Abstract
Mechanisms for intra-Golgi transport remain a hotly debated topic. Recently, we published data illuminating a new aspect involved in intra-Golgi transport, namely a release of free cytosolic Ca2+ ([Ca2+]cyt) from the lumen of Golgi cisternae that is fundamental for the secretion and the progression of newly synthesized proteins through the Golgi apparatus (GA). This increase in [Ca2+]cyt during the late stage of synchronous intra-Golgi transport stimulates the fusion of membranes containing cargo proteins and Golgi cisternae, allowing the progression of proteins through the GA. Subsequent restoration of the basal [Ca2+]cyt is also important for the delivery of cargo to the proper final destination. Additionally, the secretory pathway Ca2+-ATPase Ca2+ pump (SPCA1) plays an essential role at this stage. The fine regulation of membrane fusion is also important for the formation and the maintenance of the Golgi ribbon and SPCA1, which regulates [Ca2+]cyt levels, can be considered a controller of trafficking. This evidence contradicts a model of intra-Golgi transport in which permanent membrane continuity allows cargo diffusion and progression.
Key words: Ca2+, calcium, calcium pump, intra-Golgi trafficking, Golgi apparatus, SNARE, SPCA1
The plasma membrane (PM) and the membranes of many intracellular organelles separate the cytosol from environments with different free Ca2+ concentrations ([Ca2+]) and electrical potentials.1 The cytosolic [Ca2+] ([Ca2+]cyt) is around 20,000-fold lower than in the mammalian extracellular fluids, and varies between 50–100 nM, whereas in the external fluids the [Ca2+] is about 2 mM. In addition, the lumens of several cellular organelles, like the endoplasmic reticulum (ER) and the Golgi apparatus (GA), store high amount of free Ca2+, in the order of 1.0 mM and 0.3–0.4 mM, respectively,2 comparable with those in the external fluids. The [Ca2+] in all these compartments is finely regulated and the correct Ca2+ homeostasis is important for the majority of intracellular pathways, including the protein trafficking and secretion.
Membrane fusion is considered to be essential for intracellular transport. Fusion between membranes within the secretory and endocytic pathways is mediated by SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins.3,4 The SNAREs cannot induce rapid fusion of membranes, but proper level of [Ca2+]cyt is necessary.5 Recently, we reported that a transient increase in [Ca2+]cyt during the late phase of intra-Golgi transport was simultaneous with a decreases in Ca2+ stored in the lumen of the Golgi cisternae ([Ca2+]GA) and maximal [Ca2+]GA restoration ability.6 [Ca2+]GA started to decrease 7 min after the restoration of intra-Golgi transport.6 However, we do not exclude the ER as a possible source of the increased [Ca2+]cyt, mainly from the trans-ER, strictly associated with the GA.7 The release of Ca2+ from the GA rapidly flows through opened Ca2+ channels (IP3R; inositol-1,4,5-trisphosphate receptor), creating a local ‘cloud’ with an increase of [Ca2+]cyt surrounding the GA and generating a local Ca2+ signal.8 The [Ca2+]cyt can also rapidly stimulate IP3Rs.1,9 Indeed, it is also possible that the ER has a role in restoring the [Ca2+]GA that is crucial for cargo progression,10 because there are direct interactions between the ER and the GA.7 Therefore, focusing attention on the role that the GA have in regulating the Ca2+ during intra-Golgi trafficking, we can assume that the decreased [Ca2+]GA could be due to (1) an impaired pumping of Ca2+ into the GA, (2) an increased opening of the IP3R channels or (3) a combination of both of these two. Of these, we believe the third possibility to be the most plausible.
In the late phase of intra-Golgi transport, 5–7 min after the release of the temperature block in our experimental asay,6,11 the increase of [Ca2+]cyt is critical for cargo progression. One critical step is the remodelling of the more rigid trans-GA membranes, which contain more cholesterol and glycolipids,12 operated by Ca2+-sensitive and locally recruited Golgi enzymes.13 At the same stage, after tethering of fusing compartments operated by SNAREs,3,4 the destabilization of membranes within the contact region needs increased [Ca2+]cyt14,15. An increase in [Ca2+]cyt leads to the recruitment of the cytosolic phospholipase A(2) α (hereafter cPLA2) on the membranes of the GA.14,16 cPLA2 is regulated by phosphorylation and Ca2+, whereby Ca2+ binds the C2 domain of the enzyme, inducing the translocation of the cPLA2 to the GA and vesicles.13,16 Indeed, the cPLA2-C2 domain itself moves to the GA during the passage of vesicular stomatitis virus G (VSVG) through the trans-Golgi.13,16 The enzymatic activity of cPLA2 or its binding to the membranes, could be responsible for the increased sensitivity to [Ca2+]cyt of the secretory pathway membranes that are tightly attached one to each other by SNAREs. An increase in [Ca2+]cyt is also required during the release of the trans-SNARE complex,5 which allows formation of the intercisternal connections.17 However, the [Ca2+]cyt increase observed during the late stage of intra-Golgi transport has to quickly return to its basal levels of 50–100 nM, allowing the exit of cargo from the GA.7,18–20 All of the cellular Ca2+-ATPase pumps, including the secretory-pathway Ca2+-transport ATPase pump type 1 (SPCA1), which probably have a main role in the GA, act to reduce this otherwise ‘toxic’ level of Ca2+ from the cytosol.
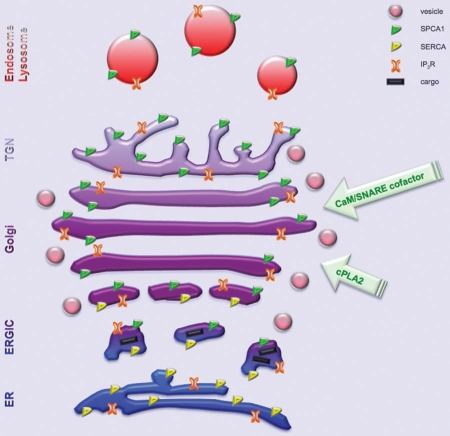
The mammalian Ca2+-ATPase pumps accumulate Ca2+ into different compartments against the Ca2+ gradient, using ATP as the source of energy. This group of pumps includes four isoforms of the plasma-membrane Ca2+-ATPases (PMCA),21 three sarco-(endo)plasmic reticulum Ca2+-ATPases (SERCA),22 and two located on the GA and the secretory granules Ca2+-ATPases (SPCAs).23 SERCAs are specifically located on the ER and the most cis-GA;19,24,25 SPCAs are located mostly on the GA and are excluded from the ER.26,27 The two SPCA isoforms, SPCA1 and SPCA2,28–30 are differently distributed; in mammals, SPCA1 is expressed in all tissues,28 whereas SPCA2 is expressed in only a limited set of tissues.30 We recently characterized the (sub) compartmental distribution of SPCA1 along the secretory pathway.27 SPCA1 is mostly excluded from the cores of the Golgi cisternae and is mainly distributed on the lateral rims of Golgi stacks, in the non-compact zones that interconnect different Golgi stacks and in the tubular cluster of the trans-Golgi network.27 This localization suggest SPCA1 has a role regulating the local [Ca2+]cyt in (sub)compartments surrounding the GA where membrane fusions occur during protein trafficking. A schematic of SPCA1 redistribution along the secretory pathway is provided in Figure 1.
Figure 1.
Schematic representation of the (sub)compartmental distribution of SPCA1 along the secretory pathway. SPCA1 is prevalent on the lateral rims of the Golgi cisternae, in the most cis- and trans-Golgi and on the endo/lysosomal compartment, but not on ER membranes. SERCA is typically present on ER membranes and overlapping with SPCA1 distribution only in the most cis-Golgi (ERGiC and cis-Golgi). The arrival of cargo to the GA induces the release of Ca2+ from the IP3R which induces a relocation on the GA of membrane remodeling enzymes (i.e., cPLA2) as well as calmodulin (CaM) and SNARE cofactors Ca2+-sensitive; this redistribution is crucial to orchestrate the SNARE fusion machinery which coordinates the fusion events necessary for the protein trafficking through the GA. Subsequently, the restoration of the basal [Ca2+]cyt requires the activation of the SPCA1, that transfers the increased [Ca2+]cyt into the GA lumen. The diluted colors from the darker ER to the clearest TGN indicates decreasing lumenal [Ca2+].
ATP2C1 encodes for the human SPCA1 protein, with four known mRNA splice variants.31 Gene silencing of ATP2C1 inhibits the correct organization of the GA32 and affects the subsequent development of the affected tissue.33 SPCA1 depletion inhibits the exit of VSVG from the GA and delays retrograde redistribution of the GA glycosylation enzymes into the ER caused by brefeldin A (BFA);27 however, the exit of these enzymes from the ER is not affected and the decreased sensitivity of SPCA1 depleted cells to BFA is not related to GA fragmentation.27 Additionally, SPCA1 depletion by RNA interference induces GA fragmentation; these fragments lack the cis-most and trans-most cisternae and remain within the perinuclear region.27 This suggests that correct SPCA1 functioning is crucial to intra-Golgi transport and for the maintenance of the Golgi ribbon.
So how is SPCA1 involved in maintaining the Golgi ribbon? The organization of the GA depends on many factors, such as the ability of cells to maintain their Golgi stacks within a restricted space, and the normal functioning of the golgins and the SNARE/Rab machineries.34 There is a need for correct positioning and normal functioning of the centrosome, the polymerization and growing of microtubules, the presence of ER-to-GA transport and regulated levels of [Ca2+] in both cytosol and secretory compartments.34 If any of these machineries are blocked, the stacks will not fuse with each other and the Golgi ribbon will not form.35 There is evidence that SPCA1 is crucial for the maintenance of the structure of the GA and that its inactivation affects intracellular transport. Inactivation of the Hansenula polymorpha PMR1 gene (the yeast homologue of ATP2C1) reduces cell viability and functionality of the secretory pathway.36 Recently, a very similar result to ours on GA fragmentation in SPCA1-interfered HeLa cells was reported.37 All these observations support our data, confirming that SPCA1 inactivation inhibits the correct organization and functioning of the GA.37
However, our observations contradict some other previous reports describing SPCA1-depleted cells in which GA appears not to be fragmented33,38 and protein trafficking is unaffected,33 although the exit of GPI-anchored proteins and VSVG from the ER was inhibited.38 Okunade et al.33 used several criteria to determine there were no effects to transport in SPCA1-depleted mice, including observations made of clathrin-coated buds, complexes of cell junctions, desmosomes and the development of the basement membrane. However, these parameters have low dependency on intracellular transport, because during the long life of epithelial cells they synthesize only very small amounts of the matrix proteins involved in the formation of the basement membrane, while the cell junctional complexes are relatively inert. Similarly, clathrin-coated buds are poorly involved in anterograde intracellular transport, but mostly in endocytosis. In addition, different cellular models and species were used: in our work we used human cells27 whereas Okanude et al. used a murine model.33 SPCA1 depletion seems also to have different effects in different cellular phenotypes; for instance, the lost of one allele of the ATP2C1 gene induces Hailey-Hailey disease in humans, demonstrating that SPCA1 has a crucial role in the regulation of [Ca2+] in keratinocytes, whereas in the murine model this appears not to be the case.39
A potential explanation of how SPCA1 affects the formation of the Golgi ribbon is that the generation and maintenance of the Golgi ribbon is a membrane fusion-dependent process that is a Ca2+-mediated.2 The transient increase in [Ca2+]cyt detected during the late phase of intra-Golgi transport was co-incident with a Ca2+ efflux from the GA itself.6 Simultaneously, there was a decline in the maximal [Ca2+]GA restoration capacity, which is operated by the Golgi Ca2+ pumps.6 This temporary redistribution of Ca2+ from the GA into the cytosol during cargo movement through the GA appears to have a crucial role in intra-Golgi transport and mainly in the late Ca2+-dependent phase of SNARE-regulated fusion between the different Golgi subcompartments.6
Similarly, these [Ca2+]cyt changes will also regulate SNARE cofactors that are involved in the release of the trans-SNARE complex that is formed between two distinct compartments during cargo progression through the GA.14,40–42 However, it appears that at steady state, even a low [Ca2+]cyt is sufficient to promote many constitutive membrane-fusion interactions along the secretory pathway.7,43–47 For instance, COPI vesicles can fuse with Golgi cisternae at steady-state48 without any apparent changes in [Ca2+]cyt. The presence of SPCA1 at the lateral rims of the GA and in the non compact zones of the Golgi ribbon, where membrane fusion occurs during cargo progression, supports the involvement of SPCA1 not only in GA Ca2+ homeostasis, but also triggering (highly localized) increases in [Ca2+]cyt. It has also recently been shown that in SPCA1 depleted cells, the basal [Ca2+]cyt is about 1.5-fold lower control cells.38 If we consider that this basal [Ca2+]cyt is about 50–100 nM, in these SPCA1 depleted cells this [Ca2+]cyt could not be sufficient to drive the fusion of COPI vesicles with membranes of the GA. This is consistent with our observation that SPCA1 depleted cells accumulate COPI vesicles as a result of decreased membrane fusion events. This results in an inhibition of Golgi ribbon formation.
It is now clear that a local release of Ca2+ from the lumen of the GA is necessary for intra-Golgi transport;7 this supports a model of intra-Golgi transport where the movement of protein through the GA requires temporary membrane fusion. In fact, if all of the Golgi cisternae are already interconnected, there is no requirement for SNARE-mediated membrane fusion modulated by the [Ca2+]cyt increase during the late phase of intra-Golgi transport.
Acknowledgements
We thank Dr. J. Mylne for reading the manuscript and Dr. G. Giacchetti for assistance with artwork. We also thank Telethon Italia (grant E.1105) and INTAS (grant 99-01732) for financial support.
Abbreviations
- [Ca2+]cyt
cytosolic Ca2+ concentration
- [Ca2+]GA
Golgi Ca2+ concentration
- [Ca2+]ER
sarco-(endo)plasmic Ca2+ concentration
- COPI
coat protein type I
- ER
endoplasmic reticulum
- GA
Golgi apparatus
- IP3R
inositol-1,4,5-trisphosphate receptor
- PM
plasma membrane
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SERCA
sarco(endo)plasmic reticulum Ca2+-ATPase
- SPCA
secretory pathway Ca2+-ATPase
Footnotes
Previously published online www.landesbioscience.com/journals/cib/article/13211
References
- 1.Taylor CW, Prole DL, Rahman T. Ca(2+) channels on the move. Biochemistry. 2009;48:18–20. doi: 10.1021/bi901739t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Micaroni M. The role of calcium in intracellular trafficking. Curr Mol Med. 2010;10:763–773. doi: 10.2174/156652410793384204. [DOI] [PubMed] [Google Scholar]
- 3.Jahn R, Scheller RH. SNAREs: engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 4.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeremic A, Kelly M, Cho JA, Cho SJ, Horber JK, Jena BP. Calcium drives fusion of SNARE-apposed bilayers. Cell Biol Int. 2004;28:19–31. doi: 10.1016/j.cellbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Micaroni M, Perinetti G, Di Giandomenico D, Bianchi K, Spaar A, Mironov AA. Synchronous intra-Golgi transport induces the release of Ca2+ from the Golgi apparatus. Exp Cell Res. 2010;316:2071–2086. doi: 10.1016/j.yexcr.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Curr Opin Cell Biol. 2005;17:135–140. doi: 10.1016/j.ceb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor CW, Laude AJ. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32:321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- 10.Porat A, Elazar Z. Regulation of intra-Golgi membrane transport by calcium. J Biol Chem. 2000;275:29233–29237. doi: 10.1074/jbc.M005316200. [DOI] [PubMed] [Google Scholar]
- 11.Mironov AA, Mironov AA, Jr, Beznoussenko GV, Trucco A, Lupetti P, Smith JD, et al. ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev Cell. 2003;5:583–594. doi: 10.1016/s1534-5807(03)00294-6. [DOI] [PubMed] [Google Scholar]
- 12.Nalefski EA, Sultzman LA, Martin DM, Kriz RW, Powler PS, Knopf JL, et al. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca2+-dependent lipid-binding domain and a Ca2+-independent catalytic domain. J Biol Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- 13.San Pietro E, Capestrano M, Polishchuk EV, Di Pentima A, Trucco A, Zizza P, et al. Gourp IV phospholipase A(2)alpha controls the formation of intercisternal continuities involved in intra-Golgi transport. PLoS Biol. 2009;7:1000194. doi: 10.1371/journal.pbio.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potoff JJ, Issa Z, Manke CW, Jr, Jena BP. Ca2+-dimethylphosphate complex formation: providing insight into Ca2+-mediated local dehydration and membrane fusion in cells. Cell Biol Int. 2008;32:361–366. doi: 10.1016/j.cellbi.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jena BP. Membrane fusion: role of SNARES and calcium. Protein Pept Lett. 2009;16:712–717. doi: 10.2174/092986609788681869. [DOI] [PubMed] [Google Scholar]
- 16.Evans JH, Leslie CC. The cytosolic phospholipase A2 catalytic domain modulates association and residence time at Golgi membranes. J Biol Chem. 2004;279:6005–6016. doi: 10.1074/jbc.M311246200. [DOI] [PubMed] [Google Scholar]
- 17.Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, Di Giandomenico D, et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–1081. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- 18.Brostrom CO, Brostrom MA. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol. 1990;52:577–590. doi: 10.1146/annurev.ph.52.030190.003045. [DOI] [PubMed] [Google Scholar]
- 19.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 20.Dolman NJ, Tepikin AV. Calcium gradients and the Golgi. Cell Calcium. 2006;40:505–512. doi: 10.1016/j.ceca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Brini M. Plasma membrane Ca2+-ATPase: from a housekeeping function to a versatile signaling role. Pfluegers Arch. 2009;457:657–664. doi: 10.1007/s00424-008-0505-6. [DOI] [PubMed] [Google Scholar]
- 22.Toyoshima C. Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch Biochem Biophys. 2008;476:3–11. doi: 10.1016/j.abb.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, et al. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004;1742:103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 25.Lin P, Le-Niculescu H, Hofmeister R, McCaffery JM, Jin M, Hennemann H, et al. The mammalian calcium-binding protein, nucleobindin (CALNUC), is a Golgi resident protein. J Cell Biol. 1998;141:1515–1527. doi: 10.1083/jcb.141.7.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behne MJ, Tu CL, Aronchik I, Epstein E, Bench G, Bikle DD, et al. Human keratinocyte ATP2C1 localises to the Golgi and controls Golgi Ca2+ stores. J Invest Dermatol. 2003;121:688–694. doi: 10.1046/j.1523-1747.2003.12528.x. [DOI] [PubMed] [Google Scholar]
- 27.Micaroni M, Perinetti G, Berrie CP, Mironov AA. The SPCA1 Ca2+ pump and intracellular membrane trafficking. Traffic. 2010;11:1315–1333. doi: 10.1111/j.1600-0854.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 28.Gunteski-Hamblin AM, Clarke DM, Shull GE. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 1992;31:7600–7608. doi: 10.1021/bi00148a023. [DOI] [PubMed] [Google Scholar]
- 29.Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+, Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J Biol Chem. 2005;280:11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- 30.Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, Raeymaekers L, et al. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi localized pump with high affinity for Ca2+ ions. J Biol Chem. 2005;280:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- 31.Fairclough RJ, Dode L, Vanoevelen J, Andersen JP, Missiaen L, Raeymaekers L, et al. Effect of Hailey-Hailey disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1) J Biol Chem. 2003;278:24721–24730. doi: 10.1074/jbc.M300509200. [DOI] [PubMed] [Google Scholar]
- 32.Van Baelen K, Vanoevelen J, Callewaert G, Parys JB, De Smedt H, Raeymaekers L, et al. The contribution of the SPCA1 Ca2+ pump to the Ca2+ accumulation in the Golgi apparatus of HeLa cells assessed via RNA-mediated interference. Biochem Biophys Res Commun. 2003;306:430–436. doi: 10.1016/s0006-291x(03)00977-x. [DOI] [PubMed] [Google Scholar]
- 33.Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, et al. Loss of the ATP2C1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis and mid-gestational death in homozygous embryos and squamous cell tumours in adult heterozygotes. J Biol Chem. 2007;282:26517–26527. doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
- 34.De Matteis MA, Mironov AA, Beznoussenko GV. The Golgi ribbon and the function of the golgins. In: Mironov AA, Pavelka M, editors. The Golgi Apparatus—State of the Art 110 Years after Camillo Golgi's Discovery. Wien-New York: Springer; 2008. pp. 223–246. [Google Scholar]
- 35.Mironov AA, Beznoussenko GV. Molecular mechanisms responsible for the formation of Golgi ribbon. Histol Histopathol. 2011 doi: 10.14670/HH-26.117. In press. [DOI] [PubMed] [Google Scholar]
- 36.Agaphonov MO, Plotnikova TA, Fokina AV, Romanova NV, Packeiser AN, Kang HA, et al. Inactivation of the Hansenula polymorpha PMR1 gene affects cell viability and functioning of the secretory pathway. FEMS Yeast Res. 2007;7:1145–1152. doi: 10.1111/j.1567-1364.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 37.Lissandron V, Podini P, Pizzo P, Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci USA. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sepulveda MR, Vanoevelen J, Raeymaekers L, Mata AM, Wuytack F. Silencing the SPCA1 (secretory pathway Ca2+-ATPase isoform 1) impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J Neurosci. 2009;29:12174–12182. doi: 10.1523/JNEUROSCI.2014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callewaert G, Parys JB, De Smedt H, Raeymakers L, Wuytack F, Vanoevelen J, et al. Similar Ca2+-signalling properties in keratinocytes and in COS-1 cells overexpressing the secretory-pathway Ca2+-ATPase SPCA1. Cell Calcium. 2003;34:157–162. doi: 10.1016/s0143-4160(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 40.Peters C, Mayer A. Ca2+ calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 41.Chapman ER. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- 42.Szule JA, Jarvis SE, Hibbert JE, Spafford JD, Braun JE, Zamponi GW, et al. Calcium-triggered membrane fusion proceeds independently of specific presynaptic proteins. J Biol Chem. 2003;278:24251–24254. doi: 10.1074/jbc.C300197200. [DOI] [PubMed] [Google Scholar]
- 43.Ivessa NE, De Lemos-Chiarini C, Gravotta D, Sabatini DD, Kreibich G. The Brefeldin A-induced retrograde transport from the Golgi apparatus to the endoplasmic reticulum depends on calcium sequestered to intracellular stores. J Biol Chem. 1995;270:25960–25967. doi: 10.1074/jbc.270.43.25960. [DOI] [PubMed] [Google Scholar]
- 44.Beckers CJ, Balch WE. Calcium GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989;108:1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker D, Wuestehube L, Schekman R, Botstein D, Segev N. GTP-binding Ypt1 protein and Ca2+ function independently in a cell-free protein transport reaction. Proc Natl Acad Sci USA. 1990;87:355–359. doi: 10.1073/pnas.87.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahluwalia JP, Topp JD, Weirather K, Zimmerman M, Stamnes M. A role for calcium in stabilizing transport vesicle coats. J Biol Chem. 2001;276:34148–34155. doi: 10.1074/jbc.M105398200. [DOI] [PubMed] [Google Scholar]
- 47.Stojilkovic SS. Ca2+-regulated exocytosis and SNARE function. Trends Endocrinol Metab. 2005;16:81–83. doi: 10.1016/j.tem.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Kweon HS, Beznoussenko GV, Micaroni M, Polishchuk RS, Trucco A, Martella O, et al. Golgi enzymes are enriched in perforated zones of Golgi cisternae but are depleted in COPI vesicles. Mol Biol Cel. 2004;15:4710–4724. doi: 10.1091/mbc.E03-12-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]