Abstract
Natural killer (NK) cells are immune cells that lyse virally infected and tumor cells. Initially, their cytolytic capability is induced by cytokines. Subsequently, in their decision whether to kill a potential target cell, NK cells have to distinguish between small differences in the expression of ligands that report on the viral infection or transformation of the target. NK killing requires tight coupling to the target cell and extensive NK cell polarization. Here we discuss, often in contrast to the second cytolytic immune cell type, cytotoxic T cells, how NK cell polarization is shaped by three constraints of their activation. First, NK cell have to respond to cytokines: Different priming cytokines yield dramatically divergent NK cell polarization. Second, NK cells have to distinguish small differences in ligand expression: NK cell polarization is tentative, likely to allow discriminatory recognition close to the NK cell activation threshold. A critical contributor to the tentative nature of NK cell polarization may be poorly developed spatiotemporal organization of NK cell signaling. Third, NK cells have to kill effectively: NK cell polarization is transient, allowing for efficient killing by sequential interactions of a single NK cell with numerous target cells.
Key words: lymphocyte polarization, NK cell, cytolytic T cell, innate immune system, Cdc42
Within minutes of exposure to an immune stimulant innate immune cells become activated. They rapidly acquire effector function and coordinate the activation of the adaptive immune response that requires days for the acquisition of effector function. Natural killer (NK) cells are innate cytolytic effectors. They kill virally infected and tumor target cells mostly by release of lysosome-derived cytolytic granules. NK cell function is shaped by two requirements. First, immune recognition by NK cells occurs in parallel with that of other innate immune cell types, in particular cytokine-secreting dendritic cells. For a well-coordinated immune response, NK cells have to respond to their cytokine environment. Second, NK cells are activated through recognition of viral components or by altered expression of self stress proteins.1 In particular altered self recognition requires sensitive discrimination between healthy and virally infected or tumor cells. NK cell activation occurs by cellular interactions and NK cell polarization is required for effective activation.2–4 The regulation of NK cell polarization is therefore critical for NK cell function. Using live cell imaging we have characterized NK cell polarization in time and space.5 Both requirements for effective NK cell function, responsiveness to cytokines and discriminatory recognition, are reflected in the regulation of NK cell polarization.
The expression of the cytolytic effector machinery of NK cells needs to be induced or ‘primed’ by cytokines, in particular dendritic cell-derived IL-12, IL-15 and IL-18.6–10 In humans, continuous exposure to pathogens yields constitutive modest induction of NK effector capability. In NK cells from mice housed under specific pathogen-free conditions, priming cytokines need to be added to NK cell cultures. For studies requiring in vitro priming, in particular in therapeutic applications, NK cells are mostly primed with a high concentration of the cell growth factor IL-2.11,12 Interestingly however, IL-2 is dispensable for in vivo NK cell priming.9 Comparing murine NK cells primed with IL-2 or dendritic cell-derived innate cytokines, IL-15, IL-12 plus IL-18 or all three, NK cell polarization toward target cells differs dramatically, as discussed in detail below. While IL-2 NK cells polarize rapidly and effectively, NK cells primed with innate cytokines do so tentatively, similar to human NK cells (Fig. 1).2,13 Different innate cytokines yield comparable polarization behavior. Given how fundamental polarization is for NK cell function, NK cells primed with innate cytokines versus IL-2 may have to be regarded as two different types of cytolytic effectors. It will be interesting to learn whether upon more detailed investigations, smaller differences in the polarization behavior of NK cells primed with different innate cytokines will also emerge.
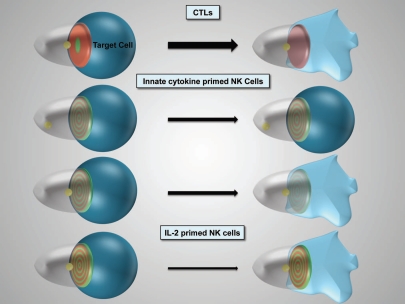
Figure 1.
Schematic representation of cytolytic effector polarization. The progression of the different cytolytic effector/target cell couples as indicated from initial polarization within seconds of tight cell coupling (left) to sustained polarization over several minutes (right) is depicted. The thickness of the arrow indicates efficacy of progression. For innate cytokine-primed NK cells two distinct cell couples fates exist. The target cell is in blue, loss of color and shape denotes lysis. The cytolytic effector is transparent. The position of the MTOC is given in yellow, interface accumulation with its spatiotemporal patterning of actin and active Cdc42 in red and green, respectively. Color intensity indicates extent of accumulation.
The rapid activation of innate immune effectors imposes unique constraints on discriminatory recognition in NK cell activation that are reflected in the features of NK cell polarization. This is most easily appreciated in comparison to cytotoxic T cells (CTL), the adaptive cytolytic effectors. To ensure lack of reactivity toward self, CTLs rely on parallel immune recognition by dendritic cells and helper T cells during their several day-long differentiation. Only when the recognition events of all three cell types match, CTLs can acquire effector capability. Mature CTLs thus come with their discriminatory recognition specificity verified. They can therefore afford to optimize their polarization for cytolytic efficacy. In target cell killing, the actual granule release lasts only seconds. Rapid execution of the killing interaction allows a cytolytic effector to kill several target cells sequentially, promoting effective population-wide killing. To maximize cytolytic efficacy, CTLs are fully polarized within seconds of target cell contact with accumulation of signaling intermediates, actin and the MTOC at the CTL/target cell interface, indicative of strong activating signaling5,14 (Fig. 1). Within just a few minutes, such polarization is largely lost and binding to other target cells occurs upon rapid establishment of a new CTL/target cell interface. Initial strong but transient polarization characterizes CTLs and is associated with effective killing. An open question is how the activating signaling that triggers polarization is lost or counteracted rapidly. As target cell contact and receptor engagement are well maintained during the loss of CTL polarization, the induction of inhibitory signaling is likely. The concept that release of the cytolytic granules could be directly related to the generation of the inhibitory signal is intriguing. Thus successful execution of a first target cell kill would set up the next. In support, we found that helper T cells that have an essentially identical signaling machinery but lack cytolytic capability maintain their initial rapid polarization much better. Therefore, comparing the dynamics of CTL and helper T cell signaling seems a promising approach to identify the signaling processes terminating CTL polarization.
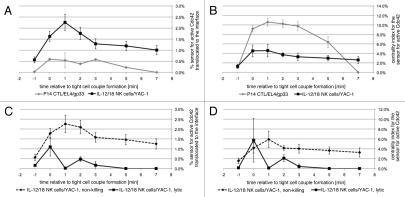
In contrast to CTLs, NK cells have to distinguish between immunogen and self, aside from the effect of priming cytokines, without the help of parallel recognition by other immune cells. Innate cytokine-primed NK cells polarize more cautiously (Fig. 1). The extent of interface actin accumulation is less and MTOC reorientation can take several minutes.15 Even in response to highly susceptible targets, only about half of the initial NK cell/target cell couples proceed the target cell lysis15 (Fig. 1). Cautious establishment of polarization is indicative of signaling processes close to the trigger threshold. We suggest that this is critical to allow the sensitive detection of small expression differences in activating and inhibitory ligands on the target cell. Notch signaling constitutes an appealing paradigm for sensitive detection of small differences in ligand expression.16 In Notch signaling engagement of ligand on the same cell, in ‘cis’, triggers different signaling pathways than ligand engagement on another cell, in ‘trans’, such that mutually exclusive signaling states can be triggered by small differences in trans ligand expression. Interestingly, ligands for many NK receptors, e.g., MHC I as the ligand for activating and inhibitory receptors of the human KIR and murine Ly49 families, are expressed both on NK cells and targets.17,18 An open question is how NK cells polarization is limited. NK cells express inhibitory receptors that can suppress cellular polarization with the ubiquitous MHC I as their ligand.19,20 However, the targets in our studies are MHC I-negative. The intensity of proximal signaling could be limited in NK cell activation. However for Cdc42, a general regulator of cellular polarization, the amount of active Cdc42 at the cytolytic effector/target cell interface is actually higher in NK cells than in CTLs (Fig. 2A).5 Interestingly however, the localization of active Cdc42 differs (Fig. 1). While active Cdc42 is enriched selectively at the center of the CTL/target cell interface, it is spread out over the NK cell/target cell interface.5 This becomes obvious when the amount of interface accumulation of active Cdc42 is normalized to its area. With such relation to accumulation area, CTLs are much more effective in generating a small area of intense Cdc42 accumulation than NK cells (Fig. 2B). Interestingly, accumulation of active Cdc42 is also moderately more focused in lytic than in non-killing NK cell/target cell couples (Fig. 2C and D). Spatiotemporal distributions have a great potential to regulate the efficiency of cell signaling, as enriching two proteins at the same time at the same place increases the likelihood that they will interact. In helper T cells clustering of signaling intermediates in distinct groups is related to effective proximal signaling.21 Accumulation of active Cdc42 at the center of the cellular interface is closely related to interface actin accumulation.22 Together these data suggest, that the ability of CTLs to generate a strong initial activating signal could be driven by efficient spatiotemporal segregation of its signaling machinery, thus enhancing the signaling efficiency. Lacking such spatiotemporal organization, NK cells may generate weaker signals from comparable stimuli. Other explanations, such as divergent composition of CTL and NK cell signaling systems, are certainly conceivable. Regardless, a promising approach to resolve the question of differential signaling intensity should be to compare CTL and NK cell signaling at the systems level with resolution in time and space.
Figure 2.
Accumulation of active Cdc42 is focused on the center of the interface. (A) The % sensor for active Cdc42 translocated to the cytolytic effector/target cell interface is given as a function of time relative to tight cell coupling with standard errors for the following interactions: P14 T cells/EL4 target cells/10 µm gp33 agonist peptide or IL-12/18 NK cells with YAC-1 target cells, as indicated. The 64, 35 cell couples were analyzed per condition. The data are part of ref. 5. (B) The % sensor for active Cdc42 translocated to the cytolytic effector/target cell interface is normalized to the size of the area of accumulation relative to the interface size. This measure is referred to as the centrality index in ref. 22. (C and D) For IL-12/18 NK cell/YAC-1 interactions, the data from (A and B) are displayed separately for non-killing (broken line) and lytic (solid line) interactions.
Tentative NK cell polarization, while favoring discriminatory recognition, likely limits cytolysis. We suggest that to compensate, the NK cell/target cell couples that progress to lysis do so rapidly, as facilitated by transient polarization. NK cell polarization in non-killing cell couples remains stable but is partial (Fig. 1). While the MTOC does not reorient toward the interface, actin and Cdc42 accumulate at the interface. In lytic cell couples, the MTOC reorients toward the target cell and interface accumulation of actin and Cdc42 is only transient, similar to CTLs (Fig. 1). Providing causal evidence for the importance of transient polarization in effective killing, interference with polarization selectively in the stably polarized, non-killing interaction, i.e., enhanced transience, promotes target cell killing.5 In an additional relation between transience of polarization and cytolytic efficacy, polarization of IL-2 primed NK cells is strong but sustained (Fig. 1). The MTOC reorients rapidly, actin and Cdc42 accumulate and remain at the interface, even though accumulation of active Cdc42 lacks central focus. Consistent with the lack of transience in polarization, IL-2 NK killing is inefficient.5
In summary, effective cytolytic function requires initial strong but transient polarization. Priming NK cells with IL-2 versus dendritic cell-derived innate cytokines generates cytolytic effectors with fundamentally different polarization behavior, strong but sustained for IL-2 NK cells, tentative but more transient for innate cytokine-primed NK cells. The necessity for unaided discriminatory NK recognition is reflected in comparably modest activating signaling in innate cytokine-primed NK cells, leading to tentative polarization with lack of progression to target cell lysis in part of the population. We speculate that inefficient spatiotemporal segregation in signaling is a key contributor to limiting the activating signal generated by NK receptor engagement. Compensating for tentative polarization, the NK cell/target cell couples that progress to killing do so rapidly as facilitated by transience of NK cell polarization.
Abbreviations
- NK cell
natural killer cell
- CTL
cytolytic T cell
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12843
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis DM. Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat Rev Immunol. 2009;9:543–555. doi: 10.1038/nri2602. [DOI] [PubMed] [Google Scholar]
- 4.Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev. 2010;235:24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinai P, Nguyen C, Schatzle JD, Wülfing C. Transience in polarization of cytolytic effectors is required for efficient killing and controlled by Cdc42. Proc Natl Acad Sci USA. 2010;107:11912–11917. doi: 10.1073/pnas.0913422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agaugue S, Marcenaro E, Ferranti B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18 or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood. 2008;112:1776–1783. doi: 10.1182/blood-2008-02-135871. [DOI] [PubMed] [Google Scholar]
- 7.Arend WP, Palmer G, Gabay C. IL-1, IL-18 and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 10.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 11.Chan CJ, Andrews DM, Smyth MJ. Can NK cells be a therapeutic target in human cancer? Eur J Immunol. 2008;38:2964–2968. doi: 10.1002/eji.200838764. [DOI] [PubMed] [Google Scholar]
- 12.Smyth MJ, Swann J, Kelly JM, Cretney E, Yokoyama WM, Diefenbach A, et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200:1325–1335. doi: 10.1084/jem.20041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kupfer A, Singer SJ. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- 15.Wülfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci USA. 2003;100:7767–7772. doi: 10.1073/pnas.1336920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprinzak D, Lakhanpal A, Lebon L, Santat LA, Fontes ME, Anderson GA, et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465:86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Back J, Chalifour A, Scarpellino L, Held W. Stable masking by H-2Dd cis ligand limits Ly49A relocalization to the site of NK cell/target cell contact. Proc Natl Acad Sci USA. 2007;104:3978–3983. doi: 10.1073/pnas.0607418104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J Immunol. 2006;177:3590–3596. doi: 10.4049/jimmunol.177.6.3590. [DOI] [PubMed] [Google Scholar]
- 20.McCann FE, Vanherberghen B, Eleme K, Carlin LM, Newsam RJ, Goulding D, et al. The size of the synaptic cleft and distinct distributions of filamentous actin, ezrin, CD43 and CD45 at activating and inhibitory human NK cell immune synapses. J Immunol. 2003;170:2862–2870. doi: 10.4049/jimmunol.170.6.2862. [DOI] [PubMed] [Google Scholar]
- 21.Singleton KL, Roybal KT, Sun Y, Fu G, Gascoigne NR, van Oers NS, et al. Spatiotemporal patterning during T cell activation is highly diverse. Sci Signal. 2009;2:15. doi: 10.1126/scisignal.2000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tskvitaria Fuller I, Seth A, Mistry N, Gu H, Rosen MK, Wülfing C. Specific patterns of Cdc42 activity are related to distinct elements of T cell polarization. J Immunol. 2006;177:1708–1720. doi: 10.4049/jimmunol.177.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]