Abstract
Research suggests that social calls are important for conveying information about food and roost location in bats. However, no studies have specifically documented calls that are used to actively attract conspecifics to roosting locations. Here we describe the cooperative signaling behavior of roost location towards flying conspecifics in Spix's disc-winged bat (Thyroptera tricolor), a species that uses a highly ephemeral roosting resource. Two types of calls were recorded during field experiments; one from flying individuals termed “inquiry calls” and another from roosting bats termed “response calls”. Inquiry calls were emitted by flying bats immediately upon release, and quickly elicited production of response calls from roosting individuals. Most flying bats entered the roost when roosting individuals responded, while very few bats entered the roost in the absence of a response. During playback experiments, we found significant differences in response rates among individuals, which could be caused by diverse intrinsic and extrinsic factors. In addition, results of our ongoing field studies suggest that the cooperative signaling behavior of roost location is important in maintaining social cohesion, and that the use of a larger home range when resources are scarcer may decrease group stability by hindering communication.
Key words: cooperation, information transfer, roost, social calls
Cooperation, a behavior performed by an individual that provides a benefit to a recipient, poses a problem to evolutionary theory because it potentially has negative fitness consequences on the performer. Thus, in the absence of a specific mechanism for the evolution of cooperation, natural selection should favor defectors. Notwithstanding, cooperative behaviors have been described in a wide diversity of taxa, including crustaceans, insects, fish, birds and mammals.1 Types of cooperative behaviors include caring for young, grooming, sharing food, defense against predators, group hunting and sharing information about predators and food location.2–11 Information transfer about food location, in particular, has been a widely recognized cooperative behavior that has apparently played a major role in the evolution of sociality in birds and bats.12,13
Even though locating adequate food patches is a critical daily task for bats, finding and securing suitable roosting sites is also important, as roosts provide protection from predators and inclement weather, and also serve as sites for social and reproductive activities.14 Bats use a wide diversity of structures for roosting, including caves, rock crevices, tree cavities and plants.15 Depending on the ephemerality of a roost and the particular needs of the bats, such as avoiding predators and parasites, roosts may be used by individuals for several years or for very short periods of less than 24 hours.16 Using such ephemeral roosts means that individuals need to locate suitable sites more often. Unfortunately, very little is known about how individuals locate sparse and ephemeral roosts. A few studies have suggested that acoustic signals may be an important cue used by bats to recruit conspecifics to roost-sites,17–19 but to date no research has examined if social calls are in fact actively used for this purpose.
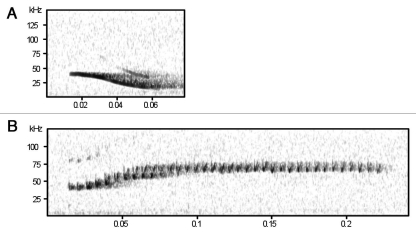
Our study of the social calls emitted by Spix's disk-winged bat (Thyroptera tricolor) provides, for the first time, conclusive evidence that a bat species uses cooperative signaling behaviors to convey information about roost location towards flying conspecifics.20 Spix's disk wing bat is a neotropical species that maintains highly cohesive social groups,21,22 yet roosts in a very ephemeral habitat—the furled leaves of members of the order Zingiberales. In a series of field experiments, we found that when individuals are looking for roosts or roostmates, they emit “inquiry calls” (Fig. 1A) that often elicit a response from individuals who have already entered a furled leaf. These “response calls” (Fig. 1B) frequently drive the flying individual into the roost. Response calls appear to be emitted by roosting individuals intentionally to aid conspecifics in the location of roosts because they are emitted only after an audible inquiry call and vocalization of response calls ceases immediately after the flying individual enters the roost.
Figure 1.
Sonograms showing (A) an inquiry call and (B) a response call.
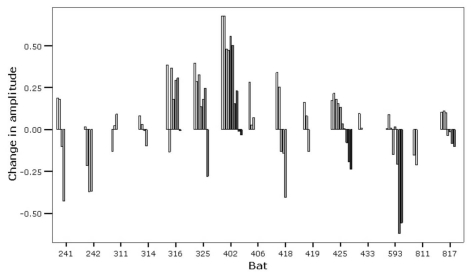
Results of acoustic trials show that flying bats typically emit an inquiry call immediately after being released, but do so rather infrequently (i.e., once every few seconds), while roosting bats emit numerous response calls in close succession. During these response bouts, the maximum amplitude of calls showed a decline with time (Fig. 2). Thus, response calls emitted right after the inquiry call were louder compared to response calls emitted later in a bout. We also observed that lactating females did not respond to inquiry calls or responded with only a single, weak call.
Figure 2.
Change in amplitude of response calls per individual. Bars indicate the difference in amplitude of first calls and subsequent calls emitted in a bout, with increasingly darker bars representing later calls.
We conducted playback experiments with 53 bats, in which an individual was placed in a tubular leaf and presented with a series of recordings of inquiry calls. We found that response rates differ considerably between individuals, with 42% of bats responding quickly (i.e., immediately after the first inquiry call was broadcasted) and vigorously (i.e., loud and repetitive calls within a bout), to inquiry call playbacks. Another 19% responded, vigorously or not, within the first 10 broadcasted calls, while another 7% responded only after the same set of inquiry calls had been repeatedly broadcasted (i.e., after 20–30 calls). Thirtytwo percent of individuals sampled never responded to any of the inquiry calls broadcasted. Response rates did not appear to be influenced by factors such as time of day, temperature or time since capture, as individuals tested consecutively often differed significantly in their response to inquiry calls. These results suggest that there may be differences in cooperative rates among individuals, and that these rates may be influenced by factors such as reproductive status. Other factors that are known to affect cooperation include dispersal patterns, resource abundance, predation risk and group size,23–26 but whether any of these are relevant in explaining cooperation rates in T. tricolor remains to be tested.
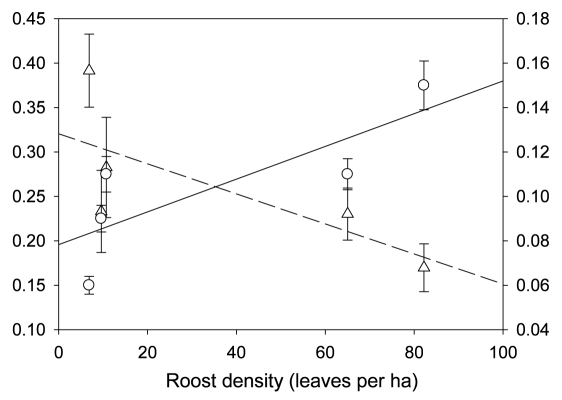
The cooperative signaling behavior of roost location towards flying conspecifics in T. tricolor may be an important means by which individuals help conspecifics reduce the costs associated with flight, such as high energetic expenditure and increased risk of predation,27,28 as these calls apparently increase the probability of finding roosts.20 These social calls may also significantly increase the ability to locate group members, particularly if they contain specific signatures unique to each individual, increasing group stability. However, when bats inhabit areas of scarce roosting resources, individuals must travel long distances to locate suitable furled leaves and their social calls may not be audible to all group members due to atmospheric attenuation,29 resulting in group fission. Our ongoing research on the social organization of T. tricolor shows that individuals use larger areas when roosts are scarcer and, as predicted, have lower association indices compared to individuals using smaller areas with abundant roosting resources (Fig. 3). Because a decrease in encounter rates is known to hinder reciprocation,30 further research is necessary to understand if the cooperative signaling behavior of roost location observed in T. tricolor is more prevalent in populations that inhabit areas with a greater abundance of furled leaves.
Figure 3.
Linear relationships between leaf density (furled leaves per hectare) and roosting home range size (in hectares) and mean association index (proportion of time that two individuals spent in association). Error bars show mean ± 1 standard error.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/13277
References
- 1.Dugatkin LA. Cooperation among animals: an evolutionary perspective. Oxford: Oxford University Press; 1997. [Google Scholar]
- 2.Russell AF, Hatchwell BJ. Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc R Soc Lond B. 2001;268:2169–2174. doi: 10.1098/rspb.2001.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumstein DT, Armitage KB. Cooperative breeding in marmots. Oikos. 1999;84:369–382. [Google Scholar]
- 4.Lehmann J, Korstjens AH, Dunbar RI. Group size, grooming and social cohesion in primates. Anim Behav. 2007;74:1617–1629. [Google Scholar]
- 5.Bulmer E, Celis P, Gil D. Parent-absent begging: evidence for sibling honesty and cooperation in the spotless starling (Sturnus unicolor) Behav Ecol. 2008;19:279–284. [Google Scholar]
- 6.Wilkinson GS. Reciprocal food sharing in the vampire bat. Nature. 1984;308:181–184. [Google Scholar]
- 7.Gilby IC, Eberly LE, Wrangham RW. Economic profitability of social predation among wild chimpanzees: individual variation promotes cooperation. Anim Behav. 2008;75:351–360. [Google Scholar]
- 8.Garay J. Cooperation in defence against a predator. J Theor Biol. 2009;257:45–51. doi: 10.1016/j.jtbi.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Wright J, Maklakov AA, Khazin V. State-dependent sentinels: an experimental study in the Arabian babbler. Proc R Soc Lond B. 2001;268:821–826. doi: 10.1098/rspb.2000.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macdonald DW. The ecology of carnivore social behaviour. Nature. 1983;301:379–384. [Google Scholar]
- 11.Wilkinson GS. Information transfer at evening bat colonies. Anim Behav. 1992;44:501–518. [Google Scholar]
- 12.Ward P, Zahavi A. The importance of certain assemblages of birds as ‘information-centres’ for food finding. Ibis. 1973;115:517–534. [Google Scholar]
- 13.Safi K, Kerth G. Comparative analyses suggest that information transfer promoted sociality in male bats in the temperate zone. Am Nat. 2007;170:465–472. doi: 10.1086/520116. [DOI] [PubMed] [Google Scholar]
- 14.Kunz TH. Roosting ecology of bats. In: Kunz TH, editor. Ecology of Bats. New York: Plenum Press; 1982. pp. 1–50. [Google Scholar]
- 15.Kunz TH, Lumsden LF. Ecology of cavity and foliage roosting bats. In: Kunz TH, Fenton MB, editors. Bat Ecology. Chicago: University of Chicago Press; 2003. pp. 3–87. [Google Scholar]
- 16.Lewis SE. Roost fidelity of bats: a review. J Mammal. 1995;76:481–496. [Google Scholar]
- 17.Kerth G, Reckardt K. Information transfer about roosts in female Bechstein's bats: an experimental field study. Proc R Soc Lond B. 2003;270:511–515. doi: 10.1098/rspb.2002.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruczynski I, Kalko EKV, Siemers BM. The sensory basis of roost finding in a forest bat, Nyctalus noctula. J Exp Biol. 2007;210:3607–3615. doi: 10.1242/jeb.009837. [DOI] [PubMed] [Google Scholar]
- 19.Ruczynski I, Kalko EKV, Siemers BM. Calls in the forest: a comparative approach to how bats find tree cavities. Ethology. 2009;115:167–177. [Google Scholar]
- 20.Chaverri G, Gillam EH, Vonhof MJ. Social calls used by a leaf-roosting bat to signal location. Biol Lett. 2010;6:441–444. doi: 10.1098/rsbl.2009.0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vonhof MJ, Whitehead H, Fenton MB. Analysis of Spix's disc-winged bat association patterns and roosting home ranges reveal a novel social structure among bats. Anim Behav. 2004;68:507–521. [Google Scholar]
- 22.Chaverri G. Comparative social network analysis in a leaf-roosting bat. Behav Ecol Sociobiol. 2010;64:1619–1630. [Google Scholar]
- 23.Krams I, Berzinš A, Krama T, Wheatcroft D, Igaune K, Rantala MJ. The increased risk of predation enhances cooperation. Proc R Soc Lond B. 2010;277:513–518. doi: 10.1098/rspb.2009.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner K. Cooperative strategies and the evolution of communication. Artif Life. 2000;6:149–179. doi: 10.1162/106454600568384. [DOI] [PubMed] [Google Scholar]
- 25.Kümmerli R, Gardner A, West SA, Griffin AS. Limited dispersal, budding dispersal and cooperation: an experimental study. Evolution. 2009;63:939–949. doi: 10.1111/j.1558-5646.2008.00548.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuki H, Hauert C, Lieberman E, Nowak MA. A simple rule for the evolution of cooperation on graphs and social networks. Nature. 2006;441:502–505. doi: 10.1038/nature04605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas SP, Suthers RA. The physiology and energetics of bat flight. J Exp Biol. 1972;57:317–335. [Google Scholar]
- 28.Fenton MB, Rautenbach IL, Smith SE, Swanepoel CM, Grosell J, van Jaarsveld J. Raptors and bats: threats and opportunities. Anim Behav. 1994;48:9–18. [Google Scholar]
- 29.Bradbury JW, Vehrencamp SL. Principles of animal communication. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- 30.Ferriere R, Michod RE. The evolution of cooperation in spatially heterogeneous populations. Am Nat. 1996;147:692–717. [Google Scholar]