Abstract
Using bioinformatics and biochemical methods in the recent past we have reported the isolation and characterization of the main components of translation initiation complex eIF4F from malaria parasite Plasmodium falciparum. We reported that eukaryotic initiation factor 4A (eIF4A), eukaryotic initiation factor 4E (eIF4E), eukaryotic initiation factor 4G (eIF4G) and poly (A) binding protein (PABP) are structurally and functionally conserved in this parasite. In the present study we report further characterization of PfeIF4A and PfeIF4E. We report that PfeIF4A and PfeIF4E are co-localized and predominantly localized in the cytoplasm. The parasite cultures treated with co-addition of PfeIF4A and PfeIF4E double stranded RNA showed ∼67% growth inhibition suggesting that inhibition of two components of the same pathway is more effective for inhibiting the proliferation of the malaria parasite Plasmodium falciparum. These observations suggest that PfeIF4A and PfeIF4E are critical for parasite growth and survival.
Key words: growth, malaria, parasite, Plasmodium falciparum, RNA interference, translation initiation factor 4A, translation initiation factor 4E
Malaria is one of the most widespread dangerous human parasitic diseases. It affects approximately 300–500 million people each year worldwide and about 2 million of infected individuals die every year. The four main species responsible for malaria in human are Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae and Plasmodium vivax.1,2 Of these four species of Plasmodium responsible for malaria, Plasmodium falciparum causes the most lethal form in humans resulting in morbidity and mortality.2 Plasmodium falciparum has become drug resistant to most of the antimalarial drugs in use and an effective vaccine is not yet available therefore the eradication of malaria has failed.3,4 For proper control of cellular growth and development, the regulation of gene expression at the level of translation initiation is important. The eukaryotic translation initiation complex eIF4F is mainly composed of the cap-binding protein eukaryotic initiation factor 4E (eIF4E), the helicase eukaryotic initiation factor 4A (eIF4A) and the multidomain scaffold protein eukaryotic initiation factor 4G (eIF4G).5 In order to understand the basic biology and mainly the process of translation initiation in Plasmodium falciparum we have bioinformatically characterized the main components of eIF4F such as eIF4E, eIF4A (PfH45), eIF4G and poly (A) binding protein (PABP) from this parasite.6 We have also cloned and functionally characterized an eIF4A homologue designated as PfH45 and eIF4E, eIF4G and PABP from Plasmodium falciparum.7,8 Similar to other systems our results have shown that PfeIF4G is the scaffold for the cap-binding complex and it binds to PfeIF4E, PfeIF4A (PfH45) and PfPABP.8 In the present study we report further characterization of PfeIF4E and PfeIF4A (PfH45) and show that these factors colocalize in the cytosol in the intraerythrocytic developmental stages of Plasmodium falciparum. We have investigated the consequences of a decrease in PfeIF4E transcript alone and in combination with PfeIF4A (PfH45) and the results show that double-stranded RNA (dsRNA) of PfeIF4E and PfeIF4A (PfH45) specifically blocks the growth of the parasite in culture. Using RNA interference assay we show that PfeIF4A and PfeIF4A (PfH45) are essentially required for the growth and survival of the parasite.
The in vivo detection of PfeIF4E and PfeIF4A (PfH45) was done by immunofluorescence assay. For this assay smears of parasitized red blood cells of asynchronous cultures were prepared and fixed by paraformaldehyde and glutaraldehyde method as described previously.7,9 The slides were washed with PBS and incubated with purified IgG of anti-PfeIF4E (raised in mice) and anti-PfeIF4A (PfH45) (raised in rabbit) at appropriate dilutions in PBS containing BSA for overnight at 4°C. The slides were washed four times with PBS for 15 min each and then incubated for 1 h at 4°C with secondary antibodies Cy3-conjugated anti-mouse IgG (Sigma) and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (Sigma) diluted in PBS containing BSA. After washing the slides were incubated in 4′,6′-di-amidino-2-phenylindole dihydrochloride (DAPI) (2 µg/ml in PBS) for nuclear staining. The slides were washed thrice with PBST (PBS, 0.5% Tween 20) for 10 min each and twice with PBS for 10 min each and mounted with anti-fade reagent (Fluroguard, BioRad, USA) and viewed under oil immersion. The images were collected using a Bio-Rad 2100 laser-scanning microscope attached to a Nikon TE 2000U microscope and the figures were prepared using Adobe Photoshop.
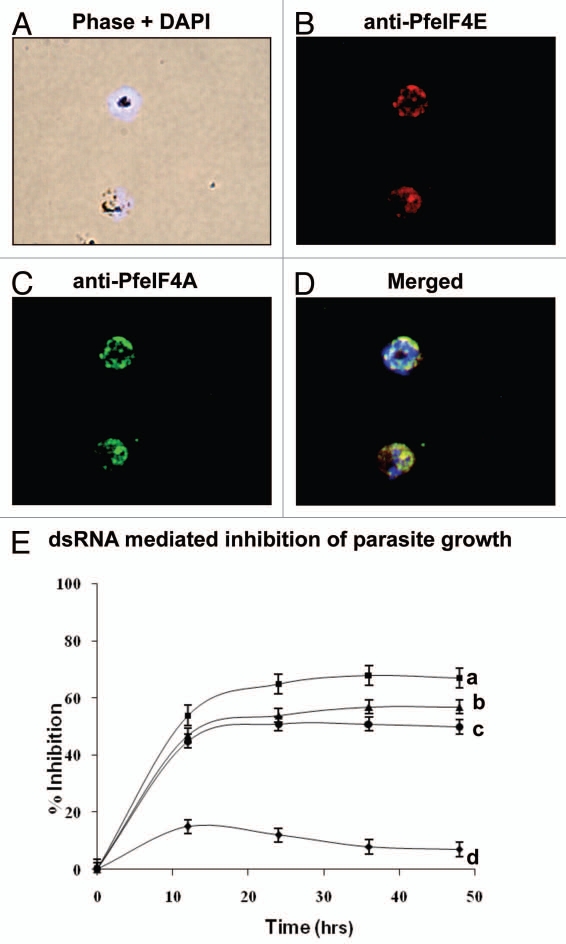
In ‘PlasmoDB’ (www.plasmodb.org) the transcriptome along with the proteome data indicate that PfeIF4E and PfeIF4A (PfH45) are expressed in all the intraerythrocytic developmental stages of Plasmodium falciparum. The purified antibodies to PfeIF4E and PfeIF4A were used to study their localization in Plasmodium falciparum by immunohistochemical methods. The fluorescence staining indicated that endogenous PfeIF4E and PfeIF4A (PfH45) co-localize and are distributed mainly in the cytoplasm in the parasite (Fig. 1B–D respectively). The nuclei were identified by DAPI staining (Fig. 1A) and the overlay of all the images is shown in Figure 1D. It has been reported that in higher eukaryotes, eIF4E is distributed primarily in the cytoplasm however a fraction is present in the nucleus also.10
Figure 1.
(A–D) Immunofluorescence assay of PfeIF4E (B) and PfeIF4A (C) respectively in fixed parasite cultures showing co-localization (D). (E) Effect of dsRNA on in vitro growth of the parasite. The relative growth of unsynchronized cultures at various time-points after treatment with dsRNA was measured and plotted. The results are represented as percent inhibition of the growth compared to control cultures. The bars indicate standard deviation. The curves are as follows: (a) cultures treated with PfeIF4A and PfeIF4E dsRNA; (b) cultures treated with PfeIF4A dsRNA; (c) cultures treated with PfeIF4E dsRNA; and (d) cultures treated with control GFP dsRNA.
The cap-binding and scanning of mRNA by the initiation complex are the two major points of regulation of translation. It is well established that RNA interference (RNAi) causes degradation of targeted endogenous RNA in many diverse organisms and several genes were “knocked down” effectively by RNAi in malaria parasite.11,12 We have previously shown that PfeIF4A (PfH45) dsRNA inhibits the growth of the parasite up to ∼50% in culture, which is due to specific downregulation in the synthesis of PfH45 protein.7 The localization data described in the previous section show that PfeIF4E and PfeIF4A (PfH45) co-localize in the cytoplasm. Therefore in order to study the effect of inhibition of the two components of the same pathway, the double-stranded RNA (dsRNAs) corresponding to PfeIF4E and PfeIF4A (PfH45), either separately or in combination were used in unsynchronized cultures. RNAs were synthesized in vitro using RiboMAX Large Scale RNA Production Systems SP6 and T7 (Promega) using linearized templates of pGEM-TGFP (green fluorescent protein), pGEM-T-PfeIF4E and pGEM-T-PfeIF4A. After the removal of template DNAs, RNAs were annealed to form (dsRNA).7 In these cultures 10 µg/ml of dsRNA of PfeIF4E or an unrelated gene GFP as control was added and the parasitemia was determined by manual counting. Each experiment was repeated at least three times with triplicate samples. It was interesting to note that the parasite growth was inhibited and it declined to ∼45% at 12 h after addition of specific dsRNA corresponding to PfeIF4A or PfeIF4E to the cultures (Fig. 1E, curve b and c respectively) but the addition of the control dsRNA had negligible effect on the parasite growth (Fig. 1E curve d). It was further noted that the change in the decline in the growth with longer incubation time was not very significant (∼50% as opposed to ∼45%; Fig. 1E, curve b and c respectively). These data show clearly that dsRNA affects the parasite growth. For studying the effect of co-addition on parasite growth, the dsRNAs corresponding to PfeIF4E and PfeIF4A (PfH45) were added to the same cultures and the effect was determined as described above. It was interesting to note that although the inhibition was not additive but the co-addition of the two RNAs did result in further inhibition of the parasite growth (∼67% as opposed to ∼45%, Fig. 1E curve a). This decline in growth is most likely due to the downregulation in the synthesis of endogenous PfeIF4E and PfeIF4A proteins.
In Caenorhabditis elegans it has been reported that IFE-3, the isoform most closely related to mammalian eIF4E-1, is essential for viability.13 We have previously reported that PfH45 is essential for parasite growth and survival.7 In the present study we have shown that PfeIF4E is required for parasite survival and both PfeIF4E and PfeIF4A dsRNAs further inhibit the growth of Plasmodium falciparum significantly. This inhibition in growth might be due to RNA interference pathway or an antisense effect.14–21 The antisense transcripts are reported to be present in intraerythrocytic cycle of the parasite and these might be acting as regulatory elements in gene transcription. The mechanism by which gene expression is modified by dsRNA in Plasmodium is still not clearly defined and the homologues of the genes of the components of the classic RNA interference pathway have not been identified in Plasmodium genome therefore this effect might be due to antisense RNA.16,17 The results reported in this manuscript are first to demonstrate that the dsRNA mediated inhibition of the activity of two components of the same pathway is more effective as compared to the inhibition rendered by individual components.
Acknowledgements
This work in R.T's laboratory is partially supported by the Department of Biotechnology and Defence Research and Development Organization grants. Infrastructural support from the Department of Biotechnology, Government of India is gratefully acknowledged.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/13396
References
- 1.Miller LH, Greenwood B. Malaria—a shadow over Africa. Science. 2002;298:121–122. doi: 10.1126/science.1078048. [DOI] [PubMed] [Google Scholar]
- 2.Tuteja R. Malaria-An overview. FEBS J. 2007;274:4670–4679. doi: 10.1111/j.1742-4658.2007.05997.x. [DOI] [PubMed] [Google Scholar]
- 3.Wiseman V, Kim M, Mutabingwa TK, Whitty CJ. Cost-effectiveness study of three antimalarial drug combinations in Tanzania. PLoS Med. 2006;3:373. doi: 10.1371/journal.pmed.0030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todryk SM, Hill AV. Malaria vaccines: the stage we are at. Nat Rev Microbiol. 2007;5:487–489. doi: 10.1038/nrmicro1712. [DOI] [PubMed] [Google Scholar]
- 5.Preiss T, Hentze MW. Starting the protein synthesis machine: eukaryotic translation initiation. Bioessays. 2003;25:1201–1211. doi: 10.1002/bies.10362. [DOI] [PubMed] [Google Scholar]
- 6.Tuteja R. Identification and bioinformatics characterization of translation initiation complex eIF4F components and poly(A)-binding protein from Plasmodium falciparum. Comm Int Biol. 2009;2:1–16. doi: 10.4161/cib.2.3.8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pradhan A, Tuteja R. Bipolar, dual Plasmodium falciparum helicase 45 expressed in the intraerythrocytic developmental cycle is required for parasite growth. J Mol Biol. 2007;373:268–281. doi: 10.1016/j.jmb.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 8.Tuteja R, Pradhan A. Isolation and functional characterization of eIF4F components and poly(A)-binding protein from Plasmodium falciparum. Parasitology Intl. 2009;58:481–485. doi: 10.1016/j.parint.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Trager W, Jensen JB. Human malaria parasite in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 10.Lejbkowicz F, Goyer C, Darveau A, Neron S, Lemieux R, Sonenberg N. A fraction of the mRNA 5′ cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc Natl Acad Sci USA. 1992;89:9612–9616. doi: 10.1073/pnas.89.20.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McRobert L, McConkey GA. RNA interference (RNAi) inhibits growth of P. falciparum. Mol Biochem Parasitol. 2002;119:273–278. doi: 10.1016/s0166-6851(01)00429-7. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra P, Dasaradhi PV, Kumar A, Mohmmed A, Agrawal N, Bhatnagar RK, et al. Double-stranded RNA-mediated gene silencing of cysteine proteases (falcipain-1 and -2) of Plasmodium falciparum. Mol Microbiol. 2002;45:1245–1254. doi: 10.1046/j.1365-2958.2002.03105.x. [DOI] [PubMed] [Google Scholar]
- 13.Keiper BD, Lamphear BJ, Deshpande AM, Jankowska-Anyszkai M, Aamodt EJ, Blumenthal T, et al. Functional characterization of five eIF4E isoforms in Caenorhabditis elegans. J Biol Chem. 2000;275:10590–10596. doi: 10.1074/jbc.275.14.10590. [DOI] [PubMed] [Google Scholar]
- 14.Gissot M, Briquet S, Refour P, Boschet C, Vaquero C. PfMyb1, a Plasmodium falciparum transcription factor, is required for intra-erythrocytic growth and controls key genes for cell cycle regulation. J Mol Biol. 2005;346:29–42. doi: 10.1016/j.jmb.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Adams B, Oldenburg A, Musiyenko A, Barik S. Characterization and expression of a PP1 serine/threonine protein phosphatase (PfPP1) from the malaria parasite, Plasmodium falciparum: demonstration of its essential role using RNA interference. Malaria J. 2002;1:5. doi: 10.1186/1475-2875-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullu E, Tschudi C, Chakraborty T. RNA interference in protozoan parasites. Cell Microbiol. 2004;6:509–519. doi: 10.1111/j.1462-5822.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 17.Militello KT, Patel V, Chessler AD, Fisher JK, Kasper JM, Gunasekera A, et al. RNA polymerase II synthesizes antisense RNA in Plasmodium falciparum. RNA. 2005;11:365–370. doi: 10.1261/rna.7940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradhan A, Tuteja R. Plasmodium falciparum DNA helicase 60: dsRNA- and antibody-mediated inhibition of the malaria parasite growth and downregulation of its enzyme activities by DNA-interacting compounds. FEBS J. 2006;273:3545–3556. doi: 10.1111/j.1742-4658.2006.05362.x. [DOI] [PubMed] [Google Scholar]
- 19.Tuteja R, Pradhan A, Sharma S. Plasmodium falciparum signal peptidase is regulated by phosphorylation and required for intra-erythrocytic growth. Mol Biochem Parasitol. 2008;157:137–147. doi: 10.1016/j.molbiopara.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Sriwilaijaroen N, Boonma S, Attasart P, Pothikasikorn J, Panyim S, Noonpakdee W. Inhibition of Plasmodium falciparum proliferation in vitro by double-stranded RNA directed against malaria histone deacetylase. Biochem Biophys Res Comm. 2009;381:144–147. doi: 10.1016/j.bbrc.2009.01.165. [DOI] [PubMed] [Google Scholar]
- 21.Crooke A, Diez A, Mason PJ, Bautista JM. Transient silencing of Plasmodium falciparum bifunctional glucose 6-phosphate dehydrogenase-6-phosphogluconolactonase. FEBS J. 2006;273:1537–1546. doi: 10.1111/j.1742-4658.2006.05174.x. [DOI] [PubMed] [Google Scholar]