Abstract
Upon starving Saccharomyces cerevisiae of glucose, the key gluconeogenic enzymes fructose-1,6-bisphosphatase (FBPase), malate dehydrogenase (MDH2), isocitrate lyase (Icl1p) and phosphoenolpyruvate carboxykinase (Pck1p) are induced. When glucose is added to cells that have been starved for 3 days, these gluconeogenic enzymes are degraded in the vacuole via the vacuole import and degradation (Vid) pathway. Moreover, it has been determined that during glucose starvation, these cargo proteins interact with the target of rapamycin complex 1 (TORC1), which is comprised of Tor1p, Tco89p, Lst8p and Kog1p. However, following glucose replenishment, Tor1p dissociates from the cargo proteins. We have determined that cells overexpressing TOR1 inhibited the phosphorylation of FBPase and its subsequent degradation in the vacuole. Interestingly, while the deletion of TCO89 inhibited FBPase degradation, it did not inhibit the phosphorylation of FBPase. Both Tor1p and Tco89p were found in endosomes originating from the plasma membrane as well as in retrograde vesicles forming from the vacuole membrane. Here we further discuss our findings and elaborate on our current model of the Vid pathway.
Key words: fructose-1,6-bisphosphatase; proteasome; vacuole; vacuole import and degradation; autophagy; target of rapamycin complex 1
Autophagy is a catabolic process by which a cell degrades its own components through the lysosome/vacuole.1 This is vital for many biological processes including cell growth, development and cell survival during stress.1–3 Moreover, deregulated autophagy has been associated with diseases such as cancer, neurodegeneration and aging.3 This catabolic process is conserved from yeast to humans and is induced in Saccharomyces cerevisiae that have been starved of nitrogen.4 The induced autophagic pathway recycles amino acids during periods of starvation.4,5 This pathway is controlled by ATG genes which are also involved in the Cvt pathway that targets aminopeptidase I from the cytosol to the vacuole and the pexophagy pathway that degrades peroxisomes.6–8 The autophagy pathway is inhibited by Tor1p and induced by rapamycin even in the absence of nitrogen starvation.9–11
We study a unique autophagy pathway in Saccharomyces cerevisiae that delivers specific cytosolic proteins to the vacuole for degradation.12–15 When yeast cells are starved of glucose, key gluconeogenic enzymes such as fructose-1,6-bisphosphatase (FBPase), malate dehydrogenase (MDH2), isocitrate lyase (Icl1p) and phosphoenolpyruvate carboxykinase (Pck1p) are induced. However, when glucose is added to the starved cells, these enzymes are inactivated and targeted for degradation to either the proteasome or the vacuole.16 The site of degradation of these gluconeogenic enzymes is dependent on the duration of starvation. For instance, when glucose is added to cells that have been starved for one day, these enzymes are degraded in the proteasome. However, when glucose is added to cells that are starved for three days, these gluconeogenic enzymes are degraded in the vacuole.17,18
The vacuolar dependent pathway utilizes specialized vesicles identified as Vid vesicles and it is in these vesicles that FBPase is sequestered.19 This sequestration requires the heat shock protein Ssa2p,20 cyclophilin A21 and Vid22p.22 The formation of Vid vesicles is blocked in cells lacking the UBC1 gene, indicative of a role in Vid vesicle formation.23 Vid24p is a peripheral protein on Vid vesicles and has been used to study the trafficking of Vid vesicles in response to glucose.24 Recent evidence suggests that the Vid pathway converges with the endocytic pathway.25 COPI coatomer proteins are also present on Vid vesicles and they recruit Vid24p to Vid vesicles. Moreover, the coatomer subunit Sec28p trafficks to endosomes when glucose is added to three day starved wild type cells.25
In order to facilitate a better understanding of the Vid pathway, it was necessary to determine how cells recognized proteins that are targeted for degradation. In this endeavor, we sought to identify cellular proteins that interacted with FBPase. Affinity chromatography was used to purify FBPase interacting proteins under established growth conditions. The bound material was subjected to MALDI analysis. This enabled us in identifying Tco89p among other cellular protein candidates. Tco89p is a member of the target of rapamycin complex 1 (TORC1) which also contains Tor1p, Kog1p and Lst8p.26
We determined that TORC1 interacted with FBPase, MDH2, Icl1p and Pck1p during glucose starvation. By kinetic analysis, it was ascertained that Tor1p was dissociated from these gluconeogenic enzymes after the addition of glucose. This suggests that Tor1p association may inhibit cargo protein degradation. This was confirmed by observing that cells overexpressing the TOR1 gene delayed FBPase degradation. However, deletion of TOR1 had little effect on FBPase degradation. On the other hand, cells lacking the TCO89 gene exhibited defective FBPase degradation by inhibiting targeting FBPase to the Vid vesicles. Cells lacking either the TOR1 gene or the TCO89 gene resulted in an increase in FBPase phosphorylation in response to glucose. Interestingly, overexpressing TOR1 inhibited FBPase phosphorylation in response to glucose. Therefore, excessive Tor1p served to inhibit FBPase phosphorylation.26
Upon examining the distribution of Tco89p and Tor1p, it was observed that both proteins were detected on endosomes forming from the plasma membrane. In addition, these proteins were also detected on vesicles emerging from the vacuole membrane. We have termed such vesicles as retrograde vesicles and the above results support our previous findings where retrograde vesicles containing Sec28p could form from the vacuole membrane.25 As such, we propose that TORC1 cycles between the plasma membrane and the vacuole. This serves to maintain the size of the vacuole. Endocytosis results in an increased influx and an expansion of the vacuole membrane. To balance this, an increased efflux must take effect to maintain the size of the vacuole. Thus, the retrograde transport provides a mechanism for maintaining the size of the vacuole. Furthermore, inhibiting the retrograde transport may also affect the anterograde transport.26
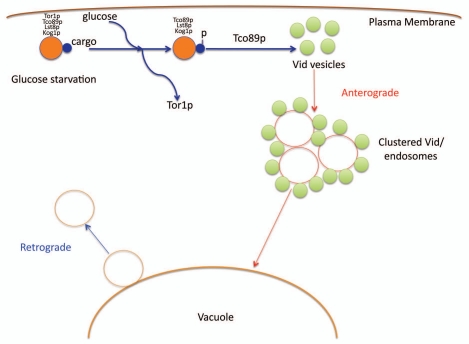
Based on our recent findings, we propose the following model (Fig. 1). During glucose starvation, cargo proteins destined for the Vid pathway associate with the TORC1 complex. After the addition of glucose, Tor1p dissociates from this complex. This facilitates the cargo proteins to be phosphorylated. Thereafter, Tco89p functions to import the cargo proteins into the Vid vesicles. As such, our present data provides the first evidence of TORC1 as a cellular factor for cargo interaction in the Vid pathway. Our experimental data further highlight the capacity of Tor1p and Tco89p of trafficking to and from the vacuole membrane. Future studies will also look to address whether Vid vesicle formation is linked to anterograde and retrograde transport pathways.
Figure 1.
A model for the vacuole import and degradation pathway. During glucose starvation, cargo proteins destined for the Vid pathway associate with the TORC1 complex. After the addition of glucose, Tor1p dissociates from this complex. This facilitates the cargo proteins to be phosphorylated. Thereafter, Tco89p functions to import the cargo proteins into the Vid vesicles. The Vid vesicles merge with endosomes to deliver the contents to the vacuole for degradation. Retrograde vesicles form from the vacuole membrane as means to maintain the size of the vacuole.
Acknowledgements
This work was supported by a NIH grant R01GM 59480 and a grant from the PA Tobacco Settlement Fund to Hui-Ling Chiang.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/13241
References
- 1.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular selfdigestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seglen PO, Gordon PB, Holen I. Non-selective autophagy. Semin Cell Biol. 1990;1:441–448. [PubMed] [Google Scholar]
- 3.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;7:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 5.Kaushik S, Cuervo AM. Chaperone-mediated autophagy. Methods Mol Biol. 2008;445:227–244. doi: 10.1007/978-1-59745-157-4_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman J, Stevens TH. Protein sorting in yeast: mutants defective in vacuolar biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986;47:1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9:65–76. [PMC free article] [PubMed] [Google Scholar]
- 10.Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn WA., Jr Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol. 1990;110:1923–1933. doi: 10.1083/jcb.110.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang HL, Schekman R. Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature. 1991;350:313–318. doi: 10.1038/350313a0. [DOI] [PubMed] [Google Scholar]
- 13.Gancedo C. Inactivation of fructose-1,6-bisphosphatase by glucose in yeast. J Bacteriol. 1971;107:401–405. doi: 10.1128/jb.107.2.401-405.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang HL, Dice JF. Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J Biol Chem. 1988;263:6797–6805. [PubMed] [Google Scholar]
- 15.Hoffman M, Chiang HL. Isolation of degradation-deficient mutants defective in the targeting of fructose-1,6-bisphosphatase into the vacuole for degradation in Saccharomyces cerevisiae. Genetics. 1996;143:1555–1566. doi: 10.1093/genetics/143.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung GC, Brown CR, Wolfe AB, Liu J, Chiang HL. Degradation of the gluconeogenic enzymes fructose-1,6-bisphosphatase and malate dehydrogenase is mediated by distinct proteolytic pathways and signaling events. J Biol Chem. 2004;279:49138–49150. doi: 10.1074/jbc.M404544200. [DOI] [PubMed] [Google Scholar]
- 17.Horak J, Wolf DH. Two distinct proteolytic systems responsible for glucose induced degradation of fructose- 1,6-bisphosphatase and the Gal2p transporter in the yeast Saccharomyces cerevisiae share the same protein components of the glucose signaling pathway. J Biol Chem. 2002;77:8248–8254. doi: 10.1074/jbc.M107255200. [DOI] [PubMed] [Google Scholar]
- 18.Regelmann J, Schule T, Josupeit FS, Horak J, Rose M, Entian KD, et al. Catabolite degradation of fructose-1,6-bisphosphatase in the yeast Saccharomyces cerevisiae: a genome-wide screen identifies eight novel GID genes and indicates the existence of two degradation pathways. Mol Biol Cell. 2003;14:1652–1663. doi: 10.1091/mbc.E02-08-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang PH, Chiang HL. Identification of novel vesicles in the cytosol to vacuole protein degradation pathway. J Cell Biol. 1997;136:803–810. doi: 10.1083/jcb.136.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown CR, McCann JA, Chiang HL. The heat shock protein Ssa2p is required for import of fructose-1,6-bisphosphatase into Vid vesicles. J Cell Biol. 2000;150:65–76. doi: 10.1083/jcb.150.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown CR, Cui DY, Hung GG, Chiang HL. Cyclophilin A mediates Vid22p function in the import of fructose-1,6-bisphosphatase into Vid vesicles. J Biol Chem. 2001;276:48017–48026. doi: 10.1074/jbc.M109222200. [DOI] [PubMed] [Google Scholar]
- 22.Brown CR, McCann JA, Hung G, Elco C, Chiang HL. sVid22p, a novel plasma membrane is required for the fructose-1,6-bisphosphatase degradation pathway. J Cell Sci. 2001;115:655–666. doi: 10.1242/jcs.115.3.655. [DOI] [PubMed] [Google Scholar]
- 23.Shieh HL, Chen Y, Brown CR, Chiang HL. Biochemical analysis of fructose-1,6-bisphosphatase import into vacuole import and degradation vesicles reveals a role for UBC1 in vesicle biogenesis. J Biol Chem. 2001;276:10398–10406. doi: 10.1074/jbc.M001767200. [DOI] [PubMed] [Google Scholar]
- 24.Chiang MC, Chiang HL. Vid24p, a novel protein localized to the fructose-1,6-bisphosphatase containing vesicles, regulates targeting of fructose-1,6-bisphosphatase from the vesicles to the vacuole for degradation. J Cell Biol. 1998;140:1347–1356. doi: 10.1083/jcb.140.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown CR, Wolfe AB, Cui D, Chiang HL. The vacuolar import and degradation pathway merges with the endocytic pathway to deliver fructose-1,6-bisphosphatase to the vacuole for degradation. J Biol Chem. 2008;283:26116–26127. doi: 10.1074/jbc.M709922200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown CR, Hung GC, Dunton DD, Chiang HL. The TOR Complex 1 is distributed in endosomes and in retrograde vesicles that form from the vacuole membrane and plays an important role in the vacuole import and degradation pathway. J Biol Chem. 2010;285:23359–23370. doi: 10.1074/jbc.M109.075143. [DOI] [PMC free article] [PubMed] [Google Scholar]