Abstract
Cell division occurs at a specific time of day in numerous species, suggesting that the circadian and cell cycles are coupled in vivo. By measuring the cell cycle rhythm in real-time, we recently showed that the circadian and cell cycles are not coupled in immortalized fibroblasts, resulting in a rapid rate of cell division even though the circadian rhythm is normal in these cells. Here we report that tumor-driven Lewis lung carcinoma (LLC) cells have perfectly temperature compensated circadian clocks, but the periods of their cell cycle gene expression rhythms are temperature-dependent, suggesting that their circadian and cell cycles are not connected. These data support our hypothesis that decoupling of the circadian and cell cycles may underlie aberrant cell division in tumor cells.
Key words: circadian, cell cycle, cancer, bioluminescence, Lewis lung carcinoma (LLC) cells, temperature compensation, cyclin B1, reporter gene, cell division
From an evolutionary perspective, the predictable daily cycles of light and temperature caused by the rotation of the earth on its axis likely had significant effects on the chemical milieu of unicellular organisms. By partitioning cellular processes into specific time domains, anticipation of periodic changes in the environment presumably increased survival and reinforced the development of endogenous circadian oscillators.1 Since cell division is critical to the survival of unicellular organisms and the integrity of DNA is susceptible to UV irradiation, the progression of the cell cycle was probably also strongly affected by daily changes in the environment, thereby imposing a need for compartmentalization of cell division into specific time domains. Indeed, multiple studies have measured diurnal fluctuations in cell division, such that mitosis occurs at a specific time of day in numerous species ranging from unicellular organisms2 to humans.3,4 In addition to protecting DNA from the mutagenic effects of UV irradiation, circadian control of cell division may serve the additional purpose of controlling the rate of cell proliferation.
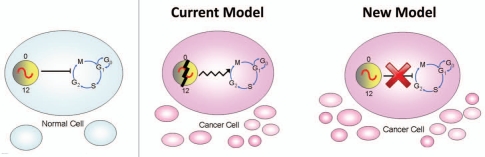
If the rate of cell proliferation is gated by the circadian system in normal cells, then what is happening in rapidly-dividing tumor cells? The current working hypothesis is that the circadian rhythm is disrupted or ameliorated in tumor cells, so that it is no longer able to regulate the rate of cell division (Fig. 1). Consistent with this model, numerous studies have demonstrated a correlation between disruption of circadian rhythms and the development of cancer. People who work irregular schedules (working sometimes during the day and sometimes at night), such as flight attendants, airline pilots and nurses, have a significantly higher risk of cancer compared to people who work normal daytime shifts.5–13 In animal studies, mice lacking the circadian genes, Period2, Period1 and Period2, Cryptochrome1 and Cryptochrome2 or 1 copy of Bmal1, have higher incidences of spontaneous and γ radiation-induced tumors compared to wild-type mice.14,15 Numerous studies have also found alterations in the expression of circadian genes in cancerous tissues.16 Therefore, multiple lines of evidence suggest that circadian dysregulation accompanies or is a prerequisite for cancer development and progression.
Figure 1.
A new model: disconnection of the circadian and cell cycles in cancer cells. In normal (non-cancerous) cells, the circadian rhythm and cell division cycle are tightly linked resulting in a 24 hour rhythm of cell division. According to the current model, since the circadian clock and cell division cycle are linked, dysregulation of the biological clock results in uncontrolled cell division which causes cancer. According to our new model, the circadian and cell cycles are disconnected from each other in tumor cells.
We recently made a new discovery that caused us to reevaluate this current model. To investigate how the circadian system regulates the cell cycle and how this process is disrupted in cancerous cells, we developed a novel approach for monitoring the cell cycle in real-time. We created immortalized fibroblasts that stably express a DNA construct in which the Cyclin B1 promoter drives the expression of destabilized green luciferase (CCNB1-dGluc).17 Since the accumulation of CYCLIN B1 protein is a prerequisite for mitosis, we have created a real-time method to measure cell division. Using this technique, we found that the circadian rhythm is not controlling the cell cycle rhythm in immortalized fibroblasts, even though the circadian rhythm is normal and functional.17 Based on these data, we proposed a novel hypothesis to account for aberrant cell division in immortalized and tumor cells: the circadian and cell cycles are tightly coupled in normal tissue, but these oscillators become disconnected in cancerous tissues, resulting in aberrant cell proliferation (Fig. 1).
The aforementioned experiments were performed in immortalized rat-1 fibroblasts. Though immortalized cells proliferate indefinitely, they are distinct from cancer cells. To test the validity of our hypothesis with respect to cancerous cells, in the current study we investigated the circadian and cell cycle rhythms in a tumor-driven cell line, Lewis lung carcinoma (LLC) cells.
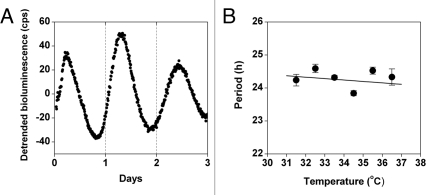
To determine if LLC cells have a circadian rhythm, we stably transfected the cells with the Period2-luciferase (Per2-luc) DNA construct18 (containing the neomycin resistance gene for selection) in which the Per2 promoter drives the expression of firefly luciferase. One day after subculturing (20% confluency), the cells were stimulated with forskolin for 30 minutes to synchronize the circadian rhythm, and then the growth media was replaced with recording media. We found that LLC cells have a robust circadian rhythm of Per2-luc expression (Fig. 2A). A fundamental property of circadian rhythms is that they are temperature compensated: the period of the rhythm is approximately 24 hrs at different ambient physiological temperatures [in contrast to most biological reactions that proceed with a temperature coefficient (Q10) ∼2 or 3, so that with every 10°C increase in temperature, the reaction rate approximately doubles or triples]. We found that the period of Per2-luc expression following forskolin stimulation is temperature compensated in LLC cells (Q10 = 1.02; Fig. 2B), suggesting that the circadian rhythm in these tumor cells is functioning normally.
Figure 2.
The circadian rhythm is temperature compensated in Lewis lung carcinoma (LLC) cells. (A and B) LLC cells stably transfected with Per2-luc were treated with forskolin for 30 minutes and then the growth media was replaced with recording media. (A) Detrended data (counts/sec) was obtained by subtracting the 24-hour moving average from the raw data. (B) The period (mean ± SEM) of Per2-luc expression in LLC cells was determined from bioluminescence recorded by the LumiCycle at 31.5°, 32.5°, 33.5°, 34.5°, 35.5° and 36.5°C (Q10= 1.02; n = at least four at each temperature).
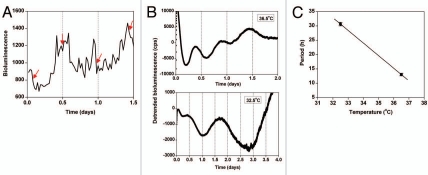
We next generated LLC cells stably transfected with CCNB1-dGluc17 to assess the cell cycle rhythm. Using a low-luminescence imaging system, we imaged CCNB1-dGluc expression in LLC cells for several days. From these images, the rhythm of CCNB1-dGluc expression and the timing of cell division (one cell visibly splitting into two cells) were determined (Fig. 3A). We found that cell division always occurs near the peak of CCNB1-dGluc expression in that cell (Fig. 3A). Quantification of bioluminescence showed that the period of the CCNB1-dGluc rhythm in individual LLC cells is ∼13 hrs (Fig. 2B; mean period ± SEM: 13.07 ± 1.06 h, n = 7). Therefore, similar to rat-1 fibroblasts, the timing of cell division in LLC cells can be monitored in real-time by measuring the CCNB1-dGluc rhythm.
Figure 3.
The period of the cell cycle gene expression rhythm is not temperature compensated in Lewis lung carcinoma (LLC) cells. (A) Representative example of the optical density of CCNB1-dGluc bioluminescence in a single LLC cell imaged at 36.5°C. The timing of cell mitosis is indicated by red arrows. Seven cells from each of two independent dishes were analyzed. (B and C) LLC cells stably expressing CCNB1-dGluc were synchronized by cold shock (22°C for 24 hrs) and then bioluminescence was recorded. Representative traces of detrended CCNB1-dGluc expression (counts/sec) at 36.5°C (top part; 12-h moving average; n = 3) or 32.5°C (bottom part; 30-h moving average; n = 3) are shown. (C) The period (mean ± Sem) of CCNB1-dGluc expression in LLC cells was determined by measuring the peak-to-peak time of two cycles at 32.5°C or the average peak-to-peak time of three cycles at 36.5°C (Q10 = 8.46).
If the circadian and cell cycles are coupled in LLC cells, then the cell cycle rhythm should be temperature compensated like the circadian rhythm. To determine if the period of the CCNB1-dGluc rhythm was affected by temperature in LLC cells, we synchronized the cell cycle rhythm with a cold pulse (22°C for 24 hours)19 and then measured bioluminescence at 36.5°C or 32.5°C (Fig. 3B). We found that the CCNB1-dGluc rhythm is not temperature compensated (Q10 = 8.46; Fig. 3C), suggesting that the circadian and cell cycles are not coupled in LLC cells.
Consistent with our finding that the circadian rhythm is normal in LLC cells, previous studies have shown that other tumor-driven cell lines, such as immortalized human osteosarcoma cells (U-2 OS)20 and peripheral neuroblastoma cells (SH-SY5Y),21 as well as explants of chemically-induced hepatocellular carcinomas,22 exhibit ∼24-hour rhythms of circadian gene expression. Therefore, the current model-that a dysfuntional circadian rhythm results in dysregulation of the cell cycle in cancer cells-cannot apply to these tumor-driven cell lines or to immortalized fibroblasts. Our novel hypothesis-that the circadian and cell cycles are decoupled in cancer cells-however, can explain how aberrant cell division can occur even when the circadian rhythm is normal. Our hypothesis is also supported by the finding that the circadian and cell cycle rhythms are disconnected in tumor-driven LLC cells even though the circadian clock is perfectly functional. To directly test our hypothesis, we must determine if the circadian and cell cycles are coupled in normal, non-cancerous cells. If not, then it is possible that the 24 hr rhythms of cell mitosis in normal cells are synchronized to the rhythmic host environment. If the circadian and cell cycles are coupled in normal cells, but they are disconnected in cancer cells, as in LLC cells, these findings open up exciting avenues for research identifying novel compounds capable of regulating the period of the cell cycle rhythm in tumor cells and could lead to novel therapeutic treatments for cancer.
Acknowledgements
We thank Dr. Muschel (University of Oxford) for the hCCNB1 promoter and Jia-Lin Liu for generating the LLC cells stably expressing Per2-luc. This work was supported by the National Institutes of Health (R01 NS051278 to S.Y.), the Research Foundation for Opto-Science & Technology (to S.Y.), the NEDO Project & Takeda Science Foundation (to Y.O.) and the Vanderbilt University Summer Research Program (to B.A.R.).
Abbreviations
- LLC cells
Lewis lung carcinoma cells
- Per2
period2
- CCNB1
cyclin B1
- dGluc
destabilized green luciferase
- luc
firefly luciferase
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12841
References
- 1.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 2.Edmunds LN. Cellular and molecular bases of biological clocks: models and mechanisms for circadian timekeeping. New York: Springer-Verlag; 1988. [Google Scholar]
- 3.Bjarnason GA, Jordan RC, Sothern RB. Circadian variation in the expression of cell cycle proteins in human oral epithelium. Am J Pathol. 1999;154:613–622. doi: 10.1016/S0002-9440(10)65306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown WR. A review and mathematical analysis of circadian rhythms in cell proliferation in mouse, rat and human epidermis. J Invest Dermatol. 1991;97:273–280. doi: 10.1111/1523-1747.ep12480379. [DOI] [PubMed] [Google Scholar]
- 5.Pukkala E, Auvinen A, Wahlberg G. Incidence of cancer among Finnish airline cabin attendants 1967–92. BMJ. 1995;311:649–652. doi: 10.1136/bmj.311.7006.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynge E. Risk of breast cancer is also increased among Danish female airline cabin attendants. BMJ. 1996;312:253. doi: 10.1136/bmj.312.7025.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wartenberg D, Stapleton CP. Risk of breast cancer is also increased among retired US female airline cabin attendants. BMJ. 1998;316:1902. doi: 10.1136/bmj.316.7148.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S. Cancer incidence in California flight attendants (United States) Cancer Causes Control. 2002;13:317–324. doi: 10.1023/a:1015284014563. [DOI] [PubMed] [Google Scholar]
- 9.Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: a population-based study (Iceland) Cancer Causes Control. 2001;12:95–101. doi: 10.1023/a:1008983416836. [DOI] [PubMed] [Google Scholar]
- 10.Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, et al. Incidence of cancer among Nordic airline pilots over five decades: occupational cohort study. BMJ. 2002;325:567. doi: 10.1136/bmj.325.7364.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Band PR, Spinelli JJ, Ng VT, Moody J, Gallagher RP. Mortality and cancer incidence in a cohort of commercial airline pilots. Aviat Space Environ Med. 1990;61:299–302. [PubMed] [Google Scholar]
- 12.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 13.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 14.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 5:10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia. 2007;9:797–800. doi: 10.1593/neo.07595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeom M, Pendergast JS, Ohmiya Y, Yamazaki S. Circadian-independent cell mitosis in immortalized fibroblasts. Proc Natl Acad Sci USA. 2010;107:9665–9670. doi: 10.1073/pnas.0914078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, et al. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci USA. 2005;102:2608–2613. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieder CL, Cole RW. Cold-shock and the Mammalian cell cycle. Cell Cycle. 2002;1:169–175. [PubMed] [Google Scholar]
- 20.Baggs JE, Price TS, DiTacchio L, Panda S, Fitzgerald GA, Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biol. 2009;7:52. doi: 10.1371/journal.pbio.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maronde E, Motzkus D. Oscillation of human period 1 (hPER1) reporter gene activity in human neuroblastoma cells in vivo. Chronobiol Int. 2003;20:671–681. doi: 10.1081/cbi-120022413. [DOI] [PubMed] [Google Scholar]
- 22.Davidson AJ, Straume M, Block GD, Menaker M. Daily timed meals dissociate circadian rhythms in hepatoma and healthy host liver. Int J Cancer. 2006;118:1623–1627. doi: 10.1002/ijc.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]