Abstract
The cellular events required for unconventional protein secretion in eukaryotic pathogens are beginning to be revealed. In fungi, extracellular release of proteins involves passage through the cell wall by mechanisms that are poorly understood. In recent years, several studies demonstrated that yeast cells produce vesicles that traverse the cell wall to release a wide range of cellular components into the extracellular space. These studies suggested that extracellular vesicle release involves components of both conventional and unconventional secretory pathways, although the precise mechanisms required for this process are still unknown. We discuss here cellular events that are candidates for regulating this interesting but elusive event in the biology of yeast cells.
Key words: secretion, extracellular vesicles, exosome, trans-cell wall transport, yeast box
Protein secretion is a widely studied cellular phenomenon. To reach the extracellular milieu, intracellularly synthesized proteins are targeted to the cell surface for release to the extracellular space.1 In mammalian cells, the plasma membrane is the final barrier to be crossed during secretion. Such processes, which involve both conventional and unconventional mechanisms, have been studied in detail and a number of excellent reviews are available in the literature.1–6
Secretory systems in microbes and mammalian cells show points of convergence and divergence.7 Fungi and prokaryotes are surrounded by thick cell walls, a key difference in comparison with mammalian and other eukaryotic cells (e.g., protozoa) that adds significant complexity to secretion systems in these organisms. A number of mechanisms have been proposed for the trans-cell wall molecular transport in prokaryotes.8 In fungi, however, the mechanisms required for passage of molecules across the cell wall are poorly understood. Recently, extracellular vesicle release has been described as a mechanism used by yeast cells to secrete many molecules across the cell wall.9–12
Extracellular vesicles produced by fungal cells share morphological and biochemical similarities with mammalian exosomes,13,14 including an ability to modulate the function of immune cells.15 Plant cells also produce exosome-like vesicles,16 supporting the notion that vesicular release is a mechanism of trans-cell wall passage shared by cell-wall containing eukaryotes. In contrast to what is observed for mammalian exosomes,17 the pathways required for extracellular vesicle biogenesis and release in both plant and fungal cells remain virtually unknown. One remarkable feature of mammalian exosomes and fungal extracellular vesicles is the abundance of cytoplasmic proteins lacking a signal peptide that directs proteins to the endoplasmic reticulum in conventional secretory processes.13,14,18–20
In a recent study, we evaluated the contribution of both conventional and unconventional pathways of secretion in the formation of extracellular vesicles in the model yeast Saccharomyces cerevisiae.21 Our approach was based on the study of mutants with defects in two major secretion pathways: conventional post-Golgi secretion2 and exosome formation, a mechanism of unconventional secretion.17 The use of this model was based on the facts that: (1) genes required for conventional, post-Golgi secretion were implicated in the formation of extracellular vesicles in fungi;22 and (2) exosomes and fungal vesicles share many similarities.18–20,23,24
Defects in the formation of multivesicular bodies (MVB) are expected to directly affect the formation of exosomes.17 Surprisingly, yeast mutants with defects in MVB formation produced similar amounts of extracellular vesicles in comparison to WT cells.21 The protein composition of vesicles from WT and mutant cells was essentially equivalent, but approximately 50% of these vesicular proteins had their abundance modified in mutant cells. Remarkably, most of the proteins (75%) found in vesicular fractions lacked signal peptides. These puzzling results indicate that, although MVB-related mutations apparently do not affect vesicle release, MVB formation is somehow related to the formation of extracellular vesicles in yeast.
Post-Golgi secretory vesicles usually fuse with the plasma membrane to release their cargo, so they are not expected to interfere with formation of vesicular structures outside the cell.6 In our analyses, however, yeast mutants lacking Sec4p, a secretory vesicle-associated Rab GTPase essential for Golgi-derived exocytosis,6 had reduced kinetics of vesicle release to the extracellular milieu.21 The fact that cells with defects in a post-Golgi event of secretion, but not with disturbed MVB formation, affected vesicle release raised an obvious and still unanswered question: how is a double layered vesicle secreted from yeast cells?
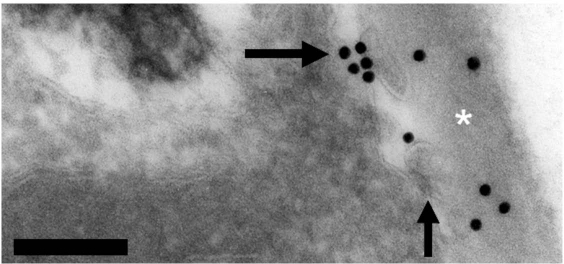
The simplest and more tangible explanation for the release of any extracellular vesicle is the fusion of MVB with the plasma membrane. However, studies by our group25,26 clearly show that double-layered vesicles can bud from the plasma membrane of yeast cells (Fig. 1). Therefore, one could speculate that proteins required for post-Golgi conventional secretion could be required for addressing vesicle components to the plasma membrane. Vesicles would then be formed by membrane budding and sequential transfer to the cell wall and extracellular space. That would be consistent with previous hypotheses raising the possibility that formation of extracellular vesicles can involve membrane budding.4 Budding from the plasma membrane would also be in line with the complex vesicle composition including cytoplasmic elements, as observed in our analyses.13,14,21 It remains unknown how these vesicles traverse the cell wall, but many cell wall degrading enzymes were observed in extracellular vesicle samples obtained from S. cerevisiae cultures.21 We hypothesize that these enzymes could hydrolyze cell wall components to facilitate vesicle passage through this cellular barrier.
Figure 1.
Cryptococcus neoformans, a yeast pathogen, produce vesicle-like structures (arrows) that apparently bud from the plasma membrane to be deposited at the cell wall, as evidenced from transmission electron microscopy. Gold labeling represents reactivity of fungal glucosylceramide with human antibodies. Scale bar, 0.1 µm. Asterisk denotes the cell wall. For experimental details, see Rodrigues and colleagues.26 modified from Barreto-Bergter et al.25 courtesy of Dr. Kildare Miranda.
The methods currently used for vesicle purification do not discriminate between vesicles of different origins. This implies that heterogeneous preparations are obtained during vesicle isolation. In this context, the possibility that the collection of mutations analyzed in our recent study21 is affecting different types of vesicles cannot be ruled out. The current knowledge on how fungal extracellular vesicles are formed, in fact, strongly suggests the involvement of multiple—and perhaps still unknown—pathways of secretion.9,11,13 As recently described in independent studies, unconventional protein secretion can also involve autophagosomes,27,28 which are intracellular structures whose functions were initially attributed to many catabolic steps.29
In autophagy, cytosolic material is sequestered by an expanding membrane compartment, the phagophore, resulting in the formation of a double-membrane vesicle, the autophagosome.29 Autophagosomes then fuse with the lysosome/vacuole where, as initially supposed, the sequestered material is degraded.29 Independent studies, however, have shown that yeast cells can also direct the autophagic content for secretion, in a process called exophagy.27–29 In fact, the autophagic machinery participates in the packaging and delivery of the soluble yeast protein acyl-Coenzyme A-binding protein Acb1 to the cell surface. Therefore, these studies suggest the existence of a vesicular mechanism that utilizes the same machinery for both secretion and degradation of cellular components. It is interesting to note that secretion of Acb1 from yeast as well as secretion of the Dictyostelium discoideum Acb1 homologue, AcbA, depends on the Golgi associated protein GRASP,27,28,30 which is apparently required for extracellular vesicle release in yeast cells.21 These observations add to an already long list of candidates that can regulate vesicle formation in yeast cells.
After our initial description of fungal extracellular vesicles in 2007,12 eight different studies showing their functions in fungal physiology or pathogenesis have been reported in the literature.13–15,21,22,31–33 Vesicle release has been associated to protein and polysaccharide secretion,12–14,22,32 surface architecture,31 virulence,10,12,13 pigmentation33 and modulation of macrophage function.15 Despite their apparent multiple functions in yeast, the cellular components controlling their biogenesis and release remain elusive. We emphasize the supposition that the methods currently used for preparation of extracellular fractions containing vesicles may co-isolate vesicular compartments of different cellular origins, which limit the application of studies based on the generation of punctual mutations. Post-Golgi components required for conventional secretion, proteins involved in MVB formation, GRASP and even autophagy-related events may be involved in the formation of extracellular vesicles. Although much progress has been made in the last three years, the route to understand how fungal extracellular vesicles are formed still seems long and laborious.
Acknowledgements
D.L.O. is a Ph.D. student at Instituto de Bioquímica Médica (Federal University of Rio de Janeiro, Brazil). L.N. and M.L.R. are supported by grants from the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). A.C. is supported by NIH awards HL059842, AI033774, AI052733, AI033142. A.J.G. and L.N. were supported in part by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (NIH D43-TW007129). J.D.N. is supported in part by NIH AI52733-06A1 and AI056070-01A2 and a Hirschl/Weill-Caulier Career Scientist Award. R.J.B.C. was supported by the Training Program in Cellular and Molecular Biology and Genetics, T32 GM007491. I.C.A. was partially supported by the NIH/NCRR grant 5G12RR008124-16A1 and 5G12RR008124-16A1S1. E.S.N was partially supported by the George A. Krutilek memorial graduate scholarship from Graduate School, University of Texas at El-Paso (UTEP). T.J.P.S. was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil. We thank Jorge J. Jó Bastos Ferreira for his invaluable suggestions on the role of vesicles in fungal physiology. We are also grateful to the Biomolecule Analysis Core Facility, Border Biomedical Research Center, UTEP, funded by NIH/NCRR grant 5G12RR00812416A1 and 5G12RR008124-16A1S1, for continuous access to mass spectrometry (LC-MS and GC-MS) instruments, which have been fundamental for several of the studies described here.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12756
References
- 1.Jeremic A. Cell secretion: an update. J Cell Mol Med. 2008;12:1151–1154. doi: 10.1111/j.1582-4934.2008.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 3.Levi SK, Glick BS. GRASPing unconventional secretion. Cell. 2007;130:407–409. doi: 10.1016/j.cell.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann JM, Malkus P, Schekman R. Out of the ER—outfitters, escorts and guides. Trends Cell Biol. 1999;9:5–7. doi: 10.1016/s0962-8924(98)01414-7. [DOI] [PubMed] [Google Scholar]
- 6.Schekman RW. Regulation of membrane traffic in the secretory pathway. Harvey Lect. 1994;90:41–57. [PubMed] [Google Scholar]
- 7.Rothblatt J, Schekman R. A hitchhiker's guide to analysis of the secretory pathway in yeast. Methods Cell Biol. 1989;32:3–36. doi: 10.1016/s0091-679x(08)61165-6. [DOI] [PubMed] [Google Scholar]
- 8.Pugsley AP, Francetic O, Driessen AJ, de Lorenzo V. Getting out: protein traffic in prokaryotes. Mol Microbiol. 2004;52:3–11. doi: 10.1111/j.1365-2958.2003.03966.x. [DOI] [PubMed] [Google Scholar]
- 9.Nosanchuk JD, Nimrichter L, Casadevall A, Rodrigues ML. A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Commun Integr Biol. 2008;1:37–39. doi: 10.4161/cib.1.1.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A. Vesicular trans-cell wall transport in fungi: a mechanism for the delivery of virulence-associated macromolecules? Lipid Insights. 2008;2008:27. doi: 10.4137/lpi.s1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009;17:158–162. doi: 10.1016/j.tim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10:1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun. 2010;78:1601–1609. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583:3363–3366. doi: 10.1016/j.febslet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 17.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 18.Aoki N, Jin-no S, Nakagawa Y, Asai N, Arakawa E, Tamura N, et al. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology. 2007;148:3850–3862. doi: 10.1210/en.2006-1479. [DOI] [PubMed] [Google Scholar]
- 19.Gatti JL, Metayer S, Belghazi M, Dacheux F, Dacheux JL. Identification, proteomic profiling and origin of ram epididymal fluid exosome-like vesicles. Biol Reprod. 2005;72:1452–1465. doi: 10.1095/biolreprod.104.036426. [DOI] [PubMed] [Google Scholar]
- 20.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, et al. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJP, Nosanchuk JD, et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE. 2010;5:11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panepinto J, Komperda K, Frases S, Park YD, Djordjevic JT, Casadevall A, et al. Sec6-dependent sorting of fungal extracellular exosomes and lac-case of Cryptococcus neoformans. Mol Microbiol. 2009;71:1165–1176. doi: 10.1111/j.1365-2958.2008.06588.x. [DOI] [PubMed] [Google Scholar]
- 23.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 24.Nicola AM, Frases S, Casadevall A. Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryot Cell. 2009;8:1373–1380. doi: 10.1128/EC.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barreto-Bergter E, Pinto MR, Rodrigijes ML, Attaur R. Structural and functional aspects of fungal glycosphingolipids. Studies in Natural Products Chemistry: Elsevier; 2006. pp. 1025–1055. [Google Scholar]
- 26.Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, de Souza W, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun. 2000;68:7049–7060. doi: 10.1128/iai.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol. 2010;188:527–536. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjithaya R, Anjard C, Loomis WF, Subramani S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions and autophagosome formation. J Cell Biol. 2010;188:537–546. doi: 10.1083/jcb.200911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahamsen H, Stenmark H. Protein secretion: unconventional exit by exophagy. Curr Biol. 2010;20:415–418. doi: 10.1016/j.cub.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell. 2007;130:524–534. doi: 10.1016/j.cell.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 31.De Jesus M, Nicola AM, Rodrigues ML, Janbon G, Casadevall A. Capsular localization of the Cryptococcus neoformans polysaccharide component galactoxylomannan. Eukaryot Cell. 2009;8:96–103. doi: 10.1128/EC.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira DL, Nimrichter L, Miranda K, Frases S, Faull KF, Casadevall A, et al. Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan. Fungal Genet Biol. 2009;46:956–963. doi: 10.1016/j.fgb.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155:3860–3867. doi: 10.1099/mic.0.032854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]