Abstract
The classical G-protein-coupled receptors (GPCRs) are characterized by their ability to interact with heterotrimeric G proteins upon activation and by structural features such as seven transmembrane spanning domains. Frizzleds (Fzs) are comparable seven transmembrane receptors (7 TMRs) that are activated via Wnts and play a critical role in embryogenesis, tissue hemostasis and oncogenicity. It remains controversial, however, whether they may be considered GPCRs. Hence, the ten members of Fzs constitute a distinct atypical family of seven-transmembrane receptors. Canonical Wnt/β-catenin signaling leads to the core process of β-catenin stabilization and, ultimately, to the translocation of β-catenin to the nucleus where it acts as a co-transcription factor and induces Wnt target gene transcription. we have documented that activation by proteinase-activated receptor1 (PAR1), a classical 7TMR, recruits dishevelled (DvL), an upstream Wnt signaling protein, to mediate β-catenin stabilization. DvL is selectively bound to activated Gα13 subunit, coupled to PAR1 following activation. Formation of the PAR1-induced DvL-Gα13 axis is carried out independently of Wnt, Fz and the co-receptor LRP5/6 (low density lipoprotein—related protein 5/6) since neither siRNA-LRP5/6 co-receptors nor the presence of SFRPs; secreted Fz receptor proteins (Wnt antagonists) affect PAR1-induced β-catenin stabilization. Similarly, PAR1 induced placenta cytotrophoblast physiological invasion process was not affected by inhibiting Wnt, but was abrogated by siRNA-DvL. we propose that DvL serves as a central mediator protein that links classical GPCRs to β-catenin stabilization in both pathological (tumor) and physiological (placenta) invasion processes.
Key words: DVL, β-catenin, GPCR, PAR1, Gα13
The hallmark feature of classical G-protein-coupled receptors (GPCRs) is their ability to interact with heterotrimeric G proteins upon activation by agonists, with subsequent receptor-β-arrestin association leading to internalization and desensitization. These properties are present in addition to structural features such as seven transmembrane spanning domains. Frizzleds (Fzs) are comparable seven transmembrane receptors that are activated via Wnts and play a critical role in embryogenesis, tissue hemostasis and oncogenicity.1,2 It remains controversial, however, whether they may be considered GPCRs, and whether the coupling of Fzs to G-proteins for downstream signaling is obligatory. Hence, the ten members of Fzs constitute a distinct atypical family of seventransmembrane receptors (7TMRs).3 Canonical Wnt/β-catenin signaling leads to the core process of β-catenin stabilization and, ultimately, to the translocation of β-catenin to the nucleus where it acts as a co-transcription factor and induces Wnt target genes. Regulation of β-catenin stability by GPCRs other than Fzs has been previously described.4–7 Yet, all these studies describe traditional G-protein-mediated signaling pathways rather than the involvement of members of classical wnt signaling that eventually lead to β-catenin stabilization.8,9 We have found that activation by proteinase-activated receptor1 (PAR1), a classical 7TMR, recruits dishevelled (DVL), an upstream Wnt signaling protein, to mediate the stability of β-catenin. DVL is selectively bound to activated Gα13 subunit, coupled to PAR1 following receptor activation. Thus, our studies identify DVL as a mediator protein that links PAR1 to β-catenin stabilization. PAR1-induced DVL-Gα13 interaction occurs independently of Wnt, Fz and the co-receptor LRP5/6 (low density lipoprotein—related protein 5/6) since neither siRNA-LRP5/6 (low density lipoprotein-related protein 5/6) co-receptors nor the presence of SFRPs; secreted Fz receptor proteins (Wnt antagonists) affects PAR1-induced β-catenin stabilization.9 Interestingly, Romero et al. recently showed that DVL is directly bound to another classical GPCR, parathyroid hormone receptor (PTH1R), which plays an important role in β-catenin stabilization. This accumulation of β-catenin was also found to be independent of Wnt signaling.10 A pattern thus emerges in which GPCRs like PAR1 or PTH1R recruit DVL, ultimately leading to β-catenin stabilization via traditional GPCRs. Our studies suggest a novel mechanism of DVL binding to activated Gα13 (Gα13-DVL) for β-catenin stabilization initiated via PAR1. We discuss herein the consequences of DVL association with factors shared by both the classical and the distinct Fz 7TMR, underscoring the significance of DVL as a critical junction protein positioned between these routes. The implications of the proposed axis of Gα13-DVL are also discussed.
Classical 7TM GPCRs and β-catenin Stabilization
Many of the classical GPCRs shown previously to lead to β-catenin stabilization act through traditional signaling pathways triggered by 7TM receptors, such as the activation of protein kinase A induced by PGE2 (prostaglandin) binding to EP2 receptor.7 Another example is the lysophosphatidic acid (LPA) and its receptors, LPA1, LPA2 or LPA3, that act by a mechanism downstream of PKC,6 which eventually leads to the inhibition of glycogen synthase kinase 3β (GSK3β) (via serine phosphorylation for its inactivation). Further examples include α-adrenergic and endothelin-1 receptors in cardiomyocytes that, once activated, recruit Akt, subsequently leading to the inactivation of GSK3β. This group also includes the EP2 (mentioned already above) and EP4 prostanoid receptors.11 The involvement of the serine/threonine kinase Akt [protein kinase B (PKB)] in GPCR signaling is well established. In fact, Akt is a major effector of the PI3K pathway, which is activated by a wide spectrum of polypeptide growth factors.12–17 Interestingly, it has been shown that Wnt-activated DVL also increases the activity of Akt which, in the presence of DVL, instigates the disruption of the axin-GSK3β complex and the inactivation of GSK3β. Inactivated GSK3β is no longer capable of tag-phosphorylating β-catenin for degradation via the E3 ligase of the ubiquitin system.18
DVL is emerging as a junction protein that is also capable of binding β-arrestins (e.g., β-arrestin1 and 2) for signaling. β-arrestins have long been recognized for their role in the internalization and desensitization of traditional GPCRs. Recently, β-arrestins have been identified as essential components of Wnt/β-catenin signaling capable of binding to DVL at the N-terminal PDZ site. This binding domain comprises the casein kinase 1 (CK1δ/ε) phosphorylation area. It is suggested that binding of β-arrestin to activated DVL directs efficient association with axin, consequently leading to DVL-linked disruption of the β-catenin “degradation complex” (e.g., axin-APC-GSK3β) and hence, to β-catenin accumulation.19–21 We have also demonstrated that β-arrestin-2 specifically binds DVL following PAR1 activation, and that activation of PAR1 leads to disruption of the “degradation complex”, with the result that axin can no longer form a complex with GSK-3β.9 It is plausible that the DVL-β-arrestin-2 complex formed by activated PAR1 subsequently associates with axin downstream, hence contributing to the disruption of the complex that sends β-catenin for degradation. DVL is thus emerging as a scaffold linker between traditional GPCR and key components in the β-catenin stabilization pathway.
β-catenin Stabilization in a Physiological InvasionProcess: The Placenta Trophoblasts
Placenta development is a tightly orchestrated process whereby anchoring to the uterus decidua is carried out by invasion of cells termed extravillous trophoblasts (EVT). These cells are a subpopulation of placenta cytotrophoblasts and are capable of forming anchoring villi that invade and reach the uterine wall, allowing direct contact with the maternal blood. The molecular machinery that governs trophoblast anchorage to maternal tissues remains largely unknown. It is however, well recognized that the molecular basis of trophoblast invasion shares many features with the process of tumor cell invasion.22,23 We have previously demonstrated that PAR1 is overexpressed in a spatiotemporally restricted manner during early gestation of placenta cytotrophoblasts and is shut off immediately thereafter when the need to invade is over.24,25 Therefore, in contrast to the continuous overexpression of PAR1 seen in malignant epithelia, PAR1 is strictly controlled in the physiological invasion process of placenta cytotrophoblasts. We have shown that activation of PAR1 markedly induces EVT invasion as well as β-catenin stabilization and its nuclear localization, demonstrated in an EVT-explant system.26 In-parallel, studies by Sonderegger et al. have demonstrated that trophopblast invasion is regulated via Wnt3A, inducing the canonical Wnt signaling pathway.27 This was manifested via the application of Wnt3A to either a trophoblastic cell line SGHPL-5 or primary extravillous trophoblasts leading to increased phosphorylation of AKT and downstream GSK3β inactivation. Moreover, luciferase activity of a canonical Wnt/TCF reporter, as well as cell migration are also elicited. Our preliminary evaluations in placenta EVT explants have indicated that PAR1-mediated functions (e.g., β-catenin stabilization and trophoblast villi invasion) are effectively inhibited in the presence of siRNA-DVL. While Wnt3A-induced β-catenin stabilization and EVT invasion are attenuated after LRP5/6 silencing (siRNA-LRP5/6), PAR1-induced β-catenin stabilization and invasion are not affected. Hence, we propose that the activation of PAR1 initiates a chain of events that ultimately lead to β-catenin stabilization and trophoblast invasion, independent of Wnt signaling. These functions of PAR1-induced placenta-EVT are abrogated in the presence of siRNA-DVL. We therefore regard DVL as a central upstream junction protein that connects PAR1 to trophoblast invasion and β-catenin stabilization (unpublished observations; Grisaru-Granovsky S and Turm H). It is proposed that while both pathways (Wnt and PAR1) act to induce β-catenin stabilization, they co-exist in a mutually exclusive manner and induce invasion in the appropriate context.
Essential Role for Gα13 and PAR1in β-catenin Stabilization
G-proteins comprise a signal transducing system that is regulated through discrete transmembrane receptors upstream and impinges on downstream effectors.28 At present, Gα subunits are classified into four distinct sub-families.29 Gα12 and its sister protein Gα13 are the most recent identified sub class, sharing 66% amino acid homology. The transformation capabilities of the family members have been demonstrated via focus-forming activity in soft agar and oncogenic properties in 3T3 NIH cells.30,31 In fact, Gα12 was termed gep oncogene since it was shown to induce neoplastic transformation of fibroblasts, as well as to stimulate mitogenic pathways in different cell types.32–35 It has been recently demonstrated that neoplastic transformation by the gep oncogene involves activation of signal transducer and activator of transcription 3 (STAT3) via PDGFR.36 A third member of the family, the Cta- Concertina (cta) gene product of Drosophila, shares close homology with Gα12 and Gα13 (53–55%) and it's absence results in the disruption of the ventral furrow during early Drosophila development.37 It appears that while Gα12 and Gα13 are part of the same family, they act in a different manner. While knock-out (KO) of the Gα13 gene causes lethality in mouse embryos at mid-gestation, in contrast, Gα12-/- mice appear viable and grow normally.38 An elegant study by the group of Coughlin SR39 showed that transgenic expression of Gα13 into the endothelium using a specific endothelial Tie promoter, rescued embryonic lethality associated with Gα13 or PAR1 gene deletion. This suggests that loss of Gα13 signaling in endothelial cells may account for the embryonic phenotype associated with PAR1 deficiency. It should be noted, however, that the rescue affected only the endothelial phenotype of Gα13-deficient mice, but did not rescue Gα13-/- mice in general, indicating that Gα13 function in cell types other than endothelial cells is important for embryonic development. In addition, unpublished observations from the group of Coughlin indicated that Gα13-/- embryos carrying the endothelial transgene are similar in phenotype to that resulting from a Wnt1-Cre-mediated deletion of Gα13. These findings indicate that Gα13 may play an as-yet undetermined role in developmental processes. Our studies on PAR1-activated Gα13 and the recruitment of DVL may be expanded in the future to demonstrate a fundamental role for the Gα13-DVL axis in developmental processes. In this respect, it has recently been shown that activated Gα13 uniquely binds and activates integrin β3 to mediate “outside-in” signaling.40 This endows Gα13 with a unique property in mediating cell-extracellular matrix interaction during adhesion. It has also been shown that activation of Wnt signaling during embryonic development is important, demonstrating that DVL associates with the actin fibers and focal adhesion plaques in mesenchymal cells, thus impinging on the rearrangement of the cytoskeleton and cell adhesion during embryonic mouse kidney development.41 We suggest that, in addition to playing a role in tumor biology, PAR1 also has a function in development. The fact that the PAR1 gene sequence is highly conserved during evolution, sharing 55–60% homology (NCBI taxonomy blast) with both zebra fish and/or Xenopus, may indicate the important putative role of the gene in development. It seems that while both Gα12 and Gα13 play a role in tumor biology, a selective part is assigned to the activated Gα13-DVL axis in β-catenin stabilization in the context of development.
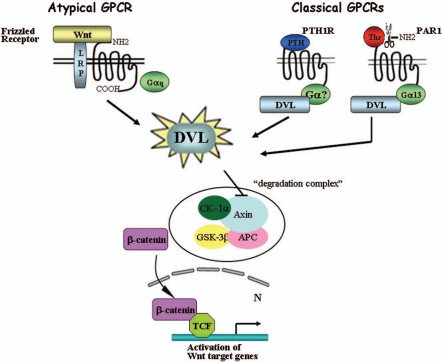
Figure 1.
DVL is a mediator of classical GPCRs for β-catenin stabilization. Wnt-induced β-catenin stabilization is mediated via frizzled receptors that are considered as atypical GPCRs. Classical GPCRs such as parathyroid hormone receptor (PTH1R) and proteinase-activated—receptor1 (PAR1) lead to β-catenin increased levels by recruiting DVL. Thus, DVL serves as a junction mediator routing the classical GPCR for β-catenin stabilization.
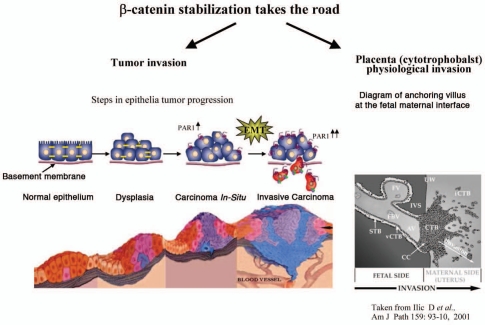
Figure 2.
PAR1 induced β-catenin stabilization in tumor- and physiological placenta- invasion processes. PAR1 induces β-catenin stabilization in either pathological (tumor) or physiological (placenta invasion) processes. Both PAR1 induced pathways are independent of Wnt signaling.
Acknowledgements
The research was supported by funds from the Israel Scientific Foundation (ISF), the Israel Cancer Research Fund (ICRF) and the Fritz Thyssen Foundation (to R.B.).
Footnotes
Previously published online www.landesbioscience.com/journals/cib/article/12979
References
- 1.Malaterre J, Ramsay RG, Mantamadiotis T. Wnt-Frizzled signalling and the many paths to neural development and adult brain homeostasis. Front Biosci. 2007;12:4–7. doi: 10.2741/2077. [DOI] [PubMed] [Google Scholar]
- 2.Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, et al. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- 3.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Shevtsov SP, Haq S, Force T. Activation of beta-catenin signaling pathways by classical G-protein-coupled receptors: mechanisms and consequences in cycling and non-cycling cells. Cell Cycle. 2006;5:2295–2300. doi: 10.4161/cc.5.20.3357. [DOI] [PubMed] [Google Scholar]
- 5.Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, et al. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci USA. 2003;100:4610–4615. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M, Zhong WW, Srivastava N, Slavin A, Yang J, Hoey T, et al. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the {beta}-catenin pathway. Proc Natl Acad Sci USA. 2005;102:6027–6032. doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 8.Force T, Woulfe K, Koch WJ, Kerkela R. Molecular scaffolds regulate bidirectional crosstalk between Wnt and classical seven-transmembrane-domain receptor signaling pathways. Sci STKE. 2007;2007:41. doi: 10.1126/stke.3972007pe41. [DOI] [PubMed] [Google Scholar]
- 9.Turm H, Maoz M, Katz V, Yin YJ, Offermanns S, Bar-Shavit R. Protease-activated receptor-1 (PAR1) acts via a novel Galpha13-dishevelled axis to stabilize beta-catenin levels. J Biol Chem. 2010;285:15137–15148. doi: 10.1074/jbc.M109.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero G, Sneddon WB, Yang Y, Wheeler D, Blair HC, Friedman PA. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. J Biol Chem. 2010;285:14756–14763. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- 12.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 13.Stambolic V, Mak TW, Woodgett JR. Modulation of cellular apoptotic potential: contributions to oncogenesis. Oncogene. 1999;18:6094–6103. doi: 10.1038/sj.onc.1203126. [DOI] [PubMed] [Google Scholar]
- 14.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 15.Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, et al. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci USA. 2008;105:8292–8297. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin YJ, Salah Z, Maoz M, Ram SC, Ochayon S, Neufeld G, et al. Oncogenic transformation induces tumor angiogenesis: a role for PAR1 activation. FASEB J. 2003;17:163–174. doi: 10.1096/fj.02-0316com. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, DeFea KA. Protease-activated receptor-2 simultaneously directs beta-arrestin-1-dependent inhibition and Galphaq-dependent activation of phosphatidylinositol 3-kinase. Biochemistry. 2006;45:9374–9385. doi: 10.1021/bi0602617. [DOI] [PubMed] [Google Scholar]
- 18.Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 19.Bryja V, Gradl D, Schambony A, Arenas E, Schulte G. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:6690–6695. doi: 10.1073/pnas.0611356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryja V, Schulte G, Arenas E. Wnt-3a utilizes a novel low dose and rapid pathway that does not require casein kinase 1-mediated phosphorylation of Dvl to activate beta-catenin. Cell Signal. 2007;19:610–616. doi: 10.1016/j.cellsig.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Bryja V, Schulte G, Rawal N, Grahn A, Arenas E. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci. 2007;120:586–595. doi: 10.1242/jcs.03368. [DOI] [PubMed] [Google Scholar]
- 22.Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, et al. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- 23.Aplin JD, Straszewski-Chavez SL, Kalionis B, Dunk C, Morrish D, Forbes K, et al. Trophoblast differentiation: progenitor cells, fusion and migration—a workshop report. Placenta. 2006;27:141–143. doi: 10.1016/j.placenta.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 25.Even-Ram SC, Grisaru-Granovsky S, Pruss D, Maoz M, Salah Z, Yong-Jun Y, et al. The pattern of expression of protease-activated receptors (PARs) during early trophoblast development. J Pathol. 2003;200:47–52. doi: 10.1002/path.1338. [DOI] [PubMed] [Google Scholar]
- 26.Grisaru-Granovsky S, Maoz M, Barzilay O, Yin YJ, Prus D, Bar-Shavit R. Protease activated receptor-1, PAR1, promotes placenta trophoblast invasion and beta-catenin stabilization. J Cell Physiol. 2009;218:512–521. doi: 10.1002/jcp.21625. [DOI] [PubMed] [Google Scholar]
- 27.Sonderegger S, Haslinger P, Sabri A, Leisser C, Otten JV, Fiala C, et al. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 151:211–220. doi: 10.1210/en.2009-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhanasekaran N, Dermott JM. Signaling by the G12 class of G proteins. Cell Signal. 1996;8:235–245. doi: 10.1016/0898-6568(96)00048-4. [DOI] [PubMed] [Google Scholar]
- 29.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 30.Chan AM, Fleming TP, McGovern ES, Chedid M, Miki T, Aaronson SA. Expression cDNA cloning of a transforming gene encoding the wild-type Galpha12 gene product. Mol Cell Biol. 1993;13:762–768. doi: 10.1128/mcb.13.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radhika V, Dhanasekaran N. Transforming G proteins. Oncogene. 2001;20:1607–1614. doi: 10.1038/sj.onc.1204274. [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Wu D, Simon MI. The transforming activity of activated Galpha12. FEBS Lett. 1993;330:319–322. doi: 10.1016/0014-5793(93)80896-3. [DOI] [PubMed] [Google Scholar]
- 33.Vara Prasad MV, Shore SK, Dhanasekaran N. Activated mutant of Galpha13 induces Egr-1, c-fos and transformation in NIH 3T3 cells. Oncogene. 1994;9:2425–2429. [PubMed] [Google Scholar]
- 34.Voyno-Yasenetskaya TA, Pace AM, Bourne HR. Mutant alpha subunits of G12 and G13 proteins induce neoplastic transformation of Rat-1 fibroblasts. Oncogene. 1994;9:2559–2565. [PubMed] [Google Scholar]
- 35.Xu N, Voyno-Yasenetskaya T, Gutkind JS. Potent transforming activity of the G13alpha subunit defines a novel family of oncogenes. Biochem Biophys Res Commun. 1994;201:603–609. doi: 10.1006/bbrc.1994.1744. [DOI] [PubMed] [Google Scholar]
- 36.Kumar RN, Shore SK, Dhanasekaran N. Neoplastic transformation by the gep oncogene, Galpha12, involves signaling by STAT3. Oncogene. 2006;25:899–906. doi: 10.1038/sj.onc.1209132. [DOI] [PubMed] [Google Scholar]
- 37.Parks GD, Lamb RA. Topology of eukaryotic type II membrane proteins: importance of N-terminal positively charged residues flanking the hydrophobic domain. Cell. 1991;64:777–787. doi: 10.1016/0092-8674(91)90507-u. [DOI] [PubMed] [Google Scholar]
- 38.Offermanns S, Mancino V, Revel JP, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science. 1997;275:533–536. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- 39.Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, et al. Essential role for Galpha13 in endothelial cells during embryonic development. Proc Natl Acad Sci USA. 2005;102:8281–8286. doi: 10.1073/pnas.0503326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong H, Shen B, Flevaris P, Chow C, Lam SC, Voyno-Yasenetskaya TA, et al. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science. 2010;327:340–343. doi: 10.1126/science.1174779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres MA, Nelson WJ. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J Cell Biol. 2000;149:1433–1442. doi: 10.1083/jcb.149.7.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]