Abstract
Plant chemicals mediating interactions with insect herbivores seem a likely target for manipulation by insectvectored plant pathogens. Yet, little is currently known about the chemical ecology of insect-vectored diseases or their effects on the ecology of vector and nonvector insects. We recently reported that a widespread plant pathogen, Cucumber mosaic virus (CMV), greatly reduces the quality of host-plants (squash) for aphid vectors, but that aphids are nevertheless attracted to the odors of infected plants—which exhibit elevated emissions of a volatile blend otherwise similar to the odor of healthy plants. This finding suggests that exaggerating existing host-location cues can be a viable vector attraction strategy for pathogens that otherwise reduce host quality for vectors. Here we report additional data regarding the effects of CMV infection on plant interactions with a common nonvector herbivore, the squash bug, Anasa tristis, which is a pest in this system. We found that adult A. tristis females preferred to oviposit on healthy plants in the field, and that healthy plants supported higher populations of nymphs. Collectively, our recent findings suggest that CMV-induced changes in host plant chemistry influence the behavior of both vector and non-vector herbivores, with significant implications both for disease spread and for broader community-level interactions.
Key words: vector behavior, Cucumber mosaic virus, volatiles, parasite manipulation, Anasa tristis, Cucurbita pepo
Because the transmission dynamics of insect-vectored diseases are determined by complex interactions among pathogens, hosts and vectors,1 the elucidation of these interactions has potentially important implications for human health and agriculture.2,3 A number of recent studies have shown that vector-borne pathogens can induce chemical and physical changes in their primary hosts that affect interactions between hosts and vectors, with significant implications for disease transmission.2–7 More generally, pathogen effects on host phenotypes have been shown to have broader implications for ecology and ecosystem function—for example by affecting interactions between hosts and other (vector or non-vector) organisms in ways that can modify food webs, alter the outcome of competitive interactions and influence flows of energy and nutrients.3,8 In plant systems, only a handful of studies have explored the effects of pathogens on chemically mediated interactions between plants and insects,5,9–13 in contrast to the extensive literature that has examined the effects of herbivore-induced changes in plant chemistry on plant-herbivore-natural enemy interactions.14,15
Recently, we began investigating the effects of a widespread plant pathogen, CMV, on the plant chemistry and chemically mediated ecology of common squash (Cucurbita pepo), a frequent host for CMV that also interacts with a variety of aphid vectors and other non-vector insects (Fig. 1).
Figure 1.

Components of the study system: (A) CMV-infected squash, (B) Healthy squash, (C) Wingless aphid morph (Myzus persicae), (D) Winged aphid morph (M. persicae), (E) Squash bug eggs (Anasa tristis), (F) Various instars of squash bug nymphs (A. tristis).
CMV is a widespread and economically important plant antagonist16,17 belonging to a class of pathogens—nonpersistent viruses—that are particularly difficult to control because they are rapidly transmitted from infected to healthy hosts through brief probes of epidermal plant tissue by aphid vectors.18,19 This transmission mechanism contrasts with that of persistent viruses, which require sustained aphid feeding in the phloem of infected plants for acquisition and are transmitted when an infected aphid again feeds on a susceptible host.20,21 The few previous studies that examined effects of plant viruses on host chemistry and vector behavior focused on persistently transmitted viruses (e.g., Potato leaf roll virus and Barley yellow dwarf virus, two serious agricultural pests) and found a tendency toward enhanced quality of infected plants for aphid vectors—apparently leading to preferential aphid colonization and feeding on infected plants, rapid population growth, and the eventual dispersal of infected aphids to new, healthy, hosts.10,22,23 Consistent with the enhanced quality of infected plants for aphids, previous studies also found that aphids were attracted to the distinctive odors of virus-infected plants.5,9,11–13
In contrast to these previous studies on persistent viruses, our results revealed a very different pattern of virus-induced changes in plant phenotype, and one more favorable to CMV's non-persistent mode of transmission.24 We found that CMV greatly reduced plant quality for aphids: aphid population growth was dramatically reduced on CMV-infected plants, and aphids quickly dispersed from CMV-infected plants, which also rarely sustained aphid colonies in the field. Nevertheless, aphids were preferentially attracted to the odors of CMV-infected plants compared to those of healthy plants, likely because infected plants exhibit greatly elevated emissions of a volatile blend that is otherwise similar to the odor of healthy plants—in contrast to previous findings for persistent viruses, which induced characteristic odor profiles.5,9,13 Thus, CMV-infected plants may be attractive to aphid vectors because they mimic the odors of large, healthy plants.
While too few systems have been explored to draw broad conclusions, the contrast between our findings for CMV and those previously reported for persistent viruses suggest that transmission mechanism may be an important factor shaping pathogen effects on host plant phenotypes that influence interactions with insect vectors. Although the effects of CMV on host plant chemistry appear to be quite different from those previously reported for persistent viruses, the overall pattern appears conducive to transmission in both cases. Persistent viruses require sustained aphid feeding for transmission and appear to induce plant phenotypes that attract and then arrest aphids, while CMV, which like other non-persistent viruses benefits from frequent aphid movement among plants, induces a phenotype that attracts aphids then causes rapid dispersal. The potential generality of this pattern is supported by work on other non-persistent viruses demonstrating similar reductions in plant quality for aphid vectors.25,26
In addition to the implications for disease transmission discussed above, pathogen-induced changes in plant phenotypes can have important impacts on ecological interactions with other, non-vector insects. For example, infection can alter plant nutritional quality and defense status (e.g., levels of secondary compounds or the induction of defense-related phytohormones), which can influence the distribution and abundance of herbivores feeding on a given plant species.27,28 A few studies have documented such effects—for instance, Colorado potato beetles had increased survival on tomato plants infected with Tobacco mosaic virus,29 while local infection of tobacco by the same virus decreased weight gain of tobacco hornworm caterpillars on systemic leaves.30 However, such interactions have rarely been examined under field conditions. Expanding upon our findings regarding the effects of CMV on interactions between squash plants and aphid vectors, we conducted a field study exploring the effects of CMV infection on plant interactions with a common nonvector herbivore, the squash bug (Anasa tristis; Fig. 1E and F).
Squash bugs are important agricultural pests that feed exclusively on plants in the Cucurbitaceae family, and our study plant (C. pepo) is a preferred host. Throughout the growing season, we tracked colonization of plants by observing egg-laying by A. tristis females and feeding by nymphs on healthy and infected plants. We observed that adult squash bugs laid eggs on healthy squash plants in preference to infected plants (Fig. 2). As a consequence of this preference there were fewer nymphs on infected squash plants once eggs began hatching (Fig. 3). Although we did not directly assess developmental rates or survival of nymphs on infected and healthy plants, the clear oviposition preference for healthy plants exhibited by A. tristis females suggests that the negative impacts of CMV infection on host plant quality for two aphid species25 may also apply to squash bugs, which feed on the sap inside plant cells. Conversely, it provides further evidence that the previously documented effects on host plant phenotypes are consistently expressed under field conditions. Our current data do not allow us to determine whether A. tristis females, like aphids, are attracted to the elevated volatile emissions of infected plants and then reject them on the basis of contact/gustatory cues, or if ovipositing females are able to discriminate against infected plants at a distance. This study does, however, demonstrate that the effects of virus infection on host plant phenotypes—which may be shaped by their implications for transmission by insect vectors—can also have significant impacts on plant interactions with nonvector herbivores, with implications for community structure and dynamics.
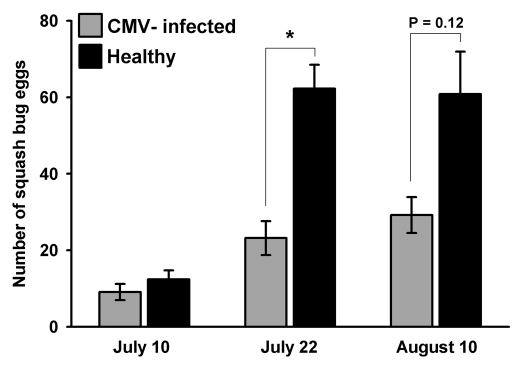
Figure 2.
Mean number of squash bug eggs on CMV-infected and healthy squash in field plots over the egg-laying period of over-wintered squash bug adults. Analysis by Kruskall-Wallis test indicates that healthy plants had significantly more eggs on July 22nd (H = 19.63 DF = 1 p = 0.000) and a similar trend persisted on August 10th (H = 2.40 DF = 1 p = 0.121).
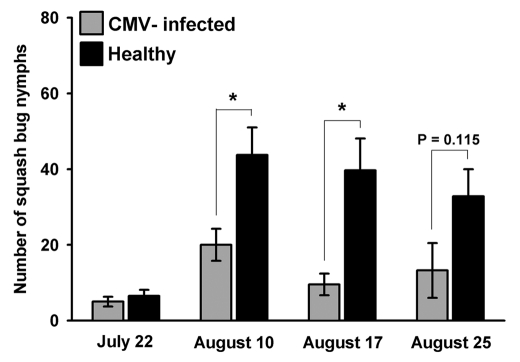
Figure 3.
Mean number of squash bug nymphs on CMV-infected and healthy squash plants during the hatching period. (Most nymphs hatched after July 22nd). Analysis by Kruskall-Wallis test indicates that healthy plants harbored significantly more nymphs on both August 10th (H = 10.90 DF = 1 p = 0.001) and August 17th (H = 9.53 DF = 1 p = 0.002), with a similar trend persisting near the end of the season, on August 25th (August 25: H = 2.49 DF = 1 p = 0.115).
Our recent findings, and those reported here, suggest that transmission mechanism is a major factor shaping the effects of plant viruses (and potentially other pathogens) on host plant phenotypes, and that these effects can have significant impacts on plant interactions with both vector and non-vector insects. Future work in this system will focus on elucidating the mechanisms underlying the observed effects of CMV on host plant quality for insect herbivores and further exploring the consequences of these effects for community- and landscape-level ecological interactions.
Acknowledgements
The pathogen CMV-FNY was kindly provided by Dr. John Murphy (Auburn University). We thank F.E. Gildow for assistance in developing the inoculation protocols; and J.C. Saunders and E. Smyers for technical assistance. Funding for this research was provided by a grant (2008-35302-04577) from the U.S. Department of Agriculture's National Research Initiative and by a Doctoral Dissertation Improvement Grant (DEB- 1011122) from the National Science Foundation.
Abbreviations
- CMV
Cucumber mosaic virus
- A. tristis
Anasa tristis
- C. pepo
Cucurbita pepo
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/13094
References
- 1.Thresh JM, Irwin ME. Epidemiology of barley yellow dwarf: a study in ecological complexity. Annu Rev Phytopathol. 1990;28:393–424. [Google Scholar]
- 2.Hurd H. Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol. 2003;48:141–161. doi: 10.1146/annurev.ento.48.091801.112722. [DOI] [PubMed] [Google Scholar]
- 3.Lefévre T, Thomas F. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infection, Genetics Evol. 2008;8:504–519. doi: 10.1016/j.meegid.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Roy BA, Raguso RA. Olfactory versus visual cues in a floral mimicry system. Oecologia. 1997;109:414–426. doi: 10.1007/s004420050101. [DOI] [PubMed] [Google Scholar]
- 5.Eigenbrode SD, Ding H, Shiel P, Berger PH. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera; Aphididae) Proc R Soc Lond B. 2002;269:455–460. doi: 10.1098/rspb.2001.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belliure B, Janssen A, Maris PC, Peters D, Sabelis MW. Herbivore arthropods benefit from vectoring plant viruses. Ecol Lett. 2005;8:70–79. [Google Scholar]
- 7.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3:298. doi: 10.1371/journal.pbio.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee MH. Parasites alter community structure. Proc Natl Acad Sci USA. 2007;104:9335–9339. doi: 10.1073/pnas.0700062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiménez-Martínez ES, Bosque-Pérez NA, Berger PH, Zemetra RS, Ding H, Eigenbrode SD. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley yellow dwarf virus-infected transgenic and untransformed wheat. Environ Entomol. 2004;33:1207–1216. [Google Scholar]
- 10.Jiménez-Martínez ES, Bosque-Pérez NA, Berger PH, Zemetra RS. Life history of the bird cherry-oat aphid, Rhopalosipum padi (Homoptera: Aphididae), on transgenic and untransformed wheat challenged with Barley yellow dwarf virus. J Econ Entomol. 2004;97:203–12. doi: 10.1093/jee/97.2.203. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan R, Alvarez JM, Eigenbrode SD, Bosque-Pérez NA. Influence of hairy nightshade Solanum sarrachoides (Sendtner) and Potato leafroll virus (Luteoviridae: Polerovirus) on the host preference of Myzus periscae (Sulzer) (Homoptera: Aphididae) Environ Entomol. 2006;35:46–53. doi: 10.1603/0046-225x(2008)37[592:eoaawh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez AE, Garzo E, Verbeek M, Vosman B, Dicke M, Tjallingii WF. Infection of potato plants with potato leafroll virus changes attraction and feeding behaviour of Myzus persicae. Entomol Exp Appl. 2007;125:135–144. [Google Scholar]
- 13.Ngumbi E, Eigenbrode SD, Bosque-Pérez NA, Ding H, Rodriguez A. Myzus persicae is arrested more by blends than by individual compounds elevated in headspace of PLRV-infected potato. J Chem Ecol. 2007;33:1733–1747. doi: 10.1007/s10886-007-9340-z. [DOI] [PubMed] [Google Scholar]
- 14.Dicke M, van Loon JJA. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl. 2000;97:237–249. [Google Scholar]
- 15.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 16.Gallitelli D. The ecology of Cucumber mosaic virus and sustainable agriculture. Virus Research. 2000;71:9–21. doi: 10.1016/s0168-1702(00)00184-2. [DOI] [PubMed] [Google Scholar]
- 17.Roossinck MJ. Cucumber mosaic virus, a model for RNA virus evolution. Molecular Plant Pathology. 2001;2:59–63. doi: 10.1046/j.1364-3703.2001.00058.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin B, Collar JL, Tjallingii WF, Fereres A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J Gen Virol. 1997;78:2701–2705. doi: 10.1099/0022-1317-78-10-2701. [DOI] [PubMed] [Google Scholar]
- 19.Wang RY, Ghabrial SA. Effect of aphid behavior on efficiency of transmission of Soybean mosaic virus by the soybean colonizing aphid, Aphis glycines. Plant Disease. 2002;86:1260–1264. doi: 10.1094/PDIS.2002.86.11.1260. [DOI] [PubMed] [Google Scholar]
- 20.Gildow FE, Rochow WF. Role of accessory salivary glands in aphid transmission of Barley yellow dwarf virus. Virology. 1980;104:97–108. doi: 10.1016/0042-6822(80)90368-2. [DOI] [PubMed] [Google Scholar]
- 21.Sylvester ES. Circulative and propagative virus transmission by aphids. Ann Rev Entomol. 1980;25:257–286. [Google Scholar]
- 22.Montllor CB, Gildow FE. Feeding responses of two grain aphids to barley yellow dwarf virus-infected oats. Entomol Exp Appl. 1980;42:63–69. [Google Scholar]
- 23.Castle SJ, Berger PH. Rates of growth and increase of Myzus persicae on virus-infected potatoes according to type of virus-vector relationship. Entomol Exp Appl. 1993;69:51–60. [Google Scholar]
- 24.Mauck KE, De Moraes CM, Mescher MC. Deceptive chemical signals attract insect vectors to inferior hosts. Proc Natl Acad Sci USA. 2010;107:3600–3605. doi: 10.1073/pnas.0907191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodge S, Powell G. Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance? Environ Entomol. 2008;37:1573–1581. doi: 10.1603/0046-225x-37.6.1573. [DOI] [PubMed] [Google Scholar]
- 26.Donaldson JR, Gratton C. Antagonistic effects of soybean viruses on soybean aphid performance. Environ Entomol. 2007;36:918–925. doi: 10.1603/0046-225x(2007)36[918:aeosvo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Culver JN, Padmanabhan MS. Virus-induced disease: altering host physiology one interaction at a time. Annu Rev Phytopathol. 2007;45:221–243. doi: 10.1146/annurev.phyto.45.062806.094422. [DOI] [PubMed] [Google Scholar]
- 28.Stout MJ, Thaler JS, Thomma BPHJ. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu Rev Entomol. 2006;51:663–689. doi: 10.1146/annurev.ento.51.110104.151117. [DOI] [PubMed] [Google Scholar]
- 29.Hare D, Dodds JA. Survival of the Colorado potato beetle on virus-infected tomato in relation to plant nitrogen and alkaloid content. Entomol Exp Appl. 1987;44:31–35. [Google Scholar]
- 30.Ajlan AM, Potter DA. Lack of effect of Tobacco mosaic virus-induced systemic acquired resistance on arthropod herbivores in tobacco. Dis Contr Pest Manag. 1992;82:647–651. [Google Scholar]