Abstract
Experience dependent alterations in neural activity are mediated by diverse forms of plasticity, which are conventionally thought to occur at either synaptic terminals and/or postsynaptic membrane, such as dendrites and cell soma. However, our recent study has revealed that plasticity is not limited to synaptic sites, but it also takes place at the site where neural activity arises, the axon initial segment (AIS), which is a highly specialized region in the axon concentrated with voltage-gated Na+ channels. We observed in an avian brainstem auditory neuron that the AIS reorganized itself to elongate after deprivation of sensory inputs, which augmented the excitability of the neuron. Notably, this elongation of AIS caused spontaneous firing in some neurons, suggesting its compensatory role to restore neural activity in the circuit. Given that the AIS is the source of neural activity, this plasticity should be a most efficient mechanism for neurons to control their activity. This finding will provide a new insight into development, maintenance and refinement of neural circuits.
Key words: auditory, axon initial segment, homeostasis, neuron, plasticity, sodium channel
Axon initial segment (AIS) is enriched with voltage-gated Na+ (Nav) channels and contributes to the induction of action potentials in neurons.1–4 AIS was classically defined as an unmyelinated region of the axon near the cell soma.5 However, our recent studies have revealed that the distribution of AIS is more diverse and delicately determined in each neuron to meet its specific need.6,7 In nucleus magnocellularis (NM) and nucleus laminaris of birds, which are the second and third order nuclei in the auditory pathway involved in the calculation of binaural temporal information for sound localization,8 the location and the length of AIS vary among neurons depending on their frequency tuning, thereby optimizing their signal processing. This implies that the distribution of AIS is optimally tuned to synaptic inputs, thus playing a critical role in regulating neural activity and function.
The optimal tuning of AIS further raised a possibility that the distribution of AIS could be modulated by presynaptic activity. This was indeed found in our latest study,9 where chicks were monoaurally deprived by removing unilateral cochlea at posthatch day 1 and the effects were examined in NM. Within 7 days after deprivation of auditory inputs, the length of Nav channel cluster at the AIS increased dramatically in the deprived side (see Fig. 1), while its location and channel density were not altered. Importantly, the Nav channel cluster was accompanied by ankyrinG, which is a membrane scaffold protein to anchor Nav channels at the AIS,10–12 indicating that the AIS structure itself was elongated after auditory deprivation.
Figure 1.
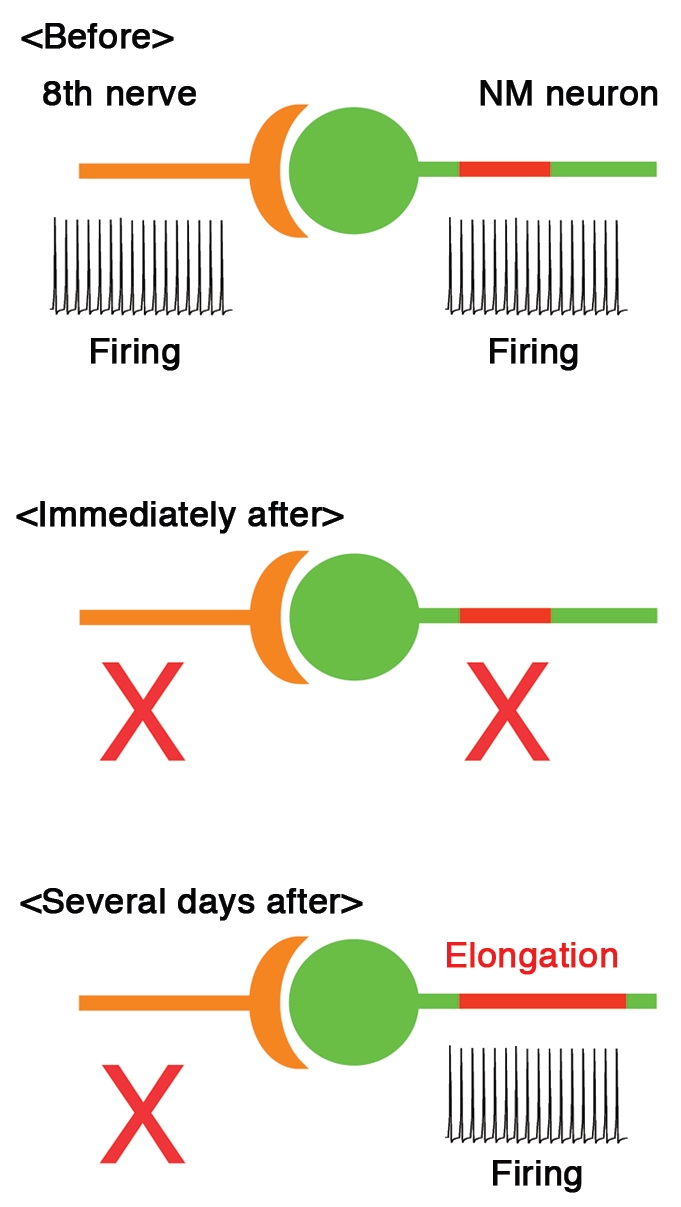
AIS plasticity compensates for the loss of auditory (8th) nerve activity in NM. Length of AIS (red) increases several days after deprivation of auditory inputs, which enhances the membrane excitability and restores firing in NM neuron (bottom). See the text for details.
Elongation of AIS also occurred with less sever manipulations, such as tympanic membrane puncture or immobilization of middle ear bones, which attenuate auditory inputs without damaging cochlea hair cells.13 Furthermore, the extent of elongation was systematically dependent on the levels of attenuation. These supported the idea that a decrease of presynaptic activity rather than a damage of cochlea itself triggers this elongation.
As expected from the elongation of AIS, auditory deprivation increased Na+ current in the axon. Accordingly, spike threshold was reduced and spikes were generated with a smaller current in the deprived side. In consequence, spontaneous firing appeared in some neurons after auditory deprivation. Thus, we showed that auditory deprivation elongates the AIS, thereby enhancing the excitability of NM neurons. This indicates that AIS has a capacity for plasticity and reorganizes itself to regulate neural activity.
What are the roles of this AIS plasticity in the auditory circuit? NM neurons are driven at high rates by auditory nerve activity in vivo,14 which completely disappears after auditory deprivation15 (see Fig. 1). Neural activity is crucial for the maintenance of neural circuits.16 Thus, the restored activity of NM neurons after auditory deprivation may indicate that the AIS plasticity works as a homeostatic mechanism to compensate for the loss of auditory nerve activity, thereby contributing to the maintenance of auditory circuits after hearing loss. Notably, auditory deprivation disrupted the sound frequency dependence of AIS length in NM, indicating that the AIS plasticity may also play a role in the optimization of circuit function through tuning the AIS. These speculations should be tested in future studies.
Activity dependent change in the distribution of AIS was also found in hippocampal pyramidal neurons in culture,17 indicating that the AIS plasticity is not specific to the auditory system, but occurs generally in the brain. Interestingly, activity affects the location of AIS rather than the length in these neurons, suggesting that the phenotype of AIS plasticity may differ depending on neuronal types and brain areas.
The shift of AIS location was sensitive to blockers of voltage gated Ca2+ channels,17 indicating that local [Ca2+]i would be important for the AIS plasticity. Consistently, it is reported that Ca2+ channels are concentrated in the AIS18 and the expression of Nav channels could be regulated by [Ca2+]i with a negative feedback mechanism.19 It is interesting to see whether the elongation of AIS is also dependent on [Ca2+]i, and if so, what determines the phenotype of AIS plasticity i.e., location or length. Additional works are also needed on detailed molecular mechanisms downstream [Ca2+]i.
It has become evident that AIS is more dynamic than previously thought and changes its distribution to regulate neuronal activity. This new form of plasticity should have the most direct impacts on neuronal excitability and play a crucial role in determining output of neurons. However, many questions remain on the characteristics, mechanisms and significance of this AIS plasticity. Unveiling these issues will reinforce our knowledge on neuronal computation as well as mechanisms to maintain homeostasis of neural circuit.
Acknowledgements
I thank R. Yamada for critically reading the manuscript. This work was supported by Grants-in-aid from MEXT (22680032) and JST PRESTO program.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/13242
References
- 1.Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- 2.Khaliq ZM, Raman IM. Relative contribution of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci. 2006;26:1935–1944. doi: 10.1523/JNEUROSCI.4664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action-potential initiation in neocortical pyramidal cells: evidence from whole-cell axon recording. J Neurophysiol. 2007;97:746–760. doi: 10.1152/jn.00922.2006. [DOI] [PubMed] [Google Scholar]
- 5.Palay SL, Sotelo C, Peters A, Orkand PM. The axon hillock and the initial segment. J Cell Biol. 1968;38:193–201. doi: 10.1083/jcb.38.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–1072. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- 7.Kuba H, Ohmori H. Roles of axonal sodium channels in precise auditory time coding at nucleus magnocellularis of the chick. J Physiol. 2009;587:87–100. doi: 10.1113/jphysiol.2008.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konishi M. Coding of auditory space. Annu Rev Neurosci. 2003;26:31–55. doi: 10.1146/annurev.neuro.26.041002.131123. [DOI] [PubMed] [Google Scholar]
- 9.Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- 10.Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol. 2007;176:509–519. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tucci DL, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: Effects of conductive and sensorineural hearing loss on N. Magnocelularis. J Comp Neurol. 1985;238:371–381. doi: 10.1002/cne.902380402. [DOI] [PubMed] [Google Scholar]
- 14.Warchol ME, Dallos P. Neural coding in the chick cochlear nucleus. J Comp Physiol A. 1990;166:721–734. doi: 10.1007/BF00240021. [DOI] [PubMed] [Google Scholar]
- 15.Born DE, Durham D, Rubel EW. Afferent influences on brainstem auditory nuclei of the chick: nucleus magnocellularis neuronal activity following cochlea removal. Brain Res. 1991;557:37–47. doi: 10.1016/0006-8993(91)90113-a. [DOI] [PubMed] [Google Scholar]
- 16.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 17.Grubb MS, Burrone J. Activity-dependent relocation of axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron. 2009;61:259–271. doi: 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman SJ, Catterall WA. Electrical activity and cytosolic calcium regulate levels of tetrodotoxinsensitive sodium channels in cultured rat muscle cells. Proc Natl Acad Sci USA. 1984;81:262–266. doi: 10.1073/pnas.81.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]


