [PubMed] [Google Scholar]
- 12.Bihan H, Espinosa C, Valdes-Socin H, et al. Long-term outcome of patients with acromegaly and congestive heart failure. J Clin Endocrinol Metab 2004;89:5308–13 [DOI] [PubMed] [Google Scholar]


