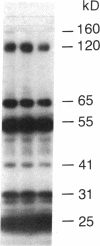
Abstract
All HIV seronegative (HIV Ab-) and most HIV seropositive (HIV Ab+) individuals' lymphocytes failed to proliferate in primary cultures in response to purified HIV or to recombinant envelope and core antigens of HIV, even in the presence of recombinant interleukin 2 (rIL-2). Most HIV Ab- and HIV Ab+ individuals' lymphocytes, however, could proliferate or be induced by rIL-2 to proliferate in response to lysates of Escherichia coli or Saccharomyces cerevisiae. These findings indicate selective defects in lymphocyte proliferative responses to HIV antigens before the development of AIDS in which lymphocytes are unable to proliferate in response to any antigens. These defects in cell-mediated immune responses to HIV antigens are likely to play an important role in the pathobiology of HIV infections. Although intact HIV or glycosylated gp120 envelope protein of HIV are involved in these defects, a non-glycosylated recombinant form of the HIV gp120 envelope (ENV2-3) and p25 core proteins did not inhibit antigen- or mitogen-driven lymphocyte proliferation.
Full text
PDF
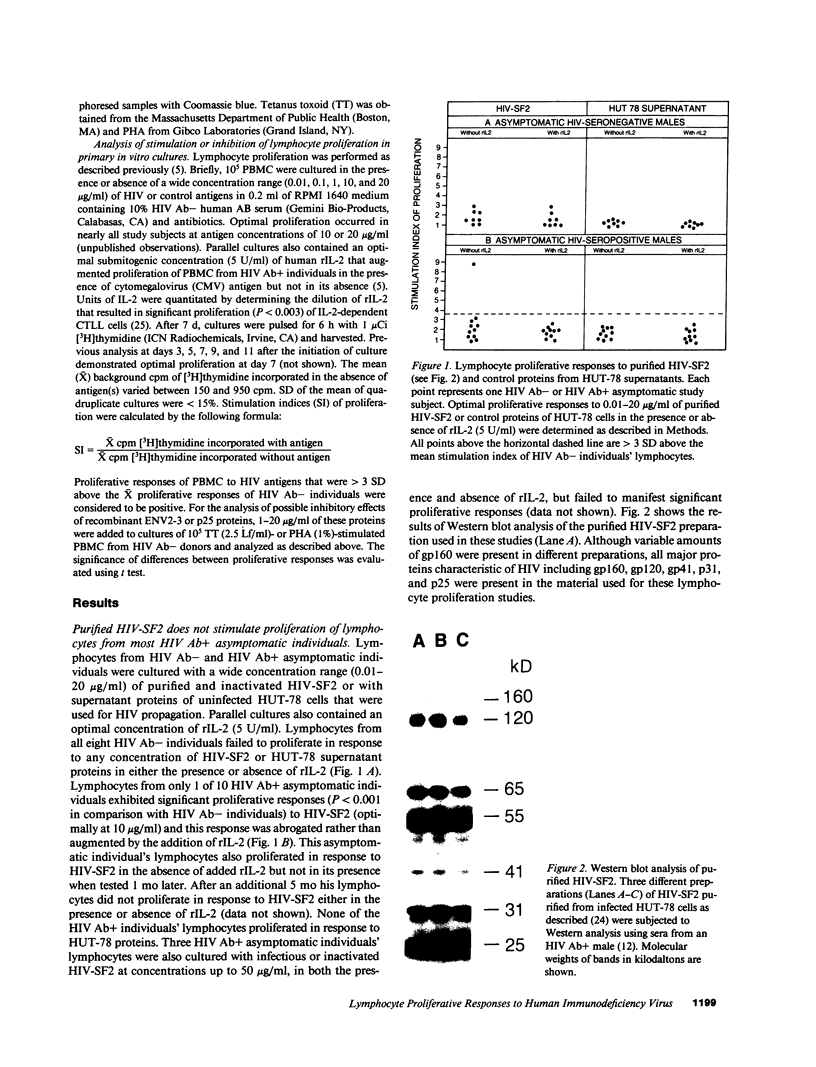
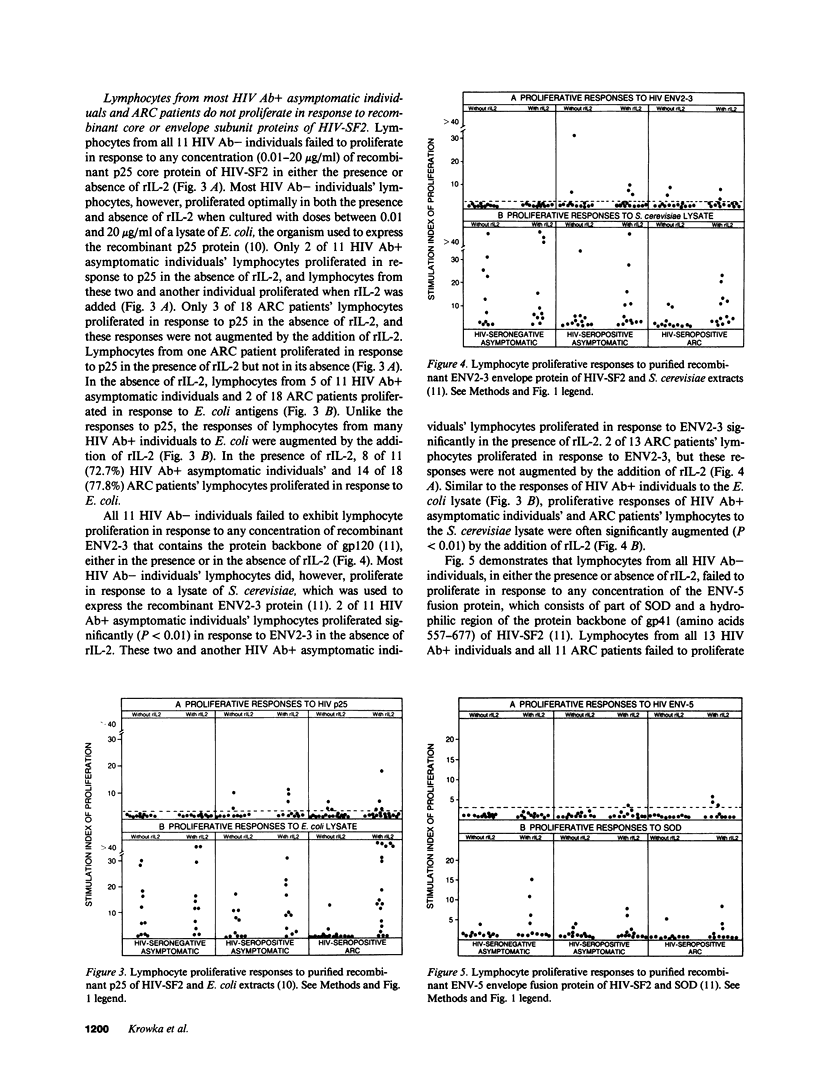



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr P. J., Steimer K. S., Sabin E. A., Parkes D., George-Nascimento C., Stephans J. C., Powers M. A., Gyenes A., Van Nest G. A., Miller E. T. Antigenicity and immunogenicity of domains of the human immunodeficiency virus (HIV) envelope polypeptide expressed in the yeast Saccharomyces cerevisiae. Vaccine. 1987 Jun;5(2):90–101. doi: 10.1016/0264-410x(87)90053-3. [DOI] [PubMed] [Google Scholar]
- Carlson J. R., Bryant M. L., Hinrichs S. H., Yamamoto J. K., Levy N. B., Yee J., Higgins J., Levine A. M., Holland P., Gardner M. B. AIDS serology testing in low- and high-risk groups. JAMA. 1985 Jun 21;253(23):3405–3408. [PubMed] [Google Scholar]
- Chanh T. C., Kennedy R. C., Kanda P. Synthetic peptides homologous to HIV transmembrane glycoprotein suppress normal human lymphocyte blastogenic response. Cell Immunol. 1988 Jan;111(1):77–86. doi: 10.1016/0008-8749(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Gluckman J. C., Klatzmann D., Cavaille-Coll M., Brisson E., Messiah A., Lachiver D., Rozenbaum W. Is there correlation of T cell proliferative functions and surface marker phenotypes in patients with acquired immune deficiency syndrome or lymphadenopathy syndrome? Clin Exp Immunol. 1985 Apr;60(1):8–16. [PMC free article] [PubMed] [Google Scholar]
- Kaminsky L. S., McHugh T., Stites D., Volberding P., Henle G., Henle W., Levy J. A. High prevalence of antibodies to acquired immune deficiency syndrome (AIDS)-associated retrovirus (ARV) in AIDS and related conditions but not in other disease states. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5535–5539. doi: 10.1073/pnas.82.16.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Krowka J., Stites D., Mills J., Hollander H., McHugh T., Busch M., Wilhelm L., Blackwood L. Effects of interleukin 2 and large envelope glycoprotein (gp 120) of human immunodeficiency virus (HIV) on lymphocyte proliferative responses to cytomegalovirus. Clin Exp Immunol. 1988 May;72(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Woody J. N., Hartzman R. J., Eckels D. D. In vitro influenza virus-specific antibody production in man: antigen-specific and HLA-restricted induction of helper activity mediated by cloned human T lymphocytes. J Immunol. 1982 Oct;129(4):1465–1470. [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Groopman J. E., Fennie C. W., Benz P. M., Capon D. J., Dowbenko D. J., Nakamura G. R., Nunes W. M., Renz M. E., Berman P. W. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science. 1986 Jul 11;233(4760):209–212. doi: 10.1126/science.3014647. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- McGrath M., Witte O., Pincus T., Weissman I. L. Retrovirus purification: method that conserves envelope glycoprotein and maximizes infectivity. J Virol. 1978 Mar;25(3):923–927. doi: 10.1128/jvi.25.3.923-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A. R., Bacchetti P., Osmond D., Krampf W., Chaisson R. E., Stites D., Wilber J., Allain J. P., Carlson J. Seropositivity for HIV and the development of AIDS or AIDS related condition: three year follow up of the San Francisco General Hospital cohort. Br Med J (Clin Res Ed) 1988 Mar 12;296(6624):745–750. doi: 10.1136/bmj.296.6624.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Nishanian P., Keith D. E., Jr, Houghton R. L., Heitjan D. F., Fahey J. L., Giorgi J. V. Antibodies to human immunodeficiency virus in human sera induce cell-mediated lysis of human immunodeficiency virus-infected cells. J Immunol. 1987 Oct 1;139(7):2458–2463. [PubMed] [Google Scholar]
- Petit A. J., Tersmette M., Terpstra F. G., de Goede R. E., van Lier R. A., Miedema F. Decreased accessory cell function by human monocytic cells after infection with HIV. J Immunol. 1988 Mar 1;140(5):1485–1489. [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Shannon K., Cowan M. J., Ball E., Abrams D., Volberding P., Ammann A. J. Impaired mononuclear-cell proliferation in patients with the acquired immune deficiency syndrome results from abnormalities of both T lymphocytes and adherent mononuclear cells. J Clin Immunol. 1985 Jul;5(4):239–245. doi: 10.1007/BF00929458. [DOI] [PubMed] [Google Scholar]
- Steimer K. S., Puma J. P., Power M. D., Powers M. A., George-Nascimento C., Stephans J. C., Levy J. A., Sanchez-Pescador R., Luciw P. A., Barr P. J. Differential antibody responses of individuals infected with AIDS-associated retroviruses surveyed using the viral core antigen p25gag expressed in bacteria. Virology. 1986 Apr 15;150(1):283–290. doi: 10.1016/0042-6822(86)90289-8. [DOI] [PubMed] [Google Scholar]
- Tong-Starksen S. E., Luciw P. A., Peterlin B. M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren B., Morfeldt-Månsson L., Biberfeld G., Moberg L., Ljungman P., Nordlund S., Bredberg-Rådén U., Werner A., Löwer J., Kurth R. Impaired specific cellular response to HTLV-III before other immune defects in patients with HTLV-III infection. N Engl J Med. 1986 Aug 7;315(6):393–394. [PubMed] [Google Scholar]
- Wahren B., Morfeldt-Månsson L., Biberfeld G., Moberg L., Sönnerborg A., Ljungman P., Werner A., Kurth R., Gallo R., Bolognesi D. Characteristics of the specific cell-mediated immune response in human immunodeficiency virus infection. J Virol. 1987 Jun;61(6):2017–2023. doi: 10.1128/jvi.61.6.2017-2023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Paradis T. J., Fuller T. C., Hirsch M. S., Schooley R. T., Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988 Apr 1;240(4848):64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- Weinhold K. J., Lyerly H. K., Matthews T. J., Tyler D. S., Ahearne P. M., Stine K. C., Langlois A. J., Durack D. T., Bolognesi D. P. Cellular anti-GP120 cytolytic reactivities in HIV-1 seropositive individuals. Lancet. 1988 Apr 23;1(8591):902–905. doi: 10.1016/s0140-6736(88)91713-8. [DOI] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L. The recovery of mice from influenza virus infection: adoptive transfer of immunity with immune T lymphocytes. Scand J Immunol. 1978;7(5):389–397. doi: 10.1111/j.1365-3083.1978.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Cheynier R., Desportes I., Leonard R., Fouchard M., Reveil B., Ittele D., Lurhuma Z., Mbayo K. A group specific anamnestic immune reaction against HIV-1 induced by a candidate vaccine against AIDS. Nature. 1988 Apr 21;332(6166):728–731. doi: 10.1038/332728a0. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Eichberg J. W., Moran P. A., McClure J., Sridhar P., Hu S. L. Proliferative and cytotoxic T cells to AIDS virus glycoproteins in chimpanzees immunized with a recombinant vaccinia virus expressing AIDS virus envelope glycoproteins. J Immunol. 1987 Aug 15;139(4):988–990. [PubMed] [Google Scholar]