Abstract
Coral reefs are in dramatic global decline due to a host of local- and global-scale anthropogenic disturbances that suppress corals and enhance seaweeds. This decline is exacerbated, and recovery made less likely, due to over-fishing of herbivores that normally limit seaweed effects on corals. Seaweeds were known to suppress coral reproduction and recruitment, but in a recent study, we demonstrated that numerous seaweeds also directly poison corals via lipid-soluble allelochemicals transferred during contact. These allelopathic interactions may limit reef recovery once seaweeds proliferate and commonly contact remaining corals. Other recent studies suggest that seaweeds may also damage corals by enhancing coral disease or via release of water-soluble compounds that stimulate damaging microbes. For some of these mechanisms, cause versus effect is not yet clear. Here, we suggest that these different mechanisms are not mutually exclusive, may interact in context-dependent ways, but need to be assessed under ecologically realistic field conditions where flow may limit impacts of some mechanisms.
Key words: competition, coral-seaweed-herbivore interaction, coral-microbe interaction, marine chemical ecology, coral reef decline
Introduction and Context
Corals are foundation species providing the topographic complexity upon which other reef organisms depend. However, corals and thus reefs, are in rapid global decline due to climate-induced bleaching,1 coral disease,2 the cascading effects of overfishing,3,4 and numerous other stresses. In addition to these demonstrated impacts, ocean acidification looms as a large problem for reefs5 and may already be slowing calcification and coral growth.6 Regardless of the initial causes of coral decline, a phase shift from corals to seaweeds is common,7,8 and the associated interactions between seaweeds and remaining corals may produce negative feedbacks limiting reef recovery.9
Despite the potentially critical role of seaweed-coral interactions on reefs, it was uncertain whether seaweeds were a cause or simply a consequence of coral decline.9 Seaweeds may proliferate following coral death from bleaching or disease because much substrate becomes available for colonization and herbivores are unable to effectively suppress this additional seaweed growth.10,11 Alternatively, studies have experimentally demonstrated seaweed blooms and coral declines following exclusion of herbivores alone,12,13 or even lowering of herbivore diversity without changing herbivore mass or density.14 The importance of these small scale experiments is bolstered by large-scale observations of seaweed growth and coral decline when fishing or herbivore disease removes herbivores across large spatial scales.3,7 Thus it appears that seaweeds may profit from coral decline, but may also directly harm corals when not limited by herbivores; however, the mechanisms and outcomes of seaweed-coral interactions are poorly understood.
Our recent study15 offers the first evidence that common seaweeds in the Caribbean and Pacific damage corals using lipid-soluble allelochemicals transferred via contact. Thus, disturbances that enhance seaweeds may cause a cascade of coral loss due to a greater frequency of coral-seaweed contact and the resulting seaweed allelopathy against corals. These interactions will be especially detrimental to small remnant corals with a high ratio of seaweed contact per unit area. Because seaweeds also suppress coral fecundity and recruitment via chemically- and physically-mediated interactions,16–20 allelopathic interactions could play a critical role in reef decline via negative feedbacks that limit reef recovery.
Mechanisms of Seaweed-Coral Interactions
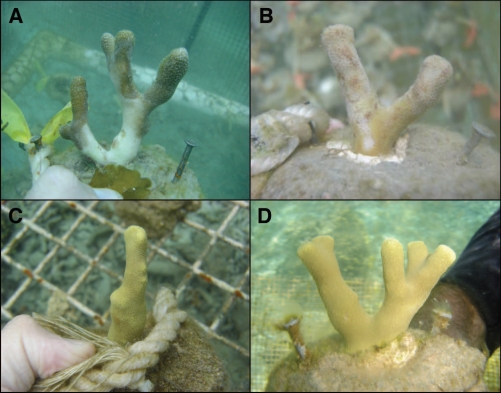
Our study15 demonstrates that seaweed allelopathy could commonly suppress corals. When corals in the genus Porites were in contact with seaweeds, those contacted by some seaweeds experienced bleaching, inhibited photosynthesis, and in some cases tissue death (Fig. 1A and B). We determined that several seaweeds produced surface-associated metabolites that caused these effects. In our study, physical effects of shading or abrasion from plastic or nylon seaweed mimics had no detectable effects and chemical effects occurred only in areas of direct contact; damage did not spread beyond these areas (Fig. 1), suggesting a direct role for surface metabolites and a limited role of microbial pathogens. Primacy of lipid-soluble compounds as allelopathic agents makes ecological sense in the sea, given the rapid advection of water-soluble compounds in high-flow environments.
Figure 1.
Bleaching of Porites corals after 20 d of contact with (A) Lobophora variegata in the Caribbean, (B) Chlorodesmis fastigiata in the tropical pacific, (C) a mimic designed to replicate the shading and abrasion of Chlorodesmis fastigiata and (D) a rope control only. Areas of corals affected were limited to areas of direct contact.
However, other studies demonstrate that seaweeds may impact corals via physical mechanisms such as shading or abrasion,20 via vectoring of coral disease,21 or via release of primary, water-soluble metabolites that stimulate damaging microbes.22–26 Therefore seaweeds may negatively impact corals via multiple mechanisms. Here, we suggest that these mechanisms need not be mutually exclusive, could interact to affect coral health in a context-dependent manner, but need to be evaluated under ecologically realistic field conditions.
Laboratory studies using still-water assays24,26 show that seaweed damage of corals is associated with microbial growth, low levels of O2 where seaweeds and corals are in near contact, and that antibiotics prevent coral death in these laboratory conditions. It is suggested that the water-soluble compounds leaking from seaweeds stimulate microbes that harm corals. The impact of antibiotics indicates a role for microbes in damaging corals, but much of the greater microbial growth and lower O2 could be a consequence rather than a cause of coral death and the greater flow typical of field conditions might limit the effect of water-soluble compounds produced by seaweeds. In our field experiments, we observed no near-contact effects or pathologies spreading beyond areas of direct contact; one would expect such effects if seaweeds were vectoring pathogens21 or stimulating damaging microbes via water-soluble metabolites.22–24 In our study, impacts of intact seaweeds could be replicated by treating corals with nonpolar metabolites from seaweed surfaces alone. Another field study also failed to detect effects of seaweeds when near corals (2–3 cm) and suggested that direct contact might be required.27 Disparities in experimental outcomes may result from the differing hydrodynamic environments of lab versus field studies; under field conditions, flow and turbulence might rapidly advect polar metabolites and select for chemical-mediation via lipophilic compounds deployed on contact. However, one can imagine some conditions (e.g., intertidal reef-flat pools at low tide) where polar metabolites from dense seaweeds could accumulate at high concentrations and affect corals. Because microbially-mediated effects of seaweeds on corals are often characterized by anoxia,24–25,28 and water flow impacts oxygen flux at micro-scales,29 indirect effects of seaweeds on microbe-coral interactions may be restricted to low-flow conditions.
Future Directions
Numerous common seaweeds chemically poison corals when in contact, even on turbulent, high-flow, reef flats.15 Knowledge of how these interactions vary among different seaweed-coral combinations and across life history stages of corals are needed to better understand how seaweeds may alter the trajectories of coral reefs during both periods of decline and recovery. Elucidation of the seaweed secondary metabolites responsible for these interactions will also benefit our knowledge of the proximate and ultimate factors shaping the function of seaweed metabolites.
Field tests of seaweed-microbe-coral interactions are needed to determine the hydrodynamic conditions under which these interactions can impact corals, and whether these effects occur alone or interact synergistically with allelopathic and physical effects of seaweeds on corals. The most parsimonious explanation for the patterns in our study is a direct allelopathic effect of seaweed non-polar metabolites, but we can not exclude the possibility that microbes played some role in the mode of action. Future research will benefit from deciphering whether altered microbial activity on corals in contact with seaweeds is a cause of coral mortality, the result of microbial decomposition of coral tissues following damage from allelochemical, physical effects of seaweeds, or a synergistic interaction of multiple mechanisms.
Currently, there are few field studies to suggest that seaweeds release primary metabolites (i.e., dissolved organic carbon (DOC)) at concentrations and scales capable of impacting coral-microbe interactions.15,23,25,27 Measurements of primary metabolite release from seaweed surfaces and transport rates across boundary layers will help to determine the scales and conditions under which seaweed primary metabolites have an effect on coral-microbe interactions. We observed no effects that one would expect from primary metabolites stimulating microbes, and others also have suggested that direct contact may be required for seaweed metabolites to affect corals.27 Moreover, there is evidence that seaweeds may serve as a reservoir and vector for coral disease;21 it is possible that direct contact not only vectors pathogens, but also chemically stresses corals, making them more susceptible to disease. It is increasingly clear that seaweeds directly damage living corals. Because of the few available studies, it is less clear whether seaweeds suppress corals primarily via allelopathy, disease transmission or mediation of microbe-coral interactions. It is likely that these mechanisms interact. A better understanding of the relative roles of these mechanisms in suppressing corals, the conditions under which each operates, when they function synergistically versus separately, and which herbivores best control the most damaging seaweeds should allow for better reef management and improved resilience of reefs following a host of present-day stresses.
Acknowledgements
This work was funded by the U.S. National Science Foundation (Grant No. 0929119) and U.S. National Institutes of Health's International Cooperative Biodiversity Groups program (Grant No. U01 TW007401).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12978
References
- 1.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 2.Harvell D, Jordan-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, et al. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography. 2007;20:172–195. [Google Scholar]
- 3.Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 4.Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 5.Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci. 2008;80:435–471. [Google Scholar]
- 6.De'ath G, Lough JM, Fabricius KE. Declining coral calcification on the Great Barrier Reef. Science. 2009;323:116–119. doi: 10.1126/science.1165283. [DOI] [PubMed] [Google Scholar]
- 7.Hughes TP. Catastrophes, phase-shifts and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 8.Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, et al. Regime shifts, resilience and biodiversity in ecosystem management. Annu Rev Ecol Syst. 2004;35:557–581. [Google Scholar]
- 9.Mumby PJ, Steneck RS. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol. 2008;23:555–563. doi: 10.1016/j.tree.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Aronson RB, Precht WF. Conservation, precaution and Caribbean reefs. Coral Reefs. 2006;25:441–450. [Google Scholar]
- 11.Mumby PJ. Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs. 2009;28:761–773. [Google Scholar]
- 12.Lewis SM. The role of herbivorous fishes in the organization of a Caribbean reef community. Ecol Monogr. 1986;56:183–200. [Google Scholar]
- 13.Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, et al. Phase shifts, herbivory and the resilience of coral reefs to climate change. Curr Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Burkepile DE, Hay ME. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci USA. 2008;105:16201–16206. doi: 10.1073/pnas.0801946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasher DB, Hay ME. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc Natl Acad Sci USA. 2010;107:9683–9688. doi: 10.1073/pnas.0912095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol-Prog Ser. 2006;323:107–117. [Google Scholar]
- 17.Foster NL, Box SJ, Mumby PJ. Competitive effects of macroalgae on the fecundity of the reef-building coral Montastraea annularis. Mar Ecol-Prog Ser. 2008;367:143–152. [Google Scholar]
- 18.Birrell CL, McCook LJ, Willis BL, Harrington L. Chemical effects of macroalgae on larval settlement of the broadcast spawning coral Acropora millepora. Mar Ecol-Prog Ser. 2008;362:129–137. [Google Scholar]
- 19.Diaz-Pulido G, Harii S, McCook LJ, Hoegh-Guldberg O. The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs. 2010;29:203–208. [Google Scholar]
- 20.Box SJ, Mumby PJ. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol-Prog Ser. 2007;342:139–149. [Google Scholar]
- 21.Nugues MM, Smith GW, Hooidonk RJ, Seabra MI, Bak RPM. Algal contact as a trigger for coral disease. Ecol Lett. 2004;7:919–923. [Google Scholar]
- 22.Kuntz NM, Kline DI, Sandin SA, Rohwer F. Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Mar Ecol-Prog Ser. 2005;294:173–180. [Google Scholar]
- 23.Kline DI, Kuntz NM, Breitbart M, Knowlton N, Rohwer F. Role of elevated organic carbon levels and microbial activity in coral mortality. Mar Ecol-Prog Ser. 2006;314:119–125. [Google Scholar]
- 24.Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, et al. Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol Lett. 2006;9:835–845. doi: 10.1111/j.1461-0248.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- 25.Haas A, Al-Zibdah M, Wild C. Effect of inorganic and organic nutrient addition on coral-algae assemblages from the northern Red Sea. J Exp Mar Biol Ecol. 2009;380:99–105. [Google Scholar]
- 26.Vermeij MJA, Smith JE, Smith CM, Thurber RV, Sandin SA. Survival and settlement success of coral planulae: independent and synergistic effects of macroalgae and microbes. Oecologia. 2009;159:325–336. doi: 10.1007/s00442-008-1223-7. [DOI] [PubMed] [Google Scholar]
- 27.Vu I, Smelick G, Harris S, Lee SC, Weil E, Whitehead RF, et al. Macroalgae has no effect on the severity and dynamics of Caribbean Yellow Band disease. PLoS ONE. 2009;4:e4514. doi: 10.1371/journal.pone.0004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barott K, Smith J, Dinsdale E, Hatay M, Sandin S, Rohwer F. Hyperspectral and physiological analyses of coral-algal interactions. PLoS ONE. 2009;4:e8043. doi: 10.1371/journal.pone.0008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mass T, Genin A, Shavit U, Grinstein M, Tchernov D. Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proc Natl Acad Sci USA. 2010;107:2527–2531. doi: 10.1073/pnas.0912348107. [DOI] [PMC free article] [PubMed] [Google Scholar]