Abstract
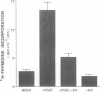
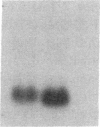
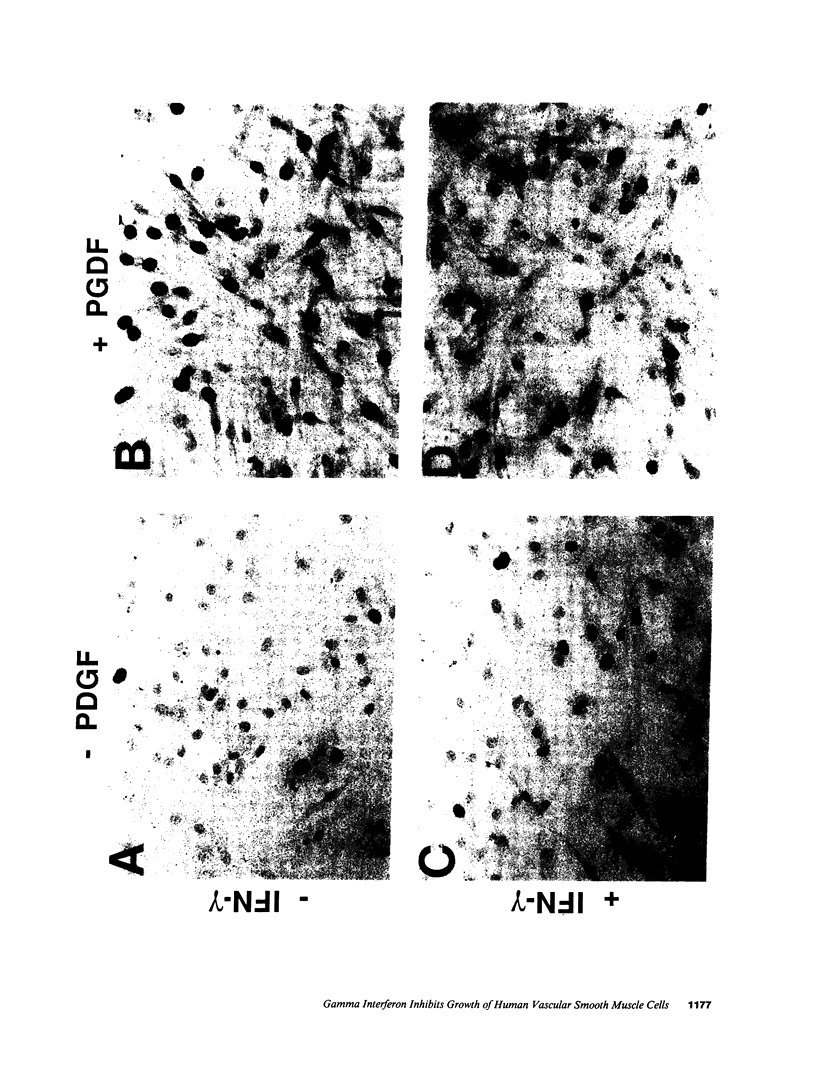
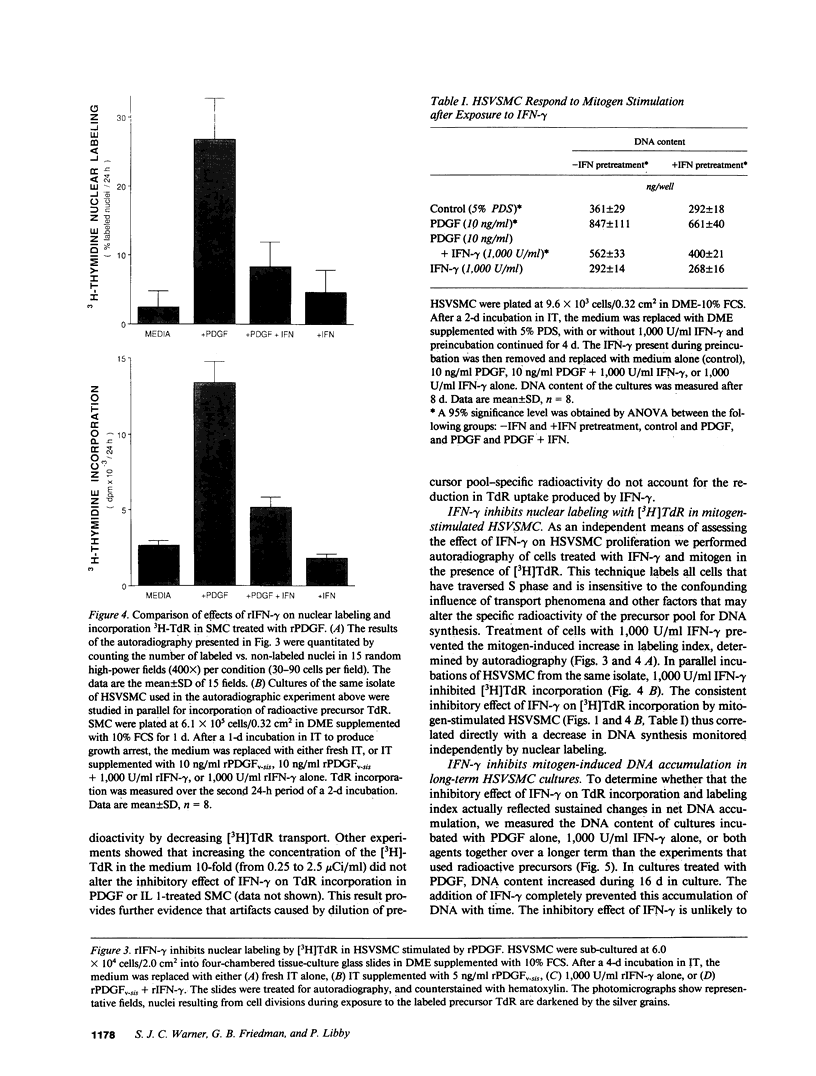
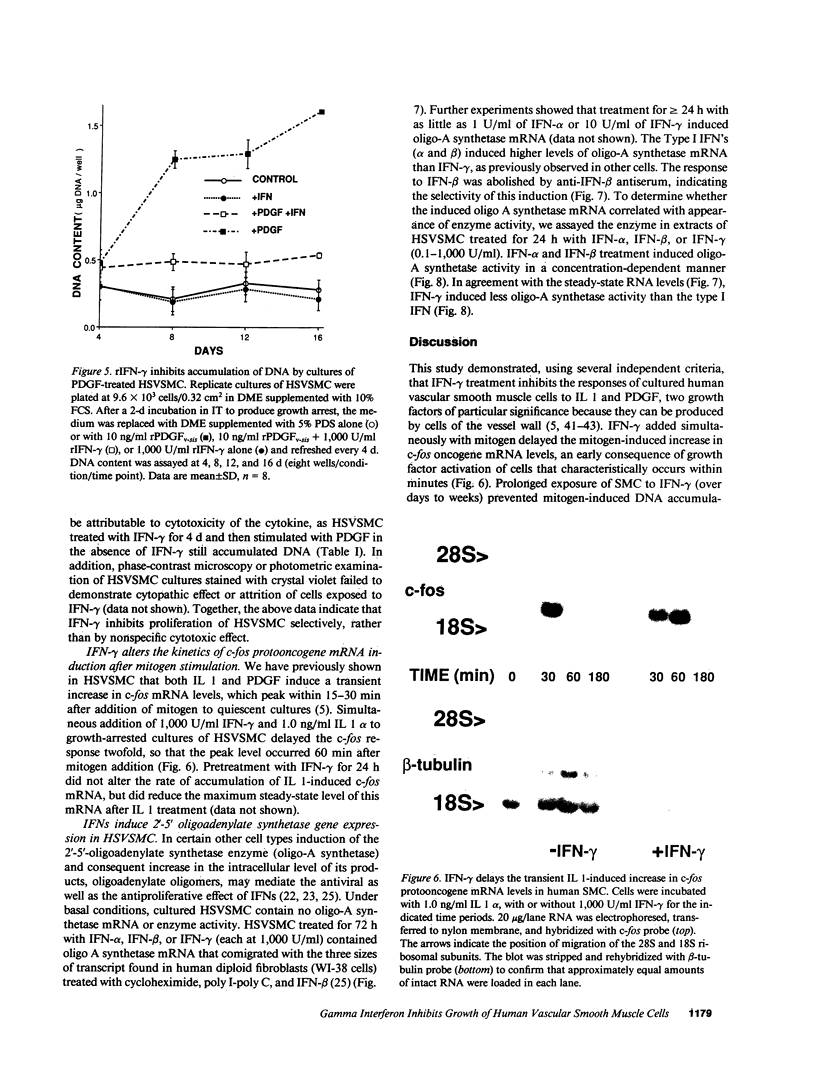
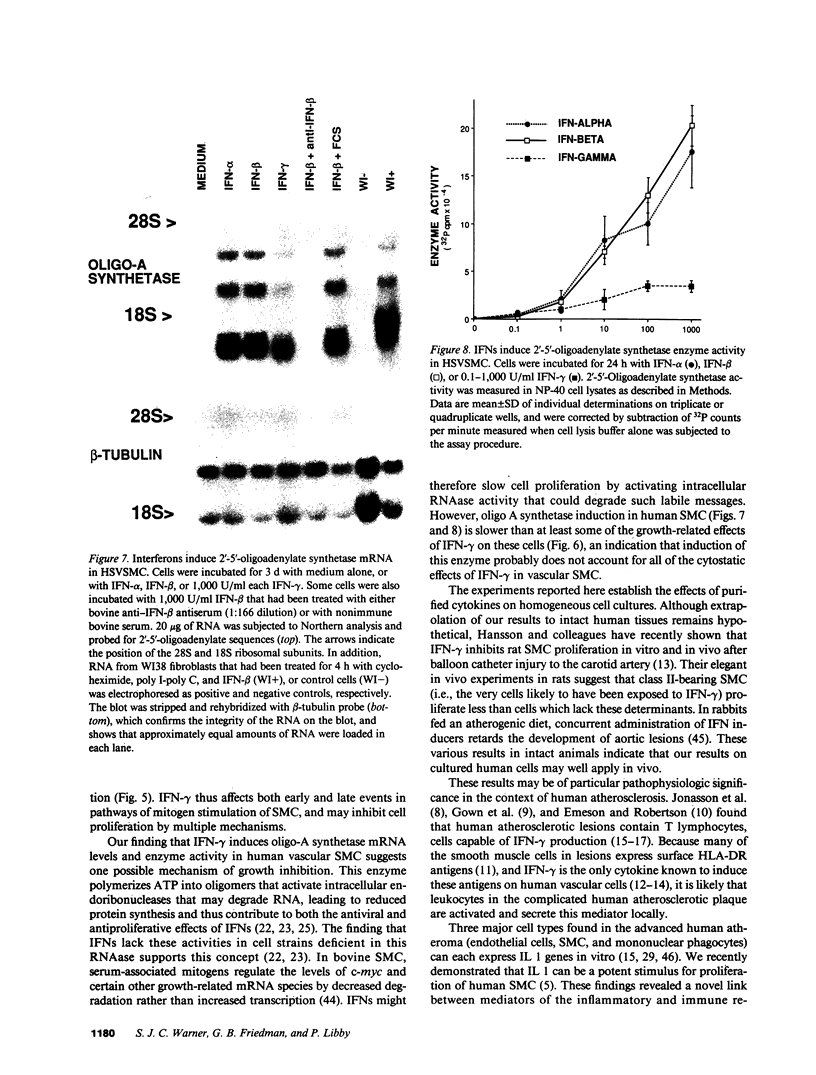
Proliferation of vascular smooth muscle cells (SMC) contributes to formation of the complicated human atherosclerotic plaque. These lesions also contain macrophages, known to secrete SMC mitogens, and T lymphocytes. Many of the SMC in the lesions express class II major histocompatibility antigens, an indication that activated T cells secrete immune IFN-gamma locally in the plaque. We therefore studied the effect of IFN-gamma on the proliferation of cultured SMC derived from adult human blood vessels. IFN-gamma (1,000 U/ml) reduced [3H]thymidine (TdR) incorporation into DNA by SMC stimulated with the well-defined mitogens IL 1 (from 15.3 +/- 0.7 to 6.2 +/- 0.7 dpm X 10(-3)/24 h) or platelet-derived growth factor (PDGF) (from 18.5 +/- 1.0 to 7.3 +/- 0.7 dpm X 10(-3)/24 h). Kinetic and nuclear labeling studies indicated that this effect of IFN-gamma was not due to altered thymidine transport or specific radioactivity of TdR in the cell. In longer term experiments (4-16 d) IFN-gamma prevented net DNA accumulation by SMC cultures stimulated by PDGF. IFN-gamma also delayed (from 30 to 60 min) the time to peak level of c-fos RNA in IL 1-treated SMC. It is unlikely that cytotoxicity caused these effects of IFN-gamma, as the inhibition of growth was reversible and we detected no cell death in SMC cultures exposed to this cytokine. Activation of 2'-5' oligoadenylate synthetase gene expression may mediate certain antiproliferative and antiviral effects of interferons. Both IFN-gamma and type I IFNs (IFN-alpha or IFN-beta) induced 2'-5' oligoadenylate synthetase mRNA and enzyme activity in SMC cultures, but with concentration dependence and time course that may not account for all of IFN-gamma's cytostatic effect on SMC. The accumulation of SMC in human atherosclerotic lesions is a long-term process that must involve altered balance between growth stimulatory and inhibitory factors. The cytostatic effect of IFN-gamma on human SMC demonstrated here may influence this balance during human atherogenesis, because T cells present in the complicated atherosclerotic plaque likely produce this cytokine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Stathakos D., Scher C. D. Isolation of a cationic polypeptide from human serum that stimulates proliferation of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2635–2639. doi: 10.1073/pnas.72.7.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benech P., Mory Y., Revel M., Chebath J. Structure of two forms of the interferon-induced (2'-5') oligo A synthetase of human cells based on cDNAs and gene sequences. EMBO J. 1985 Sep;4(9):2249–2256. doi: 10.1002/j.1460-2075.1985.tb03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dean M., Levine R. A., Ran W., Kindy M. S., Sonenshein G. E., Campisi J. Regulation of c-myc transcription and mRNA abundance by serum growth factors and cell contact. J Biol Chem. 1986 Jul 15;261(20):9161–9166. [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Einhorn S., Eldor A., Vlodavsky I., Fuks Z., Panet A. Production and characterization of interferon from endothelial cells. J Cell Physiol. 1985 Feb;122(2):200–204. doi: 10.1002/jcp.1041220206. [DOI] [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Growth factors, oncogenes, and interferons. J Exp Pathol. 1986 Summer;2(4):223–228. [PubMed] [Google Scholar]
- Friesel R., Komoriya A., Maciag T. Inhibition of endothelial cell proliferation by gamma-interferon. J Cell Biol. 1987 Mar;104(3):689–696. doi: 10.1083/jcb.104.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Claesson-Welsh L. Class II MHC antigen expression in the atherosclerotic plaque: smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol. 1986 May;64(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Clowes M. M., Clowes A. W. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988 Oct;63(4):712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3722–3726. doi: 10.1073/pnas.76.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyns A. D., Eldor A., Vlodavsky I., Kaiser N., Fridman R., Panet A. The antiproliferative effect of interferon and the mitogenic activity of growth factors are independent cell cycle events. Studies with vascular smooth muscle cells and endothelial cells. Exp Cell Res. 1985 Dec;161(2):297–306. doi: 10.1016/0014-4827(85)90087-4. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Hemken C., Brady M., Monick M. Immune interferon is a growth factor for human lung fibroblasts. Am Rev Respir Dis. 1986 Nov;134(5):1025–1028. doi: 10.1164/arrd.1986.134.5.1025. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Lapidot Y., Rapoport S., Panet A., Revel M. Antimitogenic effects of interferon and (2'-5')-oligoadenylate in synchronized 3T3 fibroblasts. FEBS Lett. 1981 Nov 16;134(2):212–216. doi: 10.1016/0014-5793(81)80604-7. [DOI] [PubMed] [Google Scholar]
- Kluger M. J., Singer R., Eiger S. M. Polymyxin B use does not ensure endotoxin-free solution. J Immunol Methods. 1985 Oct 24;83(1):201–207. doi: 10.1016/0022-1759(85)90073-0. [DOI] [PubMed] [Google Scholar]
- Kuo P. T., Wilson A. C., Goldstein R. C., Schaub R. G. Suppression of experimental atherosclerosis in rabbits by interferon-inducing agents. J Am Coll Cardiol. 1984 Jan;3(1):129–134. doi: 10.1016/s0735-1097(84)80438-6. [DOI] [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983 May;115(2):217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Salomon R. N., Birinyi L. K. Production of platelet-derived growth factor-like mitogen by smooth-muscle cells from human atheroma. N Engl J Med. 1988 Jun 9;318(23):1493–1498. doi: 10.1056/NEJM198806093182303. [DOI] [PubMed] [Google Scholar]
- Lin S. L., Kikuchi T., Pledger W. J., Tamm I. Interferon inhibits the establishment of competence in Go/S-phase transition. Science. 1986 Jul 18;233(4761):356–359. doi: 10.1126/science.3726533. [DOI] [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Tamm I. Interferon inhibition of thymidine incorporation into DNA through effects on thymidine transport and uptake. J Cell Physiol. 1984 Nov;121(2):431–436. doi: 10.1002/jcp.1041210223. [DOI] [PubMed] [Google Scholar]
- Revel M., Kimchi A., Friedman M., Wolf D., Merlin G., Panet A., Rapoport S., Lapidot Y. Cell-regulatory functions of interferon induced enzymes: antimitogenic effect of (2'-5')oligo-A, growth-related variations in (2'-5')oligo-A synthetase, and isolation of its mRNA. Tex Rep Biol Med. 1981;41:452–462. [PubMed] [Google Scholar]
- Revel M., Wallach D., Merlin G., Schattner A., Schmidt A., Wolf D., Shulman L., Kimchi A. Interferon-induced enzymes: microassays and their applications: purification and assay of (2'-5')-oligoadenylate synthetase and assay of 2'-phosphodiesterase. Methods Enzymol. 1981;79(Pt B):149–161. doi: 10.1016/s0076-6879(81)79024-4. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Nist C., Kariya B., Rivest M. J., Raines E., Callis J. Physiological quiescence in plasma-derived serum: influence of platelet-derived growth factor on cell growth in culture. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):497–508. doi: 10.1002/jcp.1040970325. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Walker L. N., Bowen-Pope D. F., Ross R., Reidy M. A. Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7311–7315. doi: 10.1073/pnas.83.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987 May 1;165(5):1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]
- West D. C., Sattar A., Kumar S. A simplified in situ solubilization procedure for the determination of DNA and cell number in tissue cultured mammalian cells. Anal Biochem. 1985 Jun;147(2):289–295. doi: 10.1016/0003-2697(85)90274-x. [DOI] [PubMed] [Google Scholar]