Abstract
Group-living species produce signals that alter the behavior and even the physiology of their social partners. Social insects possess especially sophisticated chemical communication systems that govern every aspect of colony life, including the defining feature of eusociality: reproductive division of labor. Current evidence hints at the central importance of queen pheromones, but progress has been hindered by the fact that such pheromones have only been isolated in honeybees. In a pair of papers on the ant Lasius niger, we identified and investigated a queen pheromone regulating worker sterility. The cuticular hydrocarbon 3-methylhentriacontane (3-MeC31) is correlated with queen maturity and fecundity and workers are also more likely to execute surplus queens that have low amounts of this chemical. Experiments with synthetic 3-MeC31 found that it inhibits ovarian development in queenless workers and lowers worker aggression towards objects coated with it. Production of 3-MeC31 by queens was depressed by an experimental immune challenge, and the same chemical was abundant on queenlaid eggs, suggesting that the workers' responses to the queen are conditional on her health and fecundity. Together with other studies, these results indicate that queen pheromones are honest signals of quality that simultaneously regulate multiple social behaviors.
Key words: social insect, queen pheromone, fertility signal, cuticular hydrocarbon, social physiology, primer pheromone
Parsimonious Regulation of Colony Life by Queen Pheromones
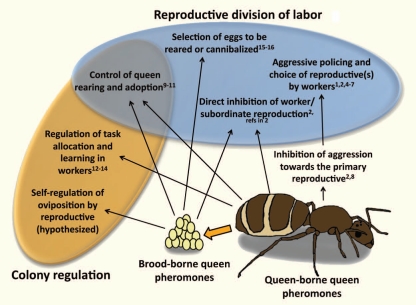
In combination with previous results, our new data1,2 suggest that queen pheromones can simultaneously regulate multiple aspects of reproductive division of labor and other colony-level processes (Fig. 1). We found that the cuticular hydrocarbon 3-MeC31 is involved in signaling queen fertility, maturity and condition,1,2 regulating worker reproduction2 and preventing worker aggression towards objects bearing the pheromone.2 Several other functions are more tentatively supported. Our results on the execution of supernumerary queens in founding associations are consistent with selective worker aggression towards the queen(s) with the least 3-MeC31.1 Workers that cannot directly identify their mother are predicted to attack queens that have produced the fewest workers, maximizing the chance that they are killing an unrelated queen,1,3 and 3-MeC31 is strongly correlated with queen productivity.
Figure 1.
Known and hypothesized functions of queen pheromones produced by queens (or other reproductive individuals) and carried on their brood. The numbers give a non-exhaustive list of studies providing evidence consistent with each function.
Queen-like chemicals have also been implicated in the aggressive response to nonpreferred reproductives, such as fertile workers and subordinate reproductives, in many other species of ants4 (especially queenless ants5), wasps6 and bees.7 Our results and those of Moore and Liebig8 imply that queen-like hydrocarbons depress worker aggression, which is seemingly incongruous with reports of these same chemicals eliciting aggression in certain contexts, e.g., when expressed by fertile workers or subordinates.4,5 This disparity suggests either (A) queen pheromone has a dose-dependent effect on aggression, i.e., weak sources of pheromone attract aggression while strong sources repel it or (B) that there are multiple cues involved in the aggressive response, such that queen pheromone excites or inhibits aggression in combination with other stimuli. The dose-dependent hypothesis seems more parsimonious, although explicit tests are needed.
I also propose that queens pheromones may be used by queens to regulate their own reproductive output with respect to external cues, e.g., the number and developmental stage of brood and the presence of other queens competing to be the sole reproductive.1 There is indirect evidence of this in L. niger: 3-MeC31 is present on queens, eggs and cocoons,1,2 and queens reduce their reproductive output when additional queens and brood are present.1
Even more queen pheromone functions have been described in other taxa. Queen adoption,9 supersedure10 and replacement10,11 behavior in ants and honeybees is thought to be regulated by queen pheromones, and in honeybees there is extensive evidence that worker task allocation,12 brain development13 and learning14 is influenced by queen pheromone exposure. Queen pheromones on the surface of eggs are likely to allow discrimination and differential rearing of eggs from different colony members, particularly queens and workers,15,16 but perhaps also from different queens.16
Perspectives for Future Research
There is now near-unanimous support for the hypothesis that social insect queen pheromones are “honest signals” of fertility or condition, and that the worker response is not counter to their own fitness interests.1,2,17–20 I therefore suggest that researchers should focus on the ultimate explanation for this honesty. There are three principal reasons why signals should be honest:18,21 (1) dishonest signaling is not selected, (2) the signal is a costly “handicap”, such that only high-quality individuals benefit from investing heavily in signaling and (3) the signal is an unfakeable “index” that is inextricably linked to the trait it is signaling. As argued elsewhere,18 hypothesis 1 is unlikely to be applicable to all social insects, including derived lineages where reproductive conflict is constrained.22 For example, in L. niger, we expect that queens in multi-queen colonies would benefit from producing dishonestly high amounts of 3-MeC31 to avoid execution by workers. At present, almost all data on putative queen pheromones appear to be equally consistent with the handicap and index hypotheses. In L. niger, our immune challenge might have depressed reproductive physiology causing a corresponding drop in pheromone production (index) or reduced condition such that pheromone production was no longer possible (handicap).2 Intriguingly, treatment with juvenile hormone reduced reproductive activity while slightly augmenting pheromone production in honeybee workers23 (which appears to falsify the index hypothesis); however, in a comparable experiment in ants both reproduction and putative queen pheromones were suppressed by juvenile hormone.24 Determining the underlying genetic architecture, biochemistry and/or fitness costs of pheromone production may be required to definitively discriminate between these hypotheses.
Our study2 shows how queen primer pheromones may be unambiguously identified, and I believe that it will be fruitful to isolate more in additional taxa. This will allow numerous novel questions to be addressed, e.g., how fast do queen pheromones evolve, are they predominantly single- or multi-component blends, and have similar pheromones evolved independently in phylogenetically-distant taxa? Answering these questions will provide insight into social evolution as a whole. For example, fast-evolving and multicomponent signals imply coevolution and possibly conflict.17,21 Convergent evolution of homologous queen pheromones would suggest that certain chemicals are particularly suited to the job: they might be particularly costly to produce (handicap hypothesis) or biochemically linked to reproduction (index hypothesis). There is tantalizing evidence that alkanes with a methyl group on the third carbon (like 3-MeC31) are also queen pheromones in other species of social insects. 3-methyl-alkanes have been correlated with fertility and/or caste in other highly-social formicine ants (Camponotus floridanus25 and Formica fusca26) and in more basal ants (Myrmecia gulosa,27 Diacamma ceylonese,28 Pachycondyla inversa29 and Platythyrea punctata30). Even more surprisingly, these compounds are characteristic of reproductives in the distantly-related termites31 and have been indirectly linked to the regulation of worker reproduction in the wasp Ropalidia marginata.32
Acknowledgements
I am grateful to all members of the Centre for Social Evolution, Copenhagen for providing a stimulating work environment. This work was supported by a Marie Curie Intra-European Fellowship (#235403; CHEMDOC).
Addendum to: Holman L, Dreier S, d'Ettorre P. Selfish strategies and honest signalling: reproductive conflicts in ant queen associations. Proc R Soc B. 2010;277:2007–2015. doi: 10.1098/rspb.2009.2311. and Holman L, Jørgensen CG, Nielsen J, d'Ettorre P. Identification of an ant queen pheromone regulating worker sterility. Proc R Soc B. 2010 doi: 10.1098/rspb.2010.0984. In press.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12976
References
- 1.Holman L, Dreier S, d'Ettorre P. Selfish strategies and honest signalling: reproductive conflicts in ant queen associations. Proc R Soc B. 2010;277:2007–2015. doi: 10.1098/rspb.2009.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman L, Jørgensen CG, Nielsen J, d'Ettorre P. Identification of an ant queen pheromone regulating worker sterility. Proc R Soc B. 2010;277:3793–3800. doi: 10.1098/rspb.2010.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balas MT. Conditions favoring queen execution in young social insect colonies. Insectes Sociaux. 2005;52:77–83. [Google Scholar]
- 4.Smith AA, Hölldober B, Liebig J. Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr Biol. 2009;19:78–81. doi: 10.1016/j.cub.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Peeters C, Liebig J. Fertility signaling as a general mechanism of regulating reproductive division of labor in ants. In: Gadau J, Fewell J, editors. Organization of Insect Societies: From Genome to Socio-Complexity. Harvard University Press; 2009. [Google Scholar]
- 6.Dapporto L, Romana Dani F, Turillazzi S. Social dominance molds cuticular and egg chemical blends in a paper wasp. Curr Biol. 2007;17:504–505. doi: 10.1016/j.cub.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Amsalem E, Twele R, Francke W, Hefetz A. Reproductive competition in the bumble-bee Bombus terrestris: do workers advertise sterility? Proc R Soc B. 2009;276:1295–1304. doi: 10.1098/rspb.2008.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore D, Liebig J. Mixed messages: fertility signaling interferes with nestmate recognition in the monogynous ant Camponotus floridanus. Behav Ecol Sociobiol. 2010;64:1011–1018. [Google Scholar]
- 9.Vander Meer RK, Alonso LE. Queen primer pheromone affects conspecific fire ant (Solenopsis invicta) aggression. Behav Ecol Sociobiol. 2002;51:122–130. [Google Scholar]
- 10.Winston ML. The Biology of the Honey Bee. Harvard University Press; 1987. [Google Scholar]
- 11.Edwards JP. Caste regulation in the pharaoh's ant Monomorium pharaonis: the influence of queens on the production of new sexual forms. Physiol Entomol. 1987;12:31–39. [Google Scholar]
- 12.Robinson GE, Winston ML, Huang Z, Pankiw T. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. 1998;44:685–692. doi: 10.1016/s0022-1910(98)00040-7. [DOI] [PubMed] [Google Scholar]
- 13.Beggs KT, Glendining KA, Marechal NM, Vergoz V, Nakamura I, Slessor KN, et al. Queen pheromone modulates brain dopamine function in worker honey bees. Proc Natl Acad Sci USA. 2007;104:2460–2464. doi: 10.1073/pnas.0608224104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergoz V, Schreurs HA, Mercer AR. Queen pheromone blocks aversive learning in young worker bees. Science. 2007;317:384–386. doi: 10.1126/science.1142448. [DOI] [PubMed] [Google Scholar]
- 15.van Zweden JS, Heinze J, Boomsma JJ, d'Ettorre P. Ant queen egg-marking signals: matching deceptive laboratory simplicity with natural complexity. PLoS One. 2009;4:4718. doi: 10.1371/journal.pone.0004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endler A, Liebig J, Hölldobler B. Queen fertility, egg marking and colony size in the ant Camponotus floridanus. Behav Ecol Sociobiol. 2006;59:1–10. [Google Scholar]
- 17.Heinze J, d'Ettorre P. Honest and dishonest communication in social Hymenoptera. J Exp Biol. 2009;212:1775–1779. doi: 10.1242/jeb.015008. [DOI] [PubMed] [Google Scholar]
- 18.van Zweden JS. The evolution of honest queen pheromones in insect societies. Commun Integr Biol. 2010;3:50–52. doi: 10.4161/cib.3.1.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Conte Y, Hefetz A. Primer pheromones in social hymenoptera. Annu Rev Entomol. 2008;53:523–542. doi: 10.1146/annurev.ento.52.110405.091434. [DOI] [PubMed] [Google Scholar]
- 20.Keller L, Nonacs P. The role of queen pheromones in social insects: queen control or queen signal? Anim Behav. 1993;45:787–794. [Google Scholar]
- 21.Maynard Smith J, Harper D. Animal Signals. Oxford University Press; 2003. [Google Scholar]
- 22.Khila A, Abouheif E. Reproductive constraint is a developmental mechanism that maintains social harmony in advanced ant societies. Proc Natl Acad Sci USA. 2008;105:17884–17889. doi: 10.1073/pnas.0807351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malka O, Katzav-Gozansky T, Hefetz A. Uncoupling fertility from fertility-associated pheromones in worker honeybees (Apis mellifera) J Insect Physiol. 2009;55:205–209. doi: 10.1016/j.jinsphys.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Cuvillier-Hot V, Lenoir A, Peeters C. Reproductive monopoly enforced by sterile police workers in a queenless ant. Behav Ecol. 2004;15:970–975. [Google Scholar]
- 25.Endler A, Liebig J, Schmitt T, Parker JE, Jones GR, Schreier P, et al. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc Natl Acad Sci USA. 2004;101:2945–2950. doi: 10.1073/pnas.0308447101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannonen M, Sledge MF, Turillazzi S, Sundström L. Queen reproduction, chemical signalling and worker behaviour in polygyne colonies of the ant Formica fusca. Anim Behav. 2002;64:477–485. [Google Scholar]
- 27.Dietemann V, Peeters C, Liebig J, Thivet V, Hölldobler B. Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc Nat Acad Sci USA. 2003;100:10341–10346. doi: 10.1073/pnas.1834281100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuvillier-Hot V, Cobb M, Malosse C, Peeters C. Sex, age and ovarian activity affect cuticular hydrocarbons in Diacamma ceylonense, a queenless ant. 2001;47:485–493. doi: 10.1016/s0022-1910(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 29.Heinze J, Stengl B, Sledge MF. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav Ecol Sociobiol. 2002;52:59–65. [Google Scholar]
- 30.Hartmann A, D'Ettorre P, Jones GR, Heinze J. Fertility signaling—the proximate mechanism of worker policing in a clonal ant. Naturwissenschaften. 2005;92:282–286. doi: 10.1007/s00114-005-0625-1. [DOI] [PubMed] [Google Scholar]
- 31.Weil T, Hoffmann K, Kroiss J, Strohm E, Korb J. Scent of a queen—cuticular hydrocarbons specific for female reproductives in lower termites. Naturwissenschaften. 2009;96:315–319. doi: 10.1007/s00114-008-0475-8. [DOI] [PubMed] [Google Scholar]
- 32.Bhadra A, Mitra A, Deshpande SA, Chandrasekhar K, Naik DG, Hefetz A, et al. Regulation of reproduction in the primitively eusocial wasp Ropalidia marginata: on the trail of the queen pheromone. J Chem Ecol. 2010;36:424–431. doi: 10.1007/s10886-010-9770-x. [DOI] [PubMed] [Google Scholar]